Abstract
To identify the most active curative treatment of Buruli ulcer, two regimens incorporating the use of rifampin (RIF) were compared with the use of RIF alone in a mouse footpad model of Mycobacterium ulcerans infection. Treatments began after footpad swelling from infection and continued for 12 weeks with five doses weekly of one of the following regimens: (i) 10 mg of RIF/kg alone; (ii) 10 mg of RIF/kg and 100 mg of amikacin (AMK)/kg; and (iii) 10 mg of RIF/kg, 100 mg of clarithromycin (CLR)/kg, and 50 mg of sparfloxacin (SPX)/kg. The activity of each regimen was assessed in terms of the reduction of the average lesion index and acid-fast bacillus (AFB) and CFU counts. All three regimens displayed various degrees of bactericidal activity against M. ulcerans. The ranking of bactericidal activity was found to be as follows: RIF-AMK > RIF-CLR-SPX > RIF. RIF-AMK was able to cure M. ulcerans-infected mice and prevent relapse 26 weeks after completion of treatment. To determine the impact of different rhythms of administration of RIF-AMK on the suppression of M.ulcerans growth, mice were given the RIF-AMK combination for 4 weeks but doses were administered either 5 days a week or twice or once weekly. After completion of treatment, the mice were kept under supervision for 30 additional weeks. M. ulcerans was considered to have grown in the footpad if swelling was visually observed and harvests contained more than 5 × 105 AFB per footpad. The proportion of mice in which growth of M. ulcerans occurred, irrespective of drug dosage, was compared with the control mice to determine the proportion of M. ulcerans killed. Each dosage of RIF-AMK was bactericidal for M. ulcerans (P < 0.001), but the effect was significantly stronger in mice treated 5 days per week. The promising results of RIF-AMK treatment in M. ulcerans-infected mice support the clinical trial that is currently in progress under World Health Organization auspices in Ghana.
Buruli ulcer or Mycobacterium ulcerans infection is the third most common mycobacterial disease after tuberculosis and leprosy. Large surgical excision of necrotic tissue, followed by skin grafting is, at present, the only treatment (21). The search for antimycobacterial drugs able to increase efficacy or substitute for surgical treatment is a priority.
Using the kinetic method designed by C. C. Shepard for testing antileprosy drug activity in the mouse footpad (17), the following antimicrobials were assessed against M. ulcerans: clarithromycin (CLR), minocycline, sparfloxacin (SPX), rifampin (RIF), rifabutin, and amikacin (AMK). RIF, rifabutin, and AMK exhibited bactericidal activity, whereas CLR and SPX exhibited bacteriostatic activity; minocycline demonstrated no activity (3).
Since the RIF-AMK combination was anticipated to be highly potent against M. ulcerans, we initially studied the curative efficacy of this combination. Treatment was given daily for 3 months after mouse footpad swelling from M. ulcerans infection had occurred. Because combined RIF-AMK requires the injection of AMK, a requirement often difficult to fulfill in the field, we tested the alternative response to two oral regimens: the use of RIF alone and the use of RIF-CLR-SPX.
The results of the first experiment indicated that the combination RIF-AMK was the most potent curative therapy against M. ulcerans. A second experiment was conducted to determine whether reducing the drug administration from daily (5 days a week) to twice weekly or once weekly for 4 weeks would retain potency. The method used was derived from the proportional bactericidal assay developed for M. leprae (2) that measures bactericidal activity of drug regimens given as preventive therapy.
MATERIALS AND METHODS
The Cu001 strain of M. ulcerans used was isolated from tissue excised from a Buruli ulcer patient in Adzopé, Ivory Coast, in June 1996, and was maintained in a mouse footpad. The footpad techniques were adapted from those developed of Shepard and McRae for the inoculation and counting of M. leprae in the mouse footpad (18, 19). Mouse footpad-grown M. ulcerans was used for inoculation. Acid-fast bacilli (AFB) in the footpad were counted, and the suspension was further diluted up to the desired concentration for footpad injection in the plantar surface. During experiments, AFB counts were performed microscopically according to the method of Shepard and McRae (18); CFU counts were made by plating appropriate dilutions of footpad suspensions onto Löwenstein-Jensen slants for incubation at 32°C for 4 months.
