Abstract
To explain the very high frequency of cystic fibrosis (CF) mutations in most populations of European descent, it has been proposed that CF heterozygotes have a survival advantage when infected with Vibrio cholerae or Escherichia coli, the toxins of which induce diarrhea by stimulation of active intestinal chloride secretion. Two assumptions underlie this hypothesis: (1) chloride conductance by the CF transmembrane conductance regulator (CFTR) is the rate-limiting step for active intestinal chloride secretion at all levels of expression, from approximately zero in patients with CF to normal levels in people who are not carriers of a mutation; and (2) heterozygotes have smaller amounts of functional intestinal CFTR than do people who are not carriers, and heterozygotes therefore secrete less chloride when exposed to secretagogues. The authors used an intestinal perfusion technique to measure in vivo basal and prostaglandin-stimulated jejunal chloride secretion in normal subjects, CF heterozygotes, and patients with CF. Patients with CF had essentially no active chloride secretion in the basal state, and secretion was not stimulated by a prostaglandin analogue. However, CF heterozygotes secreted chloride at the same rate as did people without a CF mutation. If heterozygotes are assumed to have less-than-normal intestinal CFTR function, these results mean that CFTR expression is not rate limiting for active chloride secretion in heterozygotes. The results do not support the theory that the very high frequency of CF mutations is due to a survival advantage that is conferred on heterozygotes who contract diarrheal illnesses mediated by intestinal hypersecretion of chloride.
Introduction
It is generally agreed that cystic fibrosis (CF) (MIM 219700) is the most common fatal autosomal recessive genetic defect in white persons of European descent (Romeo et al. 1989; Morral et al. 1994; Knowles et al. 1999). Approximately 1 in 3,000 white babies is born with the disease, and ∼1 in 28 healthy white adults is a carrier of a CF gene mutation. There is no evidence that this high frequency is the result of either random genetic drift or a propensity for new mutations (Rodman and Zamudio 1991; Bertranpetit and Calafell 1996). It has therefore been suggested that the high frequency of gene mutations in the white population is due to a selective advantage of being a heterozygote. There are at least two general ways in which such an advantage could be conferred. The first is increased fertility of heterozygotes, which apparently is not the case (Jorde and Lathrop 1988). The second is protection of heterozygotes against another disease.
With the discoveries that (1) CF is due to a defect in a gene product—the CF transmembrane conductance regulator (CFTR) (MIM 602421)—that normally mediates chloride permeability in epithelial cells and (2) CFTR mediates secretory diarrhea due to the toxins of Vibrio cholerae and Escherichia coli, it was logical to propose that the high frequency of CF mutations in the white population may be due to protection of heterozygotes from dehydration due to diarrheal diseases (Quinton 1982; Baxter et al. 1988; Kopelman 1993; Grubb and Gabriel 1997; Knowles et al. 1999). The fundamental assumptions underlying this heterozygote-selective-advantage hypothesis are as follows (modified from Gabriel et al. 1994): (1) chloride conductance by CFTR is the rate-limiting step for active intestinal chloride secretion at all levels of functional CFTR expression, from approximately zero in patients with CF to normal levels in people who are not carriers of a mutation; (2) heterozygotes have fewer (presumably 50%) functional intestinal CFTR chloride channels than do people who are not carriers, and they therefore secrete less chloride when exposed to secretagogues.
This theory is supported by two lines of evidence. First, the intestine of patients with CF (homozygotes for CFTR mutations) does not actively secrete chloride in response to a variety of secretagogues (Berschneider et al. 1988; Baxter et al. 1989; O’Loughlin et al. 1991; Chao et al. 1994). Second, in one study, mice that were heterozygous for CF were shown to have one half the normal amount of CFTR protein and one half the normal intestinal fluid secretion after exposure to cholera toxin (Gabriel et al. 1994).
There are two arguments against the heterozygote-selective-advantage theory. First, another study of a heterozygous mouse model did not find a defect in intestinal secretion in response to several secretagogues, including cholera toxin (Cuthbert et al. 1995). It has been suggested that this may have resulted from a milder intestinal phenotype that could have masked the difference between the responses of heterozygotic and normal mice (Grubb and Gabriel 1997). Second, CF mutations are not common in areas of the world where infectious diarrhea is most common and most lethal (Fontelo 1995). To reconcile this discrepancy, some researchers have suggested that, in certain regions of the world, there may be a disadvantage of being a heterozygote that outweighs the survival advantage from diarrhea; for example, increased salt lost in sweat in tropical climates by heterozygotes would cost more lives than would be saved when heterozygotes were exposed to diarrheal diseases (Rodman and Zamudio 1991; Quinton 1994).
Although this heterozygote-selective-advantage theory is highly appealing and is often invoked, no studies have been conducted in humans to determine whether heterozygotes have a subnormal rate of active intestinal chloride secretion. The purpose of our research was therefore to measure intestinal chloride secretion in normal subjects, parents of children with CF (i.e., heterozygotes for CF mutations), and patients with CF. Our experiment was conducted under in vivo conditions, in the jejunum. A prostaglandin analogue was used as a chloride secretagogue (Gaginella 1990; Chang and Rao 1991). For experimental use in humans, prostaglandins are advantageous because their effect on the intestine disappears soon after the agent is withdrawn, whereas the intestine continues to secrete for several days after transient exposure to cholera toxin, an effect that sometimes requires hospitalization of subjects (Schiller et al. 1997).
Subjects and Methods
This research was approved by the Institutional Review Board for Human Protection of Baylor University Medical Center. Informed consent was obtained, and the subjects were paid a fee for their participation.
Blood leukocytes or buccal cells were assayed for 86 CFTR mutations (Genzyme Genetics), which account for ∼90% of the mutations that cause CF. Because a CF mutation on one chromosome and a 5T allele on intron 8 of the CFTR gene on the other chromosome cause low CFTR expression and atypical forms of CF (Chillon et al. 1995), the poly(T) variant on intron 8 of the CFTR gene was also measured (Genzyme Genetics).
Jejunal secretion was measured by a constant-perfusion technique (Davis et al. 1980), using a triple-lumen tube and a nonabsorbable marker. The infusion site of the tube was located at the ligament of Treitz; the mixing segment was 10 cm long, and the test segment was 30 cm. The standard perfused solution contained the following: 140 mM Na+, 4 mM K+, 104 mM Cl−, 0 mM HCO3−, and 20 mM SO42−. Mannitol 40 mM was added to make the solution isotonic to plasma, and 2 g polyethylene glycol/liter was the nonabsorbable marker. During perfusion of this bicarbonate-free solution, active absorptive mechanisms by the jejunum are eliminated, and the true rate of active chloride secretion is revealed (Davis et al. 1980). The test solution was infused at a rate of 10 ml/min. Perfusion periods consisted of 50 min of equilibration and 60-min study periods, during which samples were collected for analysis by methods published elsewhere (Davis et al. 1980). The rate of absorption or secretion by the 30-cm segment of jejunum located between the proximal and distal aspiration sites was calculated using standard equations. Potential difference (PD) was measured continuously between a flowing intraluminal electrode and a subcutaneous reference electrode (Read and Fordtran 1979).
There were two study periods for each subject, the first to measure basal active chloride secretion and the second to measure the rate of active chloride secretion under stimulation by a prostaglandin analogue. For the latter purpose, we used a prostaglandin E1 agonist (misoprostol; G. D. Searle), in a concentration of 800 μg/liter. The amount of misoprostol infused during the 110-min perfusion was 880 μg. By comparison, the dose of misoprostol used clinically for prevention of gastroduodenal ulcers induced by nonsteroidal anti-inflammatory drugs is 100–200 μg orally four times per day.
Statistical significance of differences was assessed using a two-way analysis of variance on the ranks (because the data were not normally distributed), and all pairwise multiple comparisons were assessed by the Tukey test. P values <.05 were considered statistically significant.
Results
The distribution of age, ethnicity, and sex in the group of normal subjects was similar to that in the group of parents of patients with CF. None of the normal subjects had a CFTR mutation. All parents of patients with CF had a mutation on one of their CFTR alleles (table 1). None of the normal subjects or parents had a 5T allele on the CFTR intron 8. Seven of the eight patients with CF were found to have mutations on both alleles. The remaining patient (patient 6) had only one detected CFTR mutation; however, because this patient definitely has CF (lung disease and positive sweat test), we believe that she has a second mutation that was missed by the genetic analysis that was performed. All eight patients have chronic recurrent sinopulmonary infections, and all but one (patient 6) have steatorrhea due to pancreatic insufficiency. Three of the patients (patients 5, 7, and 8) had a meconium ileus as newborns, two (patients 7 and 8) had distal intestinal obstruction syndrome at ages 7 and 10 years, and another (patient 3) has hepatobiliary disease, presumably due to CF.
Table 1.
Demographics and CFTR Mutation Analysis
| Group and Subject (Sex [Age])a | Ethnicityb | CFTR Mutation Analysisc | CFTR Intron 8 Poly(T) Analysis |
| Parents: | |||
| 1 (M [28]) | White | Negative/ΔF508 | 7T/9T |
| 2 (F [32]) | White | Negative/ΔF508 | 7T/9T |
| 3 (F [44]) | White | Negative/ΔF508 | 7T/9T |
| 4 (M [36]) | AA | Negative/ΔF508 | 9T/9T |
| 5 (M [54]) | White | Negative/N1303K | 7T/9T |
| 6 (F [24]) | White | Negative/S549R | 7T/7T |
| 7 (F [30]) | AA | Negative/3120+1G-A | 7T/7T |
| Patients: | |||
| 1 (F [20]) | White | ΔF508/N1303K | 9T/9T |
| 2 (F [22]) | White | ΔF508/ΔF508 | 9T/9T |
| 3 (F [33]) | White | ΔF508/1898+1G-A | 7T/9T |
| 4 (F [27]) | White | ΔF508/ΔI507 | 7T/9T |
| 5 (M [28]) | White | ΔF508/ΔF508 | 9T/9T |
| 6 (F [31]) | White | Negative/ΔF508 | 7T/9T |
| 7 (M [18]) | White | ΔF508/M1101K | 7T/9T |
| 8 (M [18]) | White | ΔF508/M1101K | 7T/9T |
Members of the patient group had CF; members of the parent group had offspring with CF who were not necessarily included in the present study.
AA = African American.
Negative = alleles testing negative for the 86 most-common CFTR mutations.
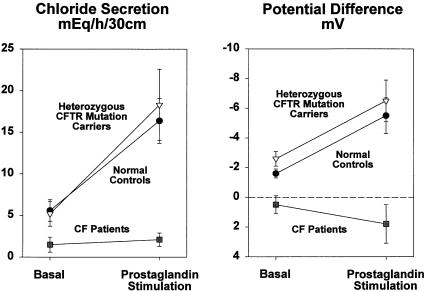
As shown in table 2 and figure 1, normal subjects in the basal state secreted chloride at a rate of 5.6 mEq/h/30 cm. The concentrations of chloride in fluid collected at the proximal and distal ends of the 30-cm test segment were higher than the chloride concentration in the infused solution and were also higher than the chloride concentration in serum. The PD was 1.5 mV (lumen negative). Therefore, chloride was secreted against both a concentration gradient and an electric gradient, establishing the active nature of chloride secretion. In contrast, sodium secretion can be explained by passive diffusion. Water was secreted at a rate of 36 ml/h/30 cm. During intraintestinal infusion of the prostaglandin analogue, the chloride secretion rate increased to 16.4 mEq/h/30 cm, and this was associated with an increase in lumen-negative PD. The increased active secretion of chloride was followed by increased passive secretion of sodium and water to maintain electric and osmolar balance (table 2).
Table 2.
Basal and Prostaglandin-Stimulated Chloride, Sodium, and Water Secretion in Subjects With and Without CFTR Mutations[Note]
| [Cl−] Levels in mEq/liter |
Secretion of |
||||||
| Subjects and Periods | Serum | Proximal End | Distal End | Cl−(mEq/h/30 cm) | Na+(mEq/h/30 cm) | H2O(ml/h/30 cm)a | PD(mV)b |
| Normal (n=7): | |||||||
| Basal | 104.6 ± .9 | 106.4 ± .4 | 109.1 ± .6 | 5.6 ± 1.3 | 5.5 ± 1.2 | 36.0 ± 10.1 | −1.5 ± .4 |
| Prostaglandin | … | 109.9 ± .8 | 114.5 ± 1.1 | 16.4 ± 2.7 | 17.2 ± 2.7 | 116.6 ± 19.4 | −5.5 ± 1.2 |
| Heterozygous CFTR mutation carriers (n=7): | |||||||
| Basal | 106.6 ± 1.0 | 108.1 ± 1.3 | 111.6 ± 1.7 | 5.2 ± 1.5 | 5.0 ± 1.7 | 26.7 ± 10.2 | −2.6 ± .5 |
| Prostaglandin | … | 112.4 ± 1.6 | 116.7 ± 2.0 | 18.3 ± 4.3 | 19.5 ± 4.5 | 127.6 ± 28.3 | −6.5 ± 1.4 |
| Patients with CF (n=8): | |||||||
| Basal | 105.8 ± .9 | 103.6 ± 1.2 | 106.2 ± 1.1 | 1.5 ± .9 | .4 ± 1.4 | −1.1 ± 8.2 | +.2 ± .5 |
| Prostaglandin | … | 103.4 ± 1.0 | 103.9 ± 1.4 | 2.1 ± .8 | 2.7 ± .7 | 17.1 ± 4.6 | +1.2 ± 1.0 |
Note.— Data are mean ± SE. None of the differences between normal and heterozygous subjects were statistically significant. All differences in secretion rates and PD of normal subjects vs. CF patients were statistically significant (P<.05).
Minus sign denotes absorption rather than secretion.
Minus sign indicates lumen-negative PD, plus sign denotes lumen-positive PD.
Figure 1 .
Basal and prostaglandin-stimulated chloride secretion and PD in normal control subjects (n=7), in heterozygous CFTR mutation carriers (n=7), and in patients with CF (n=8).
Basal and prostaglandin-stimulated levels of active chloride secretion in the heterozygotes were similar to those in normal subjects. Moreover, prostaglandin infusion in the heterozygotes was associated with a PD increase similar to that noted in the normal subjects. Four of the seven heterozygotes had a ΔF508 mutation, and three had other mutations. Basal and prostaglandin-stimulated secretion rates and PD were similar in these two subgroups.
Chloride secretion in the patients with CF was approximately zero during the basal as well as during the prostaglandin-infusion period. Moreover, in contrast to normal subjects and to heterozygotes, in whom PD was always lumen negative, the average PD in patients with CF was approximately zero under basal conditions and did not move in a negative direction when the prostaglandin analogue was administered.
Discussion
Our finding that patients with CF (homozygotes) have essentially no active chloride secretion in response to a secretagogue is in agreement with previous observations (Berschneider et al. 1988; Baxter et al. 1989; O’Loughlin et al. 1991; Chao et al. 1994) and indicates that a very low level of expression of functional intestinal CFTR protein precludes stimulation of active chloride secretion. However, we found that CFTR heterozygotes have normal rates of basal and stimulated active chloride secretion. If it is assumed that heterozygotes have less-than-normal intestinal CFTR function, our results mean that CFTR expression is not rate limiting for active chloride secretion in human heterozygotes. The rate-limiting step at the heterozygote level of CFTR expression could be one of three factors: (1) the degree to which intracellular cyclic nucleotides are increased by a secretagogue; (2) the response of transporters on the basolateral membrane of epithelial cells, which are involved in establishing the electrochemical gradients that cause chloride to exit epithelial cells through the CFTR channels (Field and Semrad 1993); or (3) the release of neurotransmitters that are apparently essential for secretagogue action (Lundgren et al. 1989; Castagliuolo et al. 1994).
The degree to which these findings refute the hypothesis that heterozygotes have a survival advantage if they contract diarrhea caused by the toxins of V. cholerae or E. coli depends on three main considerations. First, did we study an appropriate group of heterozygotes? The most common mutation in the white population is ΔF508, which accounts for ∼66% of all mutations (Knowles et al. 1999). Moreover, ΔF508 is considered to be a very old mutation (Morral et al. 1994). If the survival-advantage theory is correct, ΔF508 is therefore the most likely mutation that would confer reduced intestinal secretory capacity to heterozygotes (Cuthbert et al. 1995). Four of the heterozygotes studied in the present study had the ΔF508 mutation. Furthermore, all of the CFTR mutations in the heterozygotes in our experiment are known, when present on both alleles, to cause severe forms of CF, with a nearly complete absence of chloride conductance in epithelial tissues (The Cystic Fibrosis Genotype-Phenotype Consortium 1993; Bienvenu et al. 1996; Romey et al. 1999). We therefore believe that we studied an appropriate group of heterozygotes.
Second, does prostaglandin-induced active chloride secretion reflect secretion induced by the toxins of V. cholerae and E. coli? Toxin- and prostaglandin-induced intestinal secretion have the same profile of inhibition by neuropharmacological agents (Lundgren et al. 1989; Castagliuolo 1994). Both prostaglandin and toxins increase intracellular cAMP, which causes activation of CFTR channels (Field and Semrad 1993). Thus, CFTR channels are the final common pathway for chloride secretion for both toxins and prostaglandin. Because the CFTR defect in patients with CF is distal to the neuropharmacological and intracellular events that occur in response to various secretagogues (Hitchin et al. 1991), we believe that prostaglandin-induced secretion provides a good measurement of CFTR activity and that results obtained with prostaglandin would yield the same conclusions as do studies with toxins, provided that similar rates of chloride secretion were obtained. Petritsch and coworkers carried out a dose-response study of cholera toxin–induced chloride secretion in the jejunum of normal subjects, using methods similar to those of the present study. These workers found a maximum chloride secretion of 14.6 mEq/h/30 cm, with the largest dose of toxin tested (Petritsch et al. 1992). The prostaglandin dose that we used resulted in a chloride secretion rate of 16.4 mEq/h/30 cm. Furthermore, during administration of the prostaglandin analogue to normal subjects in our study, chloride secretion rates were somewhat higher than those observed in the jejunum of actual patients with acute cholera, as measured by a similar perfusion technique (Banwell et al. 1970). This indicates that the dose of prostaglandin analogue employed in our experiment elicited a rate of secretion that is clinically relevant to severe diarrheal diseases.
Third, our studies were conducted in the jejunum but not in the ileum or the colon. We do not believe that this limits the conclusions that can be reached from our study, because toxins of both V. cholerae and E. coli cause diarrhea mainly by inducing fluid secretion in the proximal small intestine (Banwell et al. 1970; Hamer and Gorbach 1998).
We have no good explanation for the discrepancy between our results and the study by Gabriel et al. (1994), which showed that their mouse heterozygotes had approximately one half the normal intestinal secretion rate when exposed to cholera toxin. However, Cuthbert et al. (1995) found perfectly normal responses to cholera toxin (as well as to a variety of other secretagogues) in a different heterozygote mouse model. It is possible that the Cuthbert mouse model is more relevant to human heterozygotes than is the Gabriel mouse model. However, as Cuthbert et al. observed, all mouse heterozygotes have null mutations and therefore have only one allele capable of the generation of CFTR proteins. This is unlike the situation in human heterozygotes, where one allele can produce an altered CFTR protein that may have some functional capability. Ultimately, it would appear that only a study of human heterozygotes can prove or disprove the two major assumptions of the theory.
Our results provide evidence against the theory that CF heterozygotes have a survival advantage when they contract diarrhea mediated by stimulation of active intestinal chloride secretion. Our results are obviously not applicable to protection of CF heterozygotes against other pathogenetic factors involved in the production of diarrhea.
Acknowledgments
This work was supported by U.S. Public Health Grant 5-R01-DK37172-14 from the National Institute of Diabetes, Digestive and Kidney Diseases and by the Southwest Digestive Disease Foundation. Drs. Lawrence R. Schiller, Michael Emmett, Raj K. Goyal, and Byron Cryer provided many helpful suggestions. The authors thank Diana Santa Ana for her excellent assistance in preparing the manuscript.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.gov/omim (for CF [MIM 219700] and CFTR [MIM 602421])
- Genome Database, http://gdbwww.gdb.org (for CFTR [accession number 120584])
References
- Banwell JG, Pierce NF, Mitra RC, Brigham KL, Caranasos GJ, Keimowitz RI, Fedson DS, Thomas J, Gorbach SL, Sack RB, Mondal A (1970) Intestinal fluid and electrolyte transport in human cholera. J Clin Invest 49:183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter PS, Goldhill J, Hardcastle J, Hardcastle PT, Taylor CJ (1988) Accounting for cystic fibrosis. Nature 335:211 [DOI] [PubMed] [Google Scholar]
- Baxter PS, Wilson AJ, Read NW, Hardcastle J, Hardcastle PT, Taylor CJ (1989) Abnormal jejunal potential difference in cystic fibrosis. Lancet 333:464–466 [DOI] [PubMed] [Google Scholar]
- Berschneider HM, Knowles MR, Azizkhan RG, Boucher RC, Tobey NA, Orlando RC, Powell DW (1988) Altered intestinal chloride transport in cystic fibrosis. FASEB J 2:2625–2629 [DOI] [PubMed] [Google Scholar]
- Bertranpetit J, Calafell F (1996) Genetic and geographical variability in cystic fibrosis: evolutionary considerations. Variation in the human genome. (Ciba Foundation Symposium 197). John Wiley, Chichester, United Kingdom, pp 97–118 [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Cartault F, Lesure F, Renouil M, Beldjord C, Kaplan JC (1996) A splicing mutation in intron 16 of the cystic fibrosis transmembrane conductance regulator gene, associated with severe disease, is common on Reunion Island. Hum Hered 46:168–171 [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, LaMont JT, Letourneau R, Kelly C, Connor O’Keane J, Jaffer A, Theoharides TC, Pothoulakis C (1994) Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 107:657–665 [DOI] [PubMed] [Google Scholar]
- Chang EB, Rao MC (1991) Intracellular mediators of intestinal electrolyte transport. In: Field M (ed) Diarrheal diseases. Elsevier, New York, pp 49–72 [Google Scholar]
- Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P (1994) Activation of intestinal CFTR Cl− channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J 13:1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillon M, Casals T, Mercier B, Bassas L, Lissens W, Silber S, Romey MC, Ruiz-Romero J, Verlingue C, Claustres M (1995) Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med 332:1475–1480 [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Genotype-Phenotype Consortium, The (1993) Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 329:1308–1313 [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, Halstead J, Ratcliff R, Colledge WH, Evans MJ (1995) The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study. J Physiol 482:449–454 [DOI] [PMC free article] [PubMed]
- Davis GR, Santa Ana CA, Morawski S, Fordtran JS (1980) Active chloride secretion in the normal human jejunum. J Clin Invest 66:1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Semrad CE (1993) Toxigenic diarrheas, congenital diarrheas, and cystic fibrosis: disorders of intestinal ion transport. Annu Rev Physiol 55:631–655 [DOI] [PubMed] [Google Scholar]
- Fontelo P (1995) Protection against cholera. Science 267:440 [DOI] [PubMed] [Google Scholar]
- Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ (1994) Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266:107–109 [DOI] [PubMed] [Google Scholar]
- Gaginella TS (1990) Eicosanoid-mediated intestinal secretion. In: Lebenthal E, Duffey ME (eds) Textbook of secretory diarrhea. Raven Press, New York, pp 15–30 [Google Scholar]
- Grubb BR, Gabriel SE (1997) Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Physiol 273:G258-G266 [DOI] [PubMed] [Google Scholar]
- Hamer DH, Gorbach SL (1998) Infectious diarrhea and bacterial food poisoning. In: Feldman M, Sleisenger MH, Scharschmidt BF (eds) Sleisenger & Fordtran’s gastrointestinal and liver disease, pathophysiology/diagnosis/management. WB Saunders, Philadelphia, pp 1594–1632 [Google Scholar]
- Hitchin BW, Dobson PRM, Brown BL, Hardcastle J, Hardcastle PT, Taylor CJ (1991) Measurement of intracellular mediators in enterocytes isolated from jejunal biopsy specimens of control and cystic fibrosis patients. Gut 32:893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorde LB, Lathrop GM (1988) A test of the heterozygote-advantage hypothesis in cystic fibrosis carriers. Am J Hum Genet 42:808–815 [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Friedman KJ, Silverman LM (1999) Genetics, diagnosis, and clinical phenotype. In: Yankaskas JR, Knowles MR (eds) Cystic fibrosis in adults. Lippincott-Raven, Philadelphia, pp 27–42 [Google Scholar]
- Kopelman HR (1993) Cystic fibrosis. In: Wyllie R, Hyams JS (eds) Pediatric gastrointestinal disease. WB Saunders, Philadelphia, pp 854–868 [Google Scholar]
- Lundgren O, Svanvik J, Jivegard L (1989) Enteric nervous system. I. Physiology and pathophysiology of the intestinal tract. Dig Dis Sci 34:264–283 [DOI] [PubMed] [Google Scholar]
- Morral N, Bertranpetit J, Estivill X, Nunes V, Casals T, Gimenez J, Reis A, et al (1994) The origin of the major cystic fibrosis mutation (ΔF508) in European populations. Nat Genet 7:169–175 [DOI] [PubMed] [Google Scholar]
- O'Loughlin EV, Hunt DM, Gaskin KJ, Stiel D, Bruzuszcak IM, Martin HC, Bambach C, Smith R (1991) Abnormal epithelial transport in cystic fibrosis jejunum. Am J Physiol 260:G758-G763 [DOI] [PubMed] [Google Scholar]
- Petritsch W, Eherer AJ, Holzer-Petsche U, Hinterleitner T, Beubler E, Krejs GJ (1992) Effect of cholera toxin on the human jejunum. Gut 33:1174–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton PM (1982) Abnormalities in electrolyte secretion in cystic fibrosis sweat glands due to decreased anion permeability. In: Quinton PM, Martinez JR, Hopfer U (eds) Fluid and electrolyte abnormalities in exocrine glands in cystic fibrosis. San Francisco Press, San Francisco, pp 53–76 [Google Scholar]
- ——— (1994) What is good about cystic fibrosis? Curr Biol 4:742–743 [DOI] [PubMed] [Google Scholar]
- Read NW, Fordtran JS (1979) The role of intraluminal junction potentials in the generation of the gastric potential difference in man. Gastroenterology 76:932–938 [PubMed] [Google Scholar]
- Rodman DM, Zamudio S (1991) The cystic fibrosis heterozygote: advantage in surviving cholera? Med Hypotheses 36:253–258 [DOI] [PubMed] [Google Scholar]
- Romeo G, Devoto M, Galietta LJV (1989) Why is the cystic fibrosis gene so frequent? Hum Genet 84:1–5 [DOI] [PubMed] [Google Scholar]
- Romey MC, Guittard C, Chazalette JP, Frossard P, Dawson KP, Patton MA, Casals T, Bazarbachi T, Girodon E, Rault G, Bozon D, Seguret F, Demaille J, Claustres M (1999) Complex allele [−102(T→A) +S549R(T→G)] is associated with milder forms of cystic fibrosis than allele S549R(T→G) alone. Hum Genet 105:145–150 [DOI] [PubMed] [Google Scholar]
- Schiller LR, Santa Ana CA, Porter J, Fordtran JS (1997) Glucose-stimulated sodium transport by the human intestine during experimental cholera. Gastroenterology 112:1529–1535 [DOI] [PubMed] [Google Scholar]