Abstract
Despite recent advances in the molecular genetics of type 2 diabetes, the majority of susceptibility genes in humans remain to be identified. We therefore conducted a 10-cM genomewide search (401 microsatellite markers) for type 2 diabetes–related traits in 637 members of 143 French pedigrees ascertained through multiple diabetic siblings, to map such genes in the white population. Nonparametric two-point and multipoint linkage analyzes—using the MAPMAKER-SIBS (MLS) and MAXIMUM-BINOMIAL-LIKELIHOOD (MLB) programs for autosomal markers and the ASPEX program for chromosome X markers—were performed with six diabetic phenotypes: diabetes and diabetes or glucose intolerance (GI), as well as with each of the two phenotypes associated with normal body weight (body-mass index<27 kg/m2) or early age at diagnosis (<45 years). In a second step, high-resolution genetic mapping (∼2 cM) was performed in regions on chromosomes 1 and 3 loci showing the strongest linkage to diabetic traits. We found evidence for linkage with diabetes or GI diagnosed at age <45 years in 92 affected sib pairs from 55 families at the D3S1580 locus on chromosome 3q27-qter using MAPMAKER-SIBS (MLS = 4.67, P=.000004), supported by the MLB statistic (MLB-LOD=3.43, P=.00003). We also found suggestive linkage between the lean diabetic status and markers APOA2–D1S484 (MLS = 3.04, P=.00018; MLB-LOD=2.99, P=.00010) on chromosome 1q21-q24. Several other chromosomal regions showed indication of linkage with diabetic traits, including markers on chromosome 2p21-p16, 10q26, 20p, and 20q. These results (a) showed evidence for a novel susceptibility locus for type 2 diabetes in French whites on chromosome 3q27-qter and (b) confirmed the previously reported diabetes-susceptibility locus on chromosome 1q21-q24. Saturation on both chromosomes narrowed the regions of interest down to an interval of <7 cM.
Introduction
Type 2 diabetes (MIM 125853) is a heterogeneous disorder of glucose metabolism characterized by both insulin resistance and pancreatic β-cell dysfunction. The chronic hyperglycemia is often associated with essential hypertension, obesity, and dyslipidemia in a complex metabolic syndrome leading to severe vascular complications, including blindness, end-stage renal failure, and coronary-heart disease. The prevalence of type 2 diabetes in Westernized countries is 3%–10%, and epidemiological studies predict it may reach a total of 30% in the next decade, which makes it a major health problem worldwide.
The familial clustering of type 2 diabetes, together with the higher concordance rates in MZ twins compared with DZ twins (Barnett et al. 1981; Hopper et al. 1999) and the close correspondence in hybrid populations, between genetic-admixture rates and disease prevalence (Knowler et al. 1988), suggest a genetic predisposition to type 2 diabetes, with a recurrence risk to first-degree relatives of ∼3.5 (Rich 1990). In <10% of the cases, type 2 diabetes appears to segregate in a Mendelian fashion. However, in the majority of cases, the pattern of inheritance of the disease suggests a complex genetic interaction with environmental factors.
Considerable effort has been made worldwide during the last decade to identify the underlying genetic determinants. Studies of monogenic forms of type 2 diabetes led to the identification of several diabetogenes with major effects in rare families, including the insulin, insulin-receptor, and glucokinase genes and the four transcription-factor genes encoding HNF1-α, HNF4-α, HNF1-β (hepatocyte nuclear factor), and IPF1 (insulin promoter factor 1), as well as mutations in the mitochondrial DNA (reviewed by Elbein et al. [1997]). However, these genes do not seem to play a major role in the more common forms of type 2 diabetes. Studies of candidate genes in pathways impaired in subjects with type 2 diabetes led to the identification of genes that may increase the risk for type 2 diabetes, including the IRS1 (insulin receptor substrate 1), glucagon-receptor, glycogen synthase, sulfonylurea-receptor, and IPF1 genes (Elbein et al. 1997). In any case, these genes contribute, to a modest or marginal extent, to genetic susceptibility to type 2 diabetes.
Several groups, therefore, have undertaken genomewide scans to identify loci for type 2 diabetes and related phenotypes. So far, genomewide significant linkages (Lander and Kruglyak 1995) for such susceptibility loci were identified on several distinct chromosomes in different populations. These include loci on chromosomes 2q37 (Hanis et al. 1996), 15q21 (Cox et al. 1999), 10q26 (Duggirala et al. 1999), and 3p (Ehm et al. 2000) in Mexican Americans; 12q24 (Mahtani et al. 1996) and 1q21-1q23 (Elbein et al. 1999) in whites; and 1q21-1q23 and 11q23-q25 in Pima Indians (Hanson et al. 1998). Overall, these results underscore the genetic heterogeneity of type 2 diabetes in diverse ethnic groups and the need of performing carefully designed whole-genome scans in other populations, either to identify new diabetes-susceptibility genes and/or to replicate previous findings.
To search for a major susceptibility locus (loci) contributing to type 2 diabetes in the French white population, we have completed a genomewide search for diabetes-related traits in 143 families with affected sib pairs. Here, we report evidence for a novel susceptibility locus for diabetes on chromosome 3q27-qter and independent confirmation of a locus on chromosome 1q21-q24. Subsequent fine-mapping in those regions has narrowed down the regions of interest to an interval of <7 cM.
Subjects
Families have been recruited since 1990 by means of a publicly advertised campaign for “200 families to overcome diabetes,” and details of the recruitment have been described elsewhere (Vionnet et al. 1997). Among the 402 type 2–diabetes families collected with at least two affected sibs, 145 have been initially included in the study, according to criteria the aim of which was to reduce heterogeneity of the families and to maximize the power of the study. A family was included if there were at least two diabetic subjects in the sibship being treated for diabetes (to load the families with a severe form of type 2 diabetes) and at least three individuals (parents or additional sibs) available for the study (to assist the reconstruction of parental genotypes and to avoid non-full sibs). Families presenting bilineal inheritance of type 2 diabetes or families with subjects diagnosed at age <25 years (i.e., potentially having the Maturity-Onset Diabetes of the Young [MODY] phenotype) or at age >65 years only (potential phenocopy caused by environmental factors rather than genetic ones) or families with mixture of type 1 and type 2 diabetes (potential Latent Autoimmune Diabetes of Adults [LADA]) were discarded. However, in one family, one individual was diagnosed at age 23 years and had positive anti-GAD antibodies. Nonwhite families or families with mutations in a known diabetes-susceptibility gene were also excluded from the study. Prior to genotyping of all the markers for the genome scan, 18 markers for the X chromosome (panel 28) were analyzed to check for DNA inconsistencies and Mendelian inheritance. From a preliminary selection of 145 families, 10 were discarded and were replaced by 12 additional families checked in the same manner. After the genome scan was completed, four families had to be discarded from the analyses because of recurrent Mendelian incompatibilities. All analysis results presented in this report were thus obtained in 143 families, corresponding to 148 nuclear families.