The following compounds were provided by their manufacturers: RIF, Marion Merrel-Dow, Neuilly, France; AMK, Bristol-Myers-Squibb, Paris, France; CLR, Abbott, Rungis France; SPX, Rhone DPC Europe, Anthony, France. RIF, CLR, and SPX were suspended in 0.05% agar-containing distilled water at the desired concentration. AMK was diluted in normal saline at the concentration of 10 mg/ml. The stock solutions were prepared weekly and stored at 4°C.
(i) Mouse model, infection, and treatment.
Female BALB/c mice aged 5 to 6 weeks, weighing 18 to 20 g, were purchased from Janvier Breeding Center, Le Genest-Saint-Isle, France, and housed in our animal facility for 2 days before infection. Mice were given 600 mg of cloxacillin/kg 20 to 30 min before infection to prevent possible Staphylococcus aureus superinfection. Mice were infected in the left hind footpad, and the right hind footpad was used as negative control for assessing M. ulcerans growth.
In the first experiment, 200 BALB/c mice were infected with 0.03 ml of a bacterial suspension containing 3 × 103 M. ulcerans CFU (or 3 × 104 M. ulcerans AFB). Infected mice were kept without treatment until they developed swollen footpads, usually within ca. 5 weeks. At this point, mice were randomly allocated to four groups: an untreated control group of 50 mice and three additional groups of 50 mice each, to which one of the following three regimens was administered: (i) 10 mg of RIF/kg alone; (ii) 10 mg of RIF/kg and 100 mg of AMK/kg; or (iii) 10 mg of RIF/kg, 100 mg of CLR/kg, and 50 mg of SPX/kg. Drugs were given at dosages chosen to provide an area-under-the-curve value comparable to those obtained in humans (8, 9, 13, 14). The treatments were administered by gavage 5 days a week for 12 weeks through an esophageal cannula, except for AMK, which was injected subcutaneously. After treatment completion, mice were monitored for up to 39 weeks (W39).
In the second experiment, 168 mice were randomly distributed into four groups of 42 animals each: these groups included controls (untreated) and three treatment groups that received 4 weeks of RIF-AMK therapy administered either 5 days a week or twice or once weekly. Within these four groups, there were seven subgroups of six mice each that had been infected in the left hind footpad with 0.03 ml of a serially diluted M. ulcerans suspensions containing 5 × 105, 5 × 104, 5 × 103, 5 × 102, 5 × 101, 5 × 100, or 5 × 10−1 AFB. Except for the control group, the three treatment groups began RIF-AMK therapy the day after infection. RIF was administered by gavage at 10 mg/kg (0.2 to 0.3 ml of the 1-mg/ml stock solution, according to the increase in mouse body weight). AMK was injected subcutaneously at 100 mg/kg (0.2 to 0.3 ml of the 10-mg/ml stock solution, according to the increase in mouse body weight).
After treatment completion, mice were held for 30 weeks, a period of time sufficient to permit a single surviving organism (which might exist in either the log0 or log1 serial dilution) to multiply for footpad swelling to become apparent.
(ii) Assessment of M. ulcerans growth.
In the first experiment, each drug regimen was assessed in terms of reduction of the average lesion index (ALI) and the change in the AFB and CFU counts. The lesion index was defined as follows: index 0 = normal footpad; index 1 = noninflammatory footpad swelling; index 2 = inflammatory footpad swelling; index 3 = inflammatory hind foot swelling; index 4 = inflammatory leg swelling; and index 5 = death of the mouse. Footpad examinations and mortalities were recorded on a weekly basis during the 12 weeks of treatment and then for 27 additional weeks after treatment cessation. ALI was calculated from the average of each group lesion index. The ALI varied from 0 to 5.
The day after infection, 10 mice were sacrificed to establish a baseline of AFB and CFU counts in the left footpads. After 1 week (W1), 2 weeks (W2), 4 weeks (W4), 8 weeks (W8), and 12 weeks (W12) of treatment, AFB and CFU counts were performed on samples from five footpads in each treatment group.
In the second experiment, after the 4-week period of treatment, mice were examined on a weekly basis for 34 weeks to detect any footpad swelling. If footpad swelling occurred (index 2), mice were sacrificed for AFB count. If organisms had survived the treatment and resumed multiplication after treatment completion, M. ulcerans was considered to have grown when the following conditions were associated: footpad swelling and >5 × 105 AFB harvested from the footpad. In each treatment group, the proportion of mice in which growth occurred was compared with the control group to determine the “most probable number” of AFB that gave rise to growth in order to calculate the proportion of M. ulcerans killed (2).
(iii) Statistical analysis.
In the first experiment, results were analyzed by the Student t test and Fisher exact probability correlations. P values were two tailed, and a P value of 0.05 was considered statistically significant.
In the second experiment, the proportion of viable M. ulcerans surviving treatment was determined as the median (50%) infectious dose, and the significance of the differences between the groups was calculated by using the Spearman and Korber method (20).
RESULTS
Experiment 1: activity of the three regimens utilizing RIF. (i) ALI.
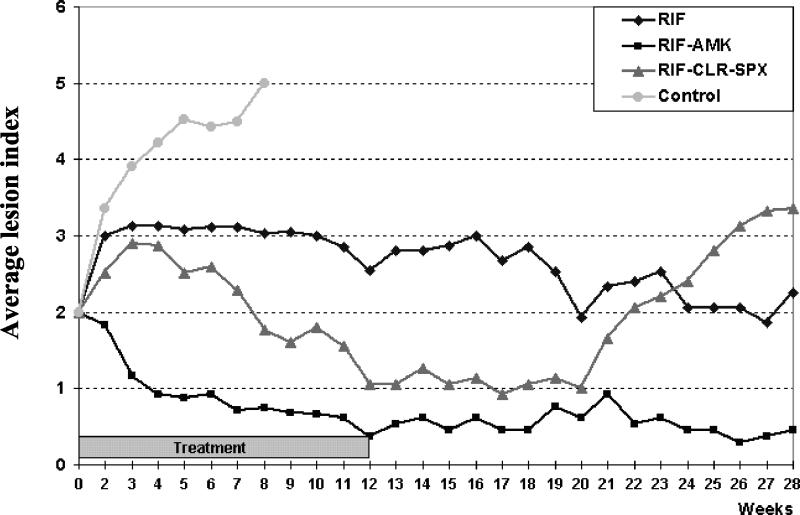
The ALI results are presented in Fig. 1. In control mice, the ALI steadily increased from inflammatory footpad swelling (index 2) to death (index 5) by W8. In treated mice, no death occurred during the 12-week treatment. In mice treated with RIF alone, the ALI increased from index 2 to index 3 between W0 and W2 and then remained stable until completion of treatment. In RIF-CLR-SPX-treated mice, the ALI also increased from index 2 to index 3 between W0 and W3. It leveled off during W3 to W4 and then steadily decreased from index 3 to index 1 between W4 and W12. In RIF-AMK-treated mice, the ALI steeply decreased from index 2 to index 1 during the first 4 weeks of treatment. Thereafter, it remained stable or decreased slowly to reach 0.5, i.e., an apparent complete cure, by W12.
FIG. 1.
Evolution of the footpad lesions (i.e., ALI) in mice infected with M. ulcerans and treated daily with RIF alone, RIF-AMK, or RIF-CLR-SPX.
During follow-up after treatment completion, the ALI remained more or less stable in RIF-treated mice at ca. index 3 or slightly decreased from W12 to the end of the follow-up at W39. The seven remaining mice were sacrificed for determination of the AFB and CFU counts. In RIF-CLR-SPX-treated mice, the ALI remained stable at around index 1 until W20 and then steadily increased to index 3.5 by W27. Because of this, the remaining 13 mice were sacrificed on W28 for determination of the AFB and CFU counts. In RIF-AMK-treated mice, the ALI remained stable at index 0.5 until the end of the 39-week follow-up, when the mice were sacrificed for determination of the AFB and CFU counts.
(ii) Enumeration of AFB in footpads.
In control mice, the mean AFB counts increased significantly (P < 0.001) from 6.22 to 7.27 log10 between W0 and W4. By W8 all of the mice were dead. In both RIF-treated mice and RIF-CLR-SPX-treated mice, the AFB counts slightly increased during the first weeks of therapy and then remained stable thereafter. In RIF-AMK-treated mice, the AFB counts remained unchanged over the course of the experiment.
(iii) Enumeration of CFU in footpads.
In control mice, the mean CFU increased from 5.7 to 6.63 log10 between W0 and W2. By W4, the cultures indicated S. aureus superinfection, and by W8 all mice had died. In treated mice, the CFU counts remained stable during the first 2 weeks of treatment. Then, by up to W8 they progressively declined to reach 0.34 log10 in RIF-AMK-treated mice, 1.72 log10 in RIF-CLR-SPX-treated mice, and 4.02 log10 in RIF-treated mice (Table 1). The CFU counts at W8 of treatment were significantly different from the pretreatment count, indicating that the drug regimens displayed various degrees of bactericidal activity in the following order: RIF-AMK > RIF-CLR-SPX > RIF alone.
TABLE 1.
Mean CFU counts in mouse footpads before and after treatment with RIF alone, RIF-CLR-SPX, or RIF-AMK
| Regimen (dosage [mg/kg/dose]) | Mean CFU count (log10 ± SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| D0 | W1 | W2 | W4 | W8 | W12 | W28 | W39 | |
| Untreated control | 5.70 ± 0.14 | 5.92 ± 0.23 | 6.63 ± 0.09 | Ca | ||||
| RIF (10) | 5.70 ± 0.14 | 5.36 ± 0.11 | 5.94 ± 0.27 | 5.90 ± 0.4 | 4.02 ± 0.40 | C | C | |
| RIF (10)-CLR (100)-SPX (50) | 5.70 ± 0.14 | 5.35 ± 0.33 | 5.89 ± 0.14 | 5.01 ± 0.31 | 1.72 ± 1.04 | C | C | |
| RIF (10)-AMK (100) | 5.70 ± 0.14 | 5.09 ± 0.18 | 5.26 ± 0.32 | 3.30 ± 0.001 | 0.34 ± 0.46 | C | C | |
C, contaminated.
Experiment 2: activities of the RIF-AMK combination given at once, twice, or five times weekly.
In untreated control mice, footpad swelling occurred at W4, W6, W8, W10, and W12 in mice infected with 105, 104, 103, 102, and 101 M. ulcerans AFB, respectively (Table 2). According to the numbers of mice with swollen footpads, the proportion of viable M. ulcerans was 73.9% in the untreated control. The proportion of viable organisms was 0.22 to 0.32% among mice treated once a week with RIF-AMK, 0.047% among mice treated twice a week, and 0.0046% among mice treated five times a week. Therefore, the killing effect of RIF-AMK was directly related to the rhythm of drug administration. The more frequently the dose was given, the greater the bactericidal activity.
TABLE 2.
Bactericidal effects against M. ulcerans of the combination of RIF (10 mg/kg) and AMK (100 mg/kg) given for 4 weeks
| Regimen | No. of footpads with multiplication of M. ulcerans/total no. of footpads with AFB inoculum ofa:
|
% M. ulcerans
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 105 | 104 | 103 | 102 | 101 | 100 | 10−1 | Viableb | Killed by treat- ment (log10)c | |
| Untreated control | 6/6 (4-5) | 5/5 (6-7) | 6/6 (8-8) | 6/6 (10-13) | 6/6 (12-17) | 2/6 (14-15) | 1/6 (17) | 73.9 | |
| RIF-AMK, one dose/wk | 6/6 (9-11) | 6/6 (11-17) | 6/6 (13-14) | 0/6 | 0/6 | 0/6 | 0/6 | 0.22-0.32 | 99.56A-99.7A (2.5)d |
| RIF-AMK, two doses/wk | 6/6 (13-18) | 5/6 (13-18) | 2/6 (19-20) | 1/6 (15) | 0/6 | 0/6 | 0/6 | 0.047 | 99.9369A,B,C (3.2) |
| RIF-AMK, five doses/wk | 6/6 (22-31) | 2/6 (24-28) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0.0046 | 99.9937A,D (4.2) |
“Multiplication” was defined as footpad swelling and harvests of ≥5 × 105 AFB per footpad. The number of weeks between infection and footpad swelling is given in parentheses.
Value was derived from the equation: percent viable M. ulcerans = 0.69/50% infectious dose.
This calculation was based on the proportions of viable bacilli between untreated controls and the treated group. Superscript letters: A, P < 0.001 between any combination RIF-AMK and untreated controls; B, P = 0.02 between the combination RIF-AMK at two doses a week and at one dose a week (minimum estimated value); C, P = 0.063 between the combination RIF-AMK at two doses a week and at one dose a week (maximum estimated value); D, P < 0.01 between the combination RIF-AMK at five doses a week and at two doses a week.
Values indicate the range from the minimum estimated value, assuming that one footpad swelling would have occurred in mice inoculated with 102 bacilli, to the maximum estimated value, assuming that five footpad swellings would have occurred in mice inoculated with 103 bacilli.
DISCUSSION
The results of both experiments demonstrated the bactericidal activity of RIF-AMK against established M. ulcerans infections. In the first experiment, 12 weeks of curative treatment with RIF-AMK given 5 days a week resulted in an apparent cure. In the second experiment, RIF-AMK was significantly more bactericidal when given 5 days a week compared to when it was given 2 days a week and was even more bactericidal when it was given 2 days a week than when given once a week. However, 4 weeks of 5-day/week therapy with RIF-AMK was clearly not long enough to completely prevent subsequent growth of M. ulcerans in infected mice. One can thus tentatively conclude that the optimal frequency of dose is daily and that the optimal duration of treatment is between 4 and 12 weeks.
Because of the bactericidal activity of the RIF-AMK against M. ulcerans, it is desirable to test its clinical activity in patients with Buruli ulcer. Both RIF and AMK are extensively used drugs: RIF is used for the treatment of tuberculosis (15) and leprosy (12), and amikacin is used for the treatment of severe bacteremia. While RIF has limited toxicity AMK, like all aminoglycoside antibiotics, has potential ototoxicity and nephrotoxicity that is dose related (7). In addition, AMK requires parenteral administration, which presents difficulties in patient delivery, along with potential transmission risk for human immunodeficiency virus under nonsterile conditions. Streptomycin (11) is a possible substitute for AMK since it has been tested against M. ulcerans in the mouse model at a daily dosage of 150 mg/kg of body weight (a dose equipotent to 15 mg/kg in humans) and exhibited bactericidal activity comparable to that of AMK, even though the activities of the two drugs have never been compared in the same experiment (3, 4, 16).
The oral combination RIF-CLR-SPX, also given 5 days a week, was clearly less effective than AMK-RIF, although it killed ca. 4 log10 M. ulcerans bacilli in 8 weeks and is an oral regimen. Therefore, it is tempting to consider testing such a combination in the treatment of Buruli ulcer. However, SPX has been withdrawn because of phototoxicity, and none of the other fluoroquinolones that have been tested against M. ulcerans appeared to be as active as SPX (1).
The results of the present study raise several issues regarding the fate of M. ulcerans in mouse tissues. The persistence of AFB, despite the clearcut decrease of CFU counts in treated mice, has analogy to the persistence of dead M. leprae in the footpads of mice treated with active drugs and is similar to findings observed in patients with lepromatous leprosy (10). Because of its immunosuppressive properties, mycolactone, the polyketide toxin secreted by M. ulcerans may inhibit the clearance of nonviable bacilli by macrophages, as does the anergy induced by M. leprae (6).
As previously reported (3), the assessment of M. ulcerans growth in footpads of mice by using Löwenstein-Jensen media was compromised by the superinfection of footpads (and consequently the culture media) with S. aureus. It appears that the best way to evaluate the efficacy of a treatment is to measure the prevention or reduction of footpad swelling in mice infected with large numbers of AFB and to relate the observation of footpad swelling combined with an AFB count greater than 5 × 105 as a surrogate marker of M. ulcerans growth in the mouse footpad. It is interesting that swelling of footpads in control mice was delayed for 2 weeks as the inoculum was diluted 10-fold. Considering that the AFB counts increased by 1 log10 in a 2-week period, the generation time of M. ulcerans can be estimated to be between 3 and 4 days, which is in agreement with previous reports (3, 5)
In conclusion, RIF-AMK is an antibiotic regimen able to cure established M. ulcerans infection in mice. The role of this combination in the treatment of M. ulcerans infection in humans, i.e., whether it can be used in addition to or as a substitute for surgery, remains to be determined.
Acknowledgments
This work was supported by the Association Française Raoul Follereau.
We thank George Kubica and Norman Morrison for editorial assistance.
REFERENCES
- 1.Bentoucha, A., J. Robert, H. Dega, N. Lounis, V. Jarlier, and J. Grosset. 2001. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans in mice. Antimicrob. Agents Chemother. 45:3109-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colston, M. J., G. R. F. Hilson, and D. K. Bannerjee. 1978. The proportional bactericidal test, a method for assessing bactericidal activity of drugs agaisnt Mycobacterium leprae in mice. Lepr