Phenotypes were determined in the light of the clinical report and the results of the latest measurements for fasting and/or oral glucose-tolerance test according to the recently established American Diabetes Association criteria for type 2 diabetes (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 1997): “diabetic” (DM) when receiving oral hypoglycemic agents or insulin ⩾1 year after the diagnosis, or when fasting glycemia was 7 mmol/l or glycemia 2 h after an oral glucose load was 11.1 mmol/l; “glucose intolerant” (GI) when fasting glycemia was 6.1–7.0 mmol/l or when 2-h glycemia was 7.8–11.1 mmol/l; “normoglycemic” (NG) when fasting glycemia was <6.10 mmol/l and 2-h glycemia was <7.8 mmol/l; “unknown” (UK) when no data were available or type 1 diabetes was present. Accordingly, the 637 individuals included in the study comprised 432 DM, 72 GI, 129 NG, and 4 UK subjects (tables 1 and 2). We designed six qualitative traits, taking into account the diabetic status, the age at diagnosis, and the body-mass index (BMI) of each subject in an attempt to stratify families into clinically more-homogeneous groups (table 3A). A maximum number of 677 affected sib pairs were analyzed with the affection status “large,” and details of the family structure are given in table 3B for all six subgroups. Both parents were available in 6 families, and one parent was available in 30 more families.
Table 1.
Clinical Characteristics of All Participants
| Participant Status | SexRatio(M:F) | Age at Time of Study(years)± SD (range) | Age at Diagnosis(years)± SD (range) | Duration of Disease(years)± SD (range) | BMI(kg/m2)± SD (range) |
| Nondiabetic (n=129) | 40:89 | 61.87±12.69 (28–91) | … | … | 25.1±4.1 (14.98–36.71) |
| Diabetic (n=432) | 198:234 | 62.85±9.6 (31–93) | 49.5±10.65 (23–83) | 13.41±9.8 (0–63) | 27.89±4.48 (15.11–45.91) |
| Glucose intolerant (n=72) | 36:36 | 61.15±9.41 (41–87) | 59.06±9.56 (41–82) | 2.08±4.49 (0–24) | 27.37±4.70 (19.23–41.77) |
Table 2.
Medication History of Diabetic Participants
| Medication History | % of Diabetic Participants |
| Diet alone | 10.7 |
| Sulfonylureas (Sf) | 24.9 |
| Biguanides (Bg) | 14.0 |
| Insulin | 12.6 |
| Sf and/or Bg + insulin | 5.3 |
| Sf + Bg | 30.9 |
| Glucor or mediator | 1.6 |
Table 3.
Description of Affection Status and Structure of the 148 Nuclear Families
| 3A. Description of Affection Status for the Six Phenotypic Groupsa | ||||||
| Affection Status | Large | Strict | Large-Lean | Strict-Lean | Large Young-Onset | Strict Young-Onset |
| Affected | NIDDM+GI | NIDDM | (NIDDM+GI) with BMI <27 | NIDDM with BMI <27 | (NIDDM+GI) with age at onset <46 | NIDDM with age at onset <46 |
| Unknown | NA | NA+GI | NA+(NIDDM+GI) with BMI >27 | NA+GI+(NIDDM with BMI >27) | NA+(NIDDM+GI) with age at onset >46 | NA+GI+(NIDDM with age at onset >46) |
| Nonaffected | NG | NG | NG | NG | NG | NG |
| 3B. No. of Families with n Affected in Each Phenotypic Groupb | ||||||
| n | Large | Strict | Large-Lean | Strict-Lean | Large Young-Onset | Strict Young-Onset |
| 2 | 43 | 66 | 37 | 40 | 41 | 45 |
| 3 | 54 | 54 | 19 | 11 | 11 | 6 |
| 4 | 30 | 18 | 5 | 5 | 3 | 3 |
| 5 | 12 | 6 | 1 | |||
| 6 | 4 | 1 | 3 | 1 | ||
| 7 | 4 | 2 | ||||
| 8 | 1 | |||||
| Total families | 148 | 147 | 65 | 57 | 55 | 54 |
| Total sib-pairs | 677 | 453 | 179 | 113 | 92 | 81 |
Affection status was determined taking into account patients' diabetic status (overt diabetes [NIDDM], GI, NG, or data not available [NA]), BMI (kg/m2), and age at diagnosis of diabetes. Three affection statuses were used for the statistical analyses: affected, nonaffected, and unknown (coded 2, 1, and 0, respectively).
Total number of sib pairs was calculated with the following formulae: (number of families with n affected, n = [2–8]) X[nX(n-1)]/2.
Methods
Genotyping
Initial 10-cM genomewide screen
Genomic DNA was isolated from leukocytes using the Puregene DNA Isolation Kit, according to the supplier's protocol (Gentra Systems). Genotyping was performed using a fluorescently labeled human linkage-mapping set (PE-LMSV2) comprising 400 highly informative microsatellite markers, with an average intermarker spacing of 9.7 cM. Multiplex PCR conditions were set up for each of the 28 panels in such a manner as to amplify the 400 markers in 87 PCRs. PCR (95°C for 12 min, followed by 30 cycles of 94°C for 15 s, 55°C for 15 s, 72°C for 30 s, and 72°C for 10 min) was performed on GeneAmp PCR system 9700 (Perkin-Elmer): 10-ml reactions; 40 ng of genomic DNA, 2.5 mM MgCl2, 0.25 mM each dNTP (Pharmacia), a variable amount (0.2–1.5 pmol) of 5′ and 3′ primers, and 0.4 U AmpliTaq Gold DNA polymerase (Perkin Elmer) in 1× PCR Buffer II (Perkin Elmer). (Multiplex PCR conditions are available from the authors on request.)
Pooled amplification products were electrophoresed through 5% polyacrylamide gels (Long Ranger Singel pack [Perkin Elmer]) for 1.5 h at 2,000 V on 24-cm plates on an ABI 377 DNA sequencer. An automated 96-channel pipettor Multimek 96 (Beckman) was used for all the pipetting steps. Semiautomated fragment sizing was performed by use of GENESCAN 3.0 software (ABI), followed by allele calling with GENOTYPER 2.1 software (ABI). Each genotype was reviewed independently by two members of the research team to confirm the accuracy of allele calling. Marker D14S68 was replaced by D14S67 because of reading difficulties. All markers but D12S310, the amplification of which failed, were included in the analysis. Two additional markers (D5S429 and D8S279) were added to the 399, to fill in gaps >20 cM. The overall data dropout rate for the 255,437 genotypes (401×637) analyzed was <4%. The average heterozygosity for the 401 markers used in the first-stage genome search was 0.79. Incompatibilities were searched for with the PED-CHECK 1.1 program (O'Connell and Weeks 1998) and inconsistencies resolved.
"Fine mapping” genotyping
After the first-stage genome search, we observed that linkage was primarily supported with markers on 3q27-qter and 1q21-q24 across most methods and phenotypes. We then saturated these regions with 16 additional genetic markers in each region, to confirm and further localize the linkage results. Each of the 32 markers was amplified individually by PCR, and genotyping was performed similarly to the genomewide scan. In addition, PCR and genotyping were performed a second time for the markers used in the genome scan and were localized in 3q27-qter and 1q21-q24 loci (seven and five markers, respectively). The overall data dropout rate for the 28,028 genotypes—16 × 2 additional + 12 “genome-scan” markers × 637 individuals—analyzed in this second step was of 0.5%, thus ensuring maximal informativity for multipoint analyses. The information content for markers used in the multipoint analyzes exceeded 0.85 for both regions.
Data Statistical Analysis
Nonparametric two-point and multipoint analyzes were performed with the programs MAPMAKER-SIBS 2.0 (MLS; Kruglyak et al. 1995), by using the “unweighted” option, and MLBGH 1.0 (Abel and Muller-Myhsok 1998), for autosomal markers. The maximum-likelihood binomial (MLB) method, which is based on the binomial distribution of parental marker alleles among affected offspring, overcomes the common problem of multiple sibs by considering the sibship as a whole. Two-point and multipoint analyzes of the chromosome X markers were performed using ASPEX (©1998 David A. Hinds and Neil Risch). Allele frequencies for the parameter file were generated from the data with DOWNFREQ 1.1 (Terwilliger et al. 1995).
Marker map positions for the genomewide scan and the high-resolution map on chromosome 3q27-qter were obtained from the sex-averaged maps compiled by Généthon and are given in Haldane centimorgans (cM). The high-resolution map order, from the Généthon map, of all markers tested at 3q27-qter was consistent with the Marshfield map order and with the results of mapping in the Genebridge 3 Radiation Hybrid Panel (Research Genetics) (data not shown). Of the 16 additional markers of chromosome 1q21-q24, 5 belonged to the Marshfield sex-averaged linkage map. For the high-resolution map on chromosome 1q21-q24, we used the map positions from the Marshfield map, since mapping in the Genebridge 3 Radiation Hybrid Panel was more consistent with the Marshfield map order than with the Généthon map. Intermarker distances in our sample were systematically checked with a program derived from VITESSE (O’Connell and Weeks 1995).
When we restrict the sample to young-onset diabetes status, the sample size is reduced to 92 sib pairs, and we observe unequal sibship sizes. It is then legitimate to investigate the reliability of the P values. We simulated the transmission of the marker keeping the familial structure and affection status. When the founders had typed parents, we used their actual genotype and simulated the allele transmission. When they were unknown, their genotypes probability was determined according to the population allele frequency and their offspring genotypes. A total of 70,000 replicates were simulated through Monte Carlo method and were analyzed with MLBGH.
Results
First-Stage Genome Scan
Table 4 summarizes results of the multipoint analyses of the 401 framework markers from the initial genome scan. We considered any region with a Znorm of 2.3 and a LOD score of 1.17 (P=.01) as “potentially interesting,” and we applied the criteria of Lander and Kruglyak to further define regions of significant (Znorm=4.11, LOD=3.6, P=.00002) or suggestive (Znorm=3.19, LOD=2.2, P=.0007) linkage. Overall, 26 of the 2,298 tests using the MLB statistics for the autosomal chromosomes (383 markers tested for the six phenotypes) gave LOD scores ⩾1.17 (P⩽.01), corresponding to 22 distinct markers that were distributed in 15 distinct genomic intervals (table 4A). The MLS statistics also gave LOD scores ⩾1.17 (P⩽.01) for those genomic intervals (table 4A), as well as for markers in nine additional regions (table 4B). Of the 108 tests performed using the MLS statistics (with ASPEX program) for the X chromosome, 2 showed indication of linkage (table 4C). Most of the multipoint results were supported by single-point analyzes (data not shown). There was a cluster of linked markers on chromosomes 3, 1, and 2. The positive chromosome 3 markers (P=.01) spanned >38 cM on the terminal q arm and gave positive results for two of six diabetes phenotypes with both statistics. Genomewide significance was observed at the D3S1580 locus when the MLS statistic was used (MLS=3.91, P=.00002 for the “large young-onset” phenotype), consistent with suggestive linkage using the MLB statistic (LOD=2.97, P=.00011). Linkage was still suggestive with both statistical methods when applying a conservative correction factor for the number of traits studied by multiplying the P value by 6 (number of phenotypes). Linkage was also suggested in this chromosomal region with the “strict-young-onset” phenotype, with both MLS and MLB statistics: at D3S1580; MLS=2.89, P=.00026, MLB-LOD=2.24, P=.00067 (table 4A). When we restricted the sample to young-onset diabetes status, the sample size was reduced to 92 sib pairs, and we observed unequal sibship sizes. However, simulation studies for the chromosome 3 results showed that the empirical P values were very close to the value expected under asymptotic conditions. Therefore, we can rely on the P values given by the MLB method.
Table 4.
Multipoint Results of the 10-cM Genome-Scan Initial Stage
| 4A. Results of MLB and MLS Multipoint Analyses for Six Diabetes-Related Phenotypes | |||||
| Intervala | Positive Markersb | Positionc(cM) | Phenotype | MLB-LOD (P) | MLS (P) |
| 1p21 | D1S206 | 141.12 | Strict-lean | 1.27 (.00777) | 1.48 (.00782) |
| 1q21-q24 | D1S484, D1S498 | 178 | Strict-lean | 2.47 (.00037) | 2.50 (.00066) |
| D1S484 | 178 | Strict | 1.23 (.00877) | 1.12 (.01911) | |
| 1q43 | D1S2785 | 276.23 | Large young-onset | 1.39 (.00575) | 1.64 (.00533) |
| 2p25 | D2S319 | 6.17 | Strict young-onset | 1.61 (.00324) | 2.02 (.00212) |
| 2p21-p16 | D2S2259, D2S391 | 75.31 | Strict | 2.28 (.0006) | 2.27 (.00116) |
| 2q24-q23 | D2S2330 | 179.22 | Large-lean | 1.25 (.00826) | 1.22 (.01507) |
| 3q27-qter | D3S1580, D3S1262, D3S1601 | 217.97 | Large young-onset | 2.97 (.00011) | 3.91 (.00002) |
| D3S1580, D3S1601 | 217.97 | Strict young-onset | 2.24 (.00067) | 2.89 (.00026) | |
| 4p16 | D4S2935, D4S412 | 12.5 | Large | 1.34 (.00643) | 1.35 (.01087) |
| 4q31 | D4S1539 | 185.99 | Large-lean | 1.87 (.00166) | 2.09 (.00175) |
| 5q31-q33 | D5S410, D5S436 | 159.43 | Large | 1.52 (.00412) | 1.67 (.00492) |
| 7q11 | D7S669 | 92.74 | Large-lean | 1.32 (.00682) | 1.68 (.00479) |
| 9q34 | D9S158 | 160.01 | Strict-lean | 1.30 (.00724) | 1.22 (.01494) |
| 10q26 | D10S1655 | 174.65 | Strict | 1.59 (.00342) | 1.24 (.01427) |
| D10S212 | 185.23 | Large | 1.22 (.00899) | 1.69 (.00472) | |
| D10S212 | 185.23 | Large-lean | 1.44 (.00504) | 1.45 (.00841) | |
| 11p15 | D11S1338 | 15.24 | Strict young-onset | 1.34 (.00648) | 1.25 (.01371) |
| 19p13 | D19S221 | 36.92 | Large young-onset | 1.20 (.00948) | 1.26 (.01358) |
| 4B. Additional Loci with MLS Score >1.17 | |||||
| Intervala | Positive Markersb | Positionc(cM) | Phenotype | MLB-LOD (P) | MLS (P) |
| 2p25 | D2S168 | 29.3 | Strict | .82 (.02621) | 1.90 (.00278) |
| 2p23 | D2S305 | 41.64 | Large | 1.16 (.0103) | 1.98 (.0023) |
| D2S305 | 41.64 | Large young-onset | .62 (.04537) | 1.60 (.00581) | |
| D2S305 | 41.64 | Strict young-onset | .80 (.02707) | 1.70 (.00459) | |
| 8q22 | D8S270 | 104.72 | Large | .97 (.01723) | 1.44 (.00865) |
| 12p11 | D12S345 | 55.56 | Large young-onset | .84 (.02447) | 1.41 (.00925) |
| 15q15 | D15S1007 | 26.78 | Strict young-onset | .74 (.03211) | 1.50 (.00753) |
| 16p11 | D16S3068 | 14.6 | Strict young-onset | 1.09 (.01253) | 1.39 (.00971) |
| 18q12 | D18S478 | 54.71 | Large young-onset | 1.09 (.0126) | 1.75 (.00401) |
| 20p13-p12 | D20S186 | 33.93 | Large young-onset | 1.14 (.01093) | 1.86 (.00312) |
| D20S186, D20S115 | 33.93 | Strict young-onset | 1.08 (.01304) | 1.68 (.00475) | |
| 20q13 | D20S178 | 66.95 | Large | .05 (.06295) | 1.72 (.00432) |
| 4C. Results of ASPEX Multipoint Analyses for Six Phenotypes | |||||
| Intervala | Positive Markersb | Positionc(cM) | Phenotype | MLB-LOD (P) | MLS (P) |
| Xp11-p21 | DXS991 | 89.34 | Large | 1.67 (.00491) | |
| DXS991 | 89.34 | Strict | 1.56 (.00643) | ||
Chromosomes locations were determined from the map available in the Genome Database and the CEDAR database.
When several markers per line are shown, the first one is the nearest to the top of the peak.
Map positions of the nearest marker to the top of the peak, obtained from sex-averaged maps compiled by Généthon.
The positive chromosome 1 markers spanned >20 cM on the q arm and also gave positive results for two diabetes phenotypes. Suggestive linkage was observed with the “strict-lean” phenotype with both the MLS and MLB statistics, and results remained potentially interesting after Bonferroni correction. Positive results for chromosome 2 markers were primarily for the “strict” phenotype, with suggestion of linkage only with the MLB analyses, and spanned an interval of 26 cM on the p arm.
Second-Stage Mapping of Chromosomes 3q and 1q
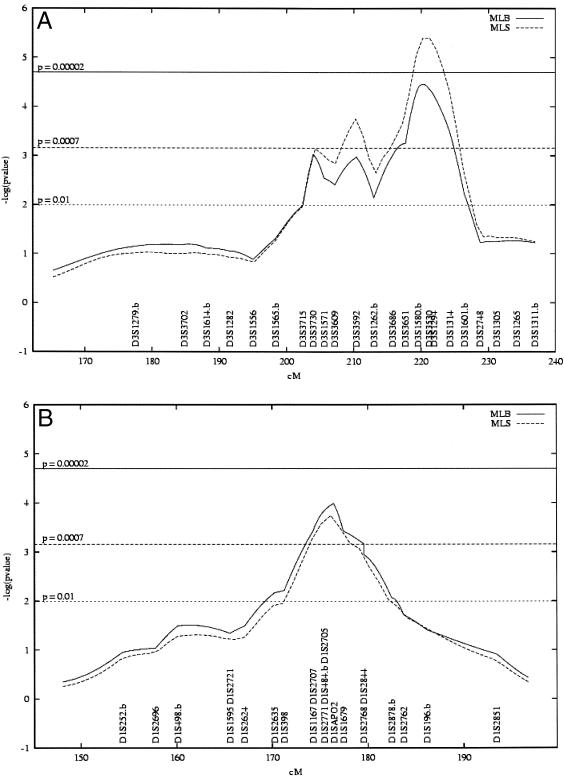
From the first-stage scan, the strongest and most consistent evidences for linkage were found for markers from chromosomes 3q27-qter and 1q21-q24, across different analytical methods and different phenotypes. We thus added 16 more markers in the 3q27-qter and 1q21-q24 regions, for totals of 23 and 21 markers with average map densities of 2.6 and 1.9 cM, respectively. Figures 1A and 1B show results from the multipoint analyzes for both MLS and MLB statistics of the saturation of 3q with the “large young-onset” phenotype and 1q with the “strict-lean” phenotype. These analyzes further supported and strengthened the findings from the initial 10-cM scan. Evidence of linkage was confirmed in the 3q27-qter region with both the “large young-onset” and “strict young-onset” phenotypes (D3S1580 ; MLS=4.67 and 3.56 (P=.000004 and .000054, respectively) MLB-LOD=3.43 and 2.65 (P=.00003 and .00024, respectively). Moreover, the high-resolution mapping identified an indication of linkage, which was not observed at the 10-cM initial genome scan mapping phase, with an additional phenotype that is the “strict” phenotype (i.e., type 2 diabetes) regardless of the age at diagnosis (453 sib pairs, MLB-LOD=1.28, P=.007).
Figure 1.
Results of the MLB and MLS multipoint analyses for the high-resolution genetic mapping at chromosome 3q27-qter with the “large young-onset” phenotype (A) and at chromosome 1q21-q24 with the “strict-lean” phenotype (B). The thresholds for putative (LOD 1.17; P=.01), suggestive (LOD 2.2; P=.0007), and significant linkage (LOD 3.6; P=.00002) are indicated by horizontal lines. Marker names followed by “.b” were also used in the first stage 10-cM genomewide screen and were fully retyped during the high-resolution mapping step (see Methods section). The horizontal axis corresponds to the partial genetic map of chromosomes 3q27-qter and 1q21-q24 intervals studied in the saturation step with high-density genetic maps. The vertical axis indicates the −log(P) obtained with multipoint analyses at each point in the studied interval.
On the other hand, although they did not reach the Lander-Kruglyak statistical thresholds for significance, results in the 1q21-q24 region were highly suggestive with the “strict-lean” phenotype (APOA2–D1S484; MLB-LOD=2.99, P=.00010; MLS=3.04, P=.00018). This saturation step with dense genetic map allowed us to restrict the 1-LOD-unit CI (Ott 1991) from 11 to 6.2 cM and from 19 to 6.5 cM for the loci on chromosomes 3 and 1, respectively.
Discussion
The present genome scan in French whites provides (1) strong evidence for a susceptibility locus for diabetes and impaired glucose tolerance with early onset (at age <46 years) on chromosome 3q27-qter and (2) suggestive evidence that there is a diabetes-susceptibility gene in French whites in a previously reported region on chromosome 1q21-q24. In our genomewide-scan design for type 2 diabetes–susceptibility genes, emphasis has been focused on power to maximize the chances for success. We used a large number of sib pairs (>200 for the “large” and “strict” phenotypes), and a 10-cM marker grid in a one-stage study yielding a power of >98% for a risk ratio (λs) of 3 and of >70% for a risk ratio (λs) of 2 (Holmans and Craddock 1997). Because genotyping errors lead to false-positive or false-negative results, automation and stringent quality-control criteria have been applied. Families were selected from among our large collection to be as homogeneous as possible, to avoid background noise caused by bilineal inheritance of the trait (DeStefano et al. 1998), other genetic forms of type 2 and/or type 1 diabetes (such as MODY or LADA), and nongenetic forms caused by environmental factors. Only families in which accurate inference of relationships among sib pairs was possible were included, to avoid reduced power to detect linkage caused by misclassification of half-sib pairs or unrelated individuals as full sibs (Boehnke and Cox 1997). Two powerful sib pair–based methods were used for analyses: the MLS method, which was previously shown to be most sensitive (Davis and Weeks 1997), and the MLB method, which takes into account sibships with several affecteds in a natural way and was shown to perform well in terms of type 1 error and power (Abel and Muller-Myhsok 1998; Abel et al. 1998).
Our best linkage result was found between markers on chromosome 3q27-qter and diabetes and impaired glucose tolerance diagnosed at age <45 years. Criteria for assessment of statistical significance in genetic-linkage studies of complex traits have been controversial. Specific critical values have been proposed; some investigators have suggested that a relatively stringent threshold of Z>3.6 is needed to obtain a genomewide P<.05 (Lander and Kruglyak 1995), whereas others have maintained that the less stringent traditional criterion of Z>3.0 is unlikely to be a false positive (Holmans et al. 1997; Morton 1998; Rao 1998). In addition, when several correlated traits have been analyzed, it is unclear how to adjust for multiple comparisons. The present analysis provides strong evidence for linkage on chromosome 3 with diabetes and impaired glucose tolerance diagnosed at age <45 years (Z=4.67) and for diabetes diagnosed at age <45 years (Z=3.56). Both two-point and multipoint MLS and MLB statistics gave consistent results that were still significant after Bonferroni correction for the number of phenotypes analyzed. In addition, the width of the peak on chromosome 3q27-qter, given by all positions providing a P value of .01 in the multipoint analysis, was broad (>20 cM), which is an argument in favor of this peak being a true positive rather than a false positive (Terwilliger et al. 1997). We found linkage on chromosome 3 through stratification of families in clinically more-homogeneous subgroups, which is known to help in identification of susceptibility loci for a complex disease (Rao 1998). The result on chromosome 3q has been observed in a subgroup of 55 non-MODY families (37% of total families) with glucose intolerance or diabetes diagnosed between age 25 and 45 years, although both parents were not affected. Therefore, this group probably represents the most genetic form of non-MODY type 2 diabetes. Results of simulation studies showed that the nominal P values obtained in this reduced sample were reliable. On the basis of a single sample, it is difficult to unambiguously distinguish true from false linkage results. However, linkage of diabetes to the chromosome 3q27-qter region is supported by (1) the consistency of results across phenotypes and analytical methods, (2) the extremely low P values, and (3) the results of our simulations. Additional support comes from replication of results either in the same population or in other populations. We could not perform a replication study in our population, because the huge size of the replication sample recommended by simulation studies (Risch and Merikangas 1996) was incompatible with the small number of similarly ascertained families remaining in our French collection. Our region of positive linkage on chromosome 3q27-qter spanned >20 cM in the first-stage genome scan and was refined to <7 cM after the fine-mapping step was conducted. This region includes several reports of positive linkage with metabolic traits; we agree with Neel and colleagues (1998) that “type 2 diabetes, essential hypertension, and obesity [are] syndromes of impaired genetic homeostasis.” Hegele et al. (1999) found an indication of linkage between D3S2418 and type 2 diabetes in a 20-cM genomewide scan in a Canadian Oji-Cree population (P=.01). They did not find evidence of linkage disequilibrium between D3S2418 and diabetes; neither did we with D3S1580 in the 55 linked families using the Monte Carlo–based χ2 implemented in CLUMP (Sham and Curtis 1995; data not shown). QTLs for impaired acute insulin response, insulin resistance in nondiabetics (Pratley et al. 1998) and in diabetics (Hanson et al. 1998), and waist:hip ratio (a measure for obesity) (Norman et al. 1998) were also suggested in this chromosomal region, in the sole Pima Indian population. Interestingly, a QTL for small low-density lipoprotein–particle phenotype, known to be a risk factor for cardiovascular disease, has been recently mapped in the same region on chromosome 3q27-qter (Rainwater et al. 1999). Several studies have reported a polymorphism in the apolipoprotein D (APOD) locus, in the region of the maximal peak of our linkage at this chromosomal region, to be associated with diabetes and obesity (Hitman et al. 1992; Vijayaraghavan et al. 1994). Although none of those previously reported linkages on chromosome 3 reached statistical significance, as did the linkages in our study, they do strengthen our results, and it is tempting to speculate that the same locus is responsible for these results.
Saturation of the chromosome 3q27-qter region in our families reduced the 1-LOD-unit confidence interval (CI) to 6.2 cM around D3S1580, which, according to the Unified Database for Human Genome Mapping, spans ∼4.5 mb, an interval of suitable size to initiate positional cloning and disequilibrium mapping efforts. This chromosomal region contains several potentially interesting genes, including the genes coding for somatostatin, apolipoprotein D, and inhibitor 2 of the synthase-activating enzyme, type 1 protein phosphatase, I-2 (PPP1R2), and the bifunctional enzyme (with enoyl-CoA-hydratase and 3-hydroxyacyl-CoA dehydrogenase activity) (MIM 182450, MIM 107740, MIM 601792, and MIM 261515, respectively).
We also found evidence of linkage between markers on chromosome 1q21-q24 and diabetes in lean patients (with BMI <27). Although not reaching the stringent statistical thresholds for significance, results on chromosome 1q were highly suggestive (P=.00010), and, again, the broad width of the peak in the initial genome scan (>20 cM) also suggested true-positive results. Linkage on chromosome 1q was also found through stratification of families, in a homogeneous subgroup of 57 families (38% of families) in which diabetes is enriched in lean patients. Since obesity is frequently associated with diabetes and is genetically determined as well, it could be a confounding variable in genetic studies of diabetes. Therefore, genetic studies of lean diabetic patients are more likely to identify true diabetes-susceptibility loci, rather than obesity-susceptibility loci. Our results on chromosome 1q confirm (and replicate) previous reports of evidence for linkage between diabetes and markers in the same region in at least two different populations. Elbein et al. (1999) reported evidence of linkage between diabetes and markers CRP/APOA2 in 19 multigenerational families of northern European ancestry from Utah using a recessive model for parametric analyzes. In a study of the Pima Indians, among whom diabetes and obesity are highly prevalent, Hanson et al. (1998) found evidence of a diabetes locus on chromosome 1q, a region that was not linked to obesity in their study. They observed a very strong evidence for linkage with diabetes (Z=4.1) at D1S2127, in the 55 sib pairs who had onset of diabetes at age <25 years, supported by comparisons of sib pairs concordant for diabetes versus discordant sib pairs at D1S1677. The finding of linkage in three different populations strengthens the evidence for a diabetes-susceptibility locus in this region.
Saturation of the chromosome 1q21-q24 region in our families reduced the 1-LOD-unit CI to 6.5 cM around APOA2/CRP, which, according to the Unified Database for Human Genome Mapping, corresponds to ∼6 mb. Indeed, positional cloning and disequilibrium-mapping efforts have already been initiated in diabetic Pima Indians to identify the chromosome 1q locus, and several of the potential candidate genes have been screened (Baier et al. 1998; Wolford et al. 1999a). These identified a linkage disequilibrium between diabetes and single-nucleotide polymorphisms near the KCNJ9 gene coding for the G-protein-coupled inward rectifier K(+) channel homolog GIRK3, suggesting that the diabetes-susceptibility gene on chromosome 1q21-q24 is closely linked to the KCNJ9 locus (Wolford et al. 1999b). Linkage disequilibrium between diabetes and chromosome 1 markers in the same region was also found in the Old Order Amish (St. Jean 1999a, 1999b). The possible role for those genes in the French families that contributed to our linkage results is under investigation.
We identified 22 other regions showing at least nominal indication of linkage (P<.01). When looking very closely at the published data, we found similar results in other studies in most of our regions. Of particular interest is our linkage result on chromosome 10q26 that largely overlaps the region of linkage with diabetes and age at onset of diabetes identified in Mexican Americans (Duggirala et al. 1999). We were therefore able to replicate their findings independently in our population.
Our suggestive linkage results on chromosome 2p21-p16 with the diabetic phenotype are supported as well by several reports for metabolic traits in that region, mostly obesity-related phenotypes, including QTLs for leptin levels, fat mass, or BMI in different populations (Comuzzie et al. 1997; Hager et al. 1998; Rotimi et al. 1999). It is therefore possible that a genetic defect localized on chromosome 2p determines the occurrence of both obesity and type 2 diabetes. It has already been reported that some loci, such as the one on chromosome 11q23-q25, harbor diabetes and obesity genes (Hanson et al. 1998).
As far as chromosome 20 is concerned, we were the first to report positive linkage on chromosome 20q in another selection of French diabetic families (Hani et al. 1996; Zouali et al. 1997). Afterward, several studies confirmed our findings (Bowden et al. 1997; Ji et al. 1997; Ghosh et al. 1999), including results of a meta-analysis of all data available worldwide for this chromosome (Boehnke et al. 1998). Obesity-related traits have also been mapped on this chromosome (Lembertas et al. 1997; Norman et al. 1998; Lee et al. 1999). From our studies and others, it seems possible that there are two diabetes- and/or obesity-susceptibility genes on chromosomes 20p and 20q. Further fine mapping and sequencing of selected candidates (HNF3-β, MC3R, CEBP-β, and Nkx2.2) are under way to elucidate the role of chromosome 20 genes in the French diabetics.
Because emphasis has been placed on power in our genomewide-scan design for type 2 diabetes–susceptibility genes, in this study, we were able to exclude linkage at the diabetes loci on chromosomes 2q37, 15q21, and 12q24—identified in Mexican-American and Finnish subjects, respectively—as well as at the diabetes-and-obesity locus on chromosome 11q23-q25 that was identified in Pima Indians or the diabetes locus recently mapped on chromosome 3p in Mexican Americans. It is therefore very likely that genes that play an important role in isolated or recently admixed populations have a marginal role, if any, in a representative admixed population such as these French whites.
In conclusion, we report the first evidence for a novel diabetes-susceptibility locus gene on chromosome 3q27-qter. Several results of positive, although not significant, linkage at this locus that were previously reported in the literature support our findings. In addition, we independently confirm linkage with diabetes in the 1q21-q24 interval (Hanson et al. 1998; Elbein et al. 1999). Other loci on chromosomes 2p21-p16, 10q26, and 20 are potentially interesting but await further studies to be confirmed.
Acknowledgments
We are indebted to all families who participated to this study. We thank Dr. Teng for technical assistance. We thank Drs. Graeme I. Bell, Jacqui Beckman, and Florence Demenais for useful comments on the manuscript. We thank Valérie Delannoy for her help with radiation-hybrid mapping. This research was supported in part by Eli Lilly and by Région Nord-Pas de Calais (to S.D.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- CEDAR, http://cedar.genetics.soton.ac.uk/pub (for chromosome locations)
- Généthon, ftp://ftp.genethon.fr/pub/Gmap/Nature-1995 (for marker maps)
- Genome Database, http://www.gdb.org (for chromosome locations)
- Marshfield, http://research.marshfieldclinic.org/genetics/ (for marker map)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for type 2 diabetes [MIM 125853], somatostatin [MIM 182450], apolipoprotein D [MIM 107740], type 1 protein phosphatase I-2 [PPP1R2; MIM 601792], bifunctional enzyme with enoyl-CoA-hydratase and 3-hydroxyacyl-CoA dehydrogenase activity [MIM 261515])
- Unified Database for Human Genome Mapping, http://bioinformatics.weizmann.ac.il/udb/
References
- Abel L, Alcais A, Mallet A (1998a) Comparison of four sib-pair linkage methods for analyzing sibships with more than two affecteds: interest of the binomial maximum likelihood approach. Genet Epidemiol 15:371–390 [DOI] [PubMed] [Google Scholar]
- Abel L, Muller-Myhsok B (1998b) Robustness and power of the maximum-likelihood-binomial and maximum-likelihood-score methods, in multipoint linkage analysis of affected-sibship data. Am J Hum Genet 63:638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier L, Wiedrich C, Dobberfuhl A, Traurig M, Thuillez P, Bogardus C, Hanson R (1998) Sequence analysis of candidate genes in a region of chromosome 1 linked to type 2 diabetes. Diabetes 47:A171 [Google Scholar]
- Barnett AH, Eff C, Leslie RDG, Pyke DA (1981) Diabetes in identical twins: a study of 200 pairs. Diabetologia 20:87–93 [DOI] [PubMed] [Google Scholar]
- Boehnke M, Cox NJ (1997) Accurate inference of relationships in sib-pair linkage studies. Am J Hum Genet 61:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke M, Cox NJ, International Type 2 Diabetes Linkage Analysis Consortium (1998) Lessons learned in a combined linkage analysis (24 datasets, >2000 families) of type 2 diabetes on chromosome 20. Paper presented at the 48th annual meeting of American Society of Human Genetics, Denver, October 27–31 [Google Scholar]
- Bowden DW, Sale M, Howard TD, Qadri A, Spray BJ, Rothschild CB, Akots G, Rich SS, Freedman BI (1997) Linkage of genetic markers on human chromosomes 20 and 12 to NIDDM in Caucasian sib-pairs with a history of diabetic nephropathy. Diabetes 46:882–886 [DOI] [PubMed] [Google Scholar]
- Comuzzie AG, Hixson JE, Almasy L, Mitchell BD, Mahaney MC, Dyer TD, Stern MP, MacCluer JW, Blangero J (1997) A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet 15:273–276 [DOI] [PubMed] [Google Scholar]
- Cox NJ, Frigge M, Nicolae DL, Concannon P, Hanis CL, Bell GI, Kong A (1999) Loci on chromosomes 2 (NIDDM1) and 15 interact to increase susceptibility to diabetes in Mexican Americans. Nat Genet 21:213–215 [DOI] [PubMed] [Google Scholar]
- Davis S, Weeks DE (1997) Comparison of nonparametric statistics for detection of linkage in nuclear families: single-marker evaluation. Am J Hum Genet 61:1431–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano AL, Nicolaou M, Cupples LA (1998) Effect of bilineal inheritance on the power of affected sib-pair linkage analysis. Paper presented at the Seventh Annual Meeting of the International Genetic Epidemiology Society, Arcachon, France, September 8–10 [Google Scholar]
- Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O'Connell P, Stern MP (1999) Linkage of type 2 diabetes mellitus and of age at onset to a genetic location on chromosome 10q in Mexican Americans. Am J Hum Genet 64:1127–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm MG, Karnoub MC, Sakul H, Gottschalk K, Holt DC, Weber JL, Vaske D, Briley D, Briley L, Kopf J, McMillen P, Nguyen Q, Reisman M, Lai EH, Joslyn G, Shepherd NS, Bell C, Wagner MJ, Burns DK (2000) Genomewide search for type 2 diabetes susceptibility genes in four Americans populations. Am J Hum Genet 66:1871–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein SC (1997) The genetics of human noninsulin-dependent (type 2) diabetes mellitus. J Nutr 127:1891S–1896S [DOI] [PubMed] [Google Scholar]
- Elbein SC, Hoffman MD, Teng K, Leppert MF, Hasstedt SJ (1999) A genome-wide search for type 2 diabetes susceptibility genes in Utah Caucasians. Diabetes 48:1175–1182 [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, The (1997) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20:1183–1197 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Watanabe RM, Hauser ER, Valle T, Magnuson VL, Erdos MR, Langefeld CD, et al (1999) Type 2 diabetes: evidence for linkage on chromosome 20 in 716 Finnish affected sib pairs. Proc Natl Acad Sci USA 96:2198–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager J, Dina C, Francke S, Dubois S, Houari M, Vatin V, Vaillant E, Lorentz N, Basdevant A, Clement K, Guy-Grand B, Froguel P (1998) A genome-wide scan for human obesity genes reveals a major susceptibility locus on chromosome 10. Nat Genet 20:304–308 [DOI] [PubMed] [Google Scholar]
- Hani EH, Zouali H, Philippi A, Beaudoin JC, Vionnet N, Passa P, Demenais F, Froguel P (1996) Indication for genetic linkage of the phosphoenolpyruvate carboxykinase (PCK1) gene region on chromosome 20q to non insulin dependent diabetes mellitus. Diabete Metab 22:451–454 [PubMed] [Google Scholar]
- Hanis CL, Boerwinkle E, Chakraborty R, Ellsworth DL, Concannon P, Stirling B, Morrison VA, et al (1996) A genome-wide search for human non-insulin-dependent (type 2) diabetes genes reveals a major susceptibility locus on chromosome 2. Nat Genet 13:161–166 [DOI] [PubMed] [Google Scholar]
- Hanson RL, Ehm MG, Pettitt DJ, Prochazka M, Thompson DB, Timberlake D, Foroud T, Kobes S, Baier L, Burns DK, Almasy L, Blangero J, Garvey WT, Bennett PH, Knowler WC (1998) An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet 63:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegele RA, Sun F, Harris SB, Anderson C, Hanley AJ, Zinman B (1999) Genome-wide scanning for type 2 diabetes susceptibility in Canadian Oji-Cree, using 190 microsatellite markers. J Hum Genet 44:10–14 [DOI] [PubMed] [Google Scholar]
- Hitman GA, McCarthy MI, Mohan V, Viswanathan M (1992) The genetics of non-insulin-dependent diabetes mellitus in South India: an overview. Ann Med 24:491–497 [DOI] [PubMed] [Google Scholar]
- Holmans P, Craddock N (1997) Efficient strategies for genome scanning using maximum-likelihood affected-sib-pair analysis. Am J Hum Genet 60:657–666 [PMC free article] [PubMed] [Google Scholar]
- Hopper JL (1999) Is type II (non-insulin-dependent) diabetes mellitus not so “genetic” after all? Diabetologia 42:125–127 [DOI] [PubMed] [Google Scholar]
- Ji L, Malecki M, Warram JH, Yang Y, Rich SS, Krowlewski AS (1997) New susceptibility locus for NIDDM is localized to human chromosome 20q. Diabetes 46: 876–881 [DOI] [PubMed] [Google Scholar]
- Knowler WC, Williams RC, Pettitt DJ, Steinberg AG (1988) Gm3,5,13,14 and type 2 diabetes mellitus: an association in American Indians with genetic admixture. Am J Hum Genet 43:520–526 [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L, Lander ES (1995) Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet 57:439–454 [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Kruglyak L (1995) Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11:241–247 [DOI] [PubMed] [Google Scholar]
- Lee JH, Reed DR, Li WD, Xu W, Joo EJ, Kilker RL, Nanthakumar E, North M, Sakul H, Bell C, Price RA (1999) Genome scan for human obesity and linkage to markers in 20q13. Am J Hum Genet 64:196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembertas AV, Pérusse L, Chagnon YC, Fisler JS, Warden CH, Purcell-Huynh DA, Dionne FT, Gagnon J, Nadeau A, Lusis AJ, Bouchard C (1997) Identification of an obesity quantitative trait locus on chromosome 2 and evidence of linkage to body fat and insulin on the human homologous region 20q. J Clin Invest 100:1240–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani MM, Widen E, Lehto M, Thomas J, McCarthy M, Brayer J, Bryant B, Chan G, Daly M, Forsblom C, Kanninen T, Kirby A, Kruglyak L, Munnelly K, Parkkonen M, Reeve-Daly MP, Weaver A, Brettin T, Duyk G, Lander ES, Groop LC (1996) Mapping of a gene for type 2 diabetes associated with an insulin secretion defect by a genome scan in Finnish families. Nat Genet 14:90–94 [DOI] [PubMed] [Google Scholar]
- Morton NE (1998) Significance levels in complex inheritance. Am J Hum Genet 62:690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel JV, Weder AB, Julius S (1998) Type II diabetes, essential hypertension, and obesity as “syndromes of impaired genetic homeostasis”: the “thrifty genotype” hypothesis enters the 21st century. Perspect Biol Med 42:44–74 [DOI] [PubMed] [Google Scholar]
- Norman RA, Tataranni PA, Pratley R, Thompson DB, Hanson RL, Prochazka M, Baier L, Ehm MG, Sakul H, Foroud T, Garvey WT, Burns D, Knowler WC, Bennett PH, Bogardus C, Ravussin E (1998) Autosomal genomic scan for loci linked to obesity and energy metabolism in Pima Indians. Am J Hum Genet 62:659–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE (1995) The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat Genet 11:402–408 [DOI] [PubMed] [Google Scholar]
- ——— (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J (1991) Analysis of human genetic linkage. Revised ed. Johns Hopkins University Press, Baltimore [Google Scholar]
- Pratley RE, Thompson DB, Prochazka M, Baier L, Mott D, Ravussin E, Sakul H, Ehm MG, Burns DK, Foroud T, Garvey WT, Hanson RL, Knowler WC, Bennett PH, Bogardus C (1998) An autosomal genomic scan for loci linked to prediabetic phenotypes in Pima Indians. J Clin Invest 101:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao DC (1998) CAT scans, PET scans and genomic scans. Genet Epidemiol 15:1–18 [DOI] [PubMed] [Google Scholar]
- Rainwater DL, Almasy L, Blangero J, Cole SA, Vandeberg JL, MacCluer JW, Hixson JE (1999) A genome search identifies major quantitative trait loci on human chromosomes 3 and 4 that influence cholesterol concentrations in small LDL particles. Arterioscler Thromb Vasc Biol 19:777–783 [DOI] [PubMed] [Google Scholar]
- Rich SS (1990) Mapping genes in diabetes: genetic epidemiological perspective. Diabetes 39:1315–1319 [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas K (1996) The future of genetic studies of complex human diseases. Science 273:1516–1517 [DOI] [PubMed] [Google Scholar]
- Rotimi CN, Comuzzie AG, Lowe WL, Luke A, Blangero J, Cooper RS (1999) The quantitative trait locus on chromosome 2 for serum leptin levels is confirmed in African-Americans. Diabetes 48:643–644 [DOI] [PubMed] [Google Scholar]
- Sham PC, Curtis D (1995) Monte Carlo tests for associations between disease and alleles at highly polymorphic loci. Ann Hum Genet 59:97–105 [DOI] [PubMed] [Google Scholar]
- St. Jean PL, Hsueh WC, Mitchell B, Pollin T, Burns D, Bell C, Wagner M, Shuldiner AR (1999a) Linkage disequilibrium between chromosome 1 markers and type 2 diabetes in the Old Order Amish. Paper presented at the Second Research Symposium on the Genetics of Diabetes, San Jose, October 17–19 [Google Scholar]
- St. Jean PL, Mitchell BD, Hsueh WC, Burns DK, Ehm MG, Wagner MJ, Bell C, Aburomia R, Nanthakumar E, Shuldiner AR (1999b) Type 2 diabetes loci in the old order Amish. Diabetes 48:A46 [Google Scholar]
- Terwilliger JD (1995) A powerful likelihood method for the analysis of linkage disequilibrium between trait loci and one or more polymorphic marker loci. Am J Hum Genet 56:777–787 [PMC free article] [PubMed] [Google Scholar]
- Terwilliger JD, Shannon WD, Lathrop MG, Nolan JP, Goldin LR, Chase GA, Weeks DE (1997) True and false positive peaks in genomewide scans: applications of length-biased sampling to linkage mapping. Am J Hum Genet 61:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Hitman GA, Kopelman PG (1994) Apolipoprotein-D polymorphism: a genetic marker for obesity and hyperinsulinemia. J Clin Endocrinol Metab 79:568–570 [DOI] [PubMed] [Google Scholar]
- Vionnet N, Hani EH, Lesage S, Philippi A, Hager J, Varret M, Stoffel M, Tanizawa Y, Chiu KC, Glaser B, Permutt MA, Passa P, Demenais F, Froguel P (1997) Genetics of non insulin dependent diabetes mellitus (NIDDM) in France: studies with 19 candidate genes in affected sib-pairs. Diabetes 46:1062–1068 [DOI] [PubMed] [Google Scholar]
- Wolford JK, Bogardus C, Hanson R, Prochazka M (1999a) Molecular analysis of a type 2 diabetes-linked region on chromosome 1q21-q23. Diabetes 48:A47 [Google Scholar]
- Wolford JK, Bogardus C, Prochazka M (1999b) Linkage disequilibrium mapping of a region on 1q21-23 linked with type 2 diabetes mellitus in Pima Indians. Paper presented at Second ADA Research Symposium on the Genetics of Diabetes, San Jose, October 17–19 [Google Scholar]
- Zouali H, Hani EH, Philippi A, Vionnet N, Beckmann J, Demenais F, Froguel P (1997) A susceptibility locus for early-onset non-insulin-dependent (type 2) diabetes mellitus maps to chromosome 20q, proximal to the phosphoenolpyruvate carboxykinase gene. Hum Mol Gen 6:1401–1408 [DOI] [PubMed] [Google Scholar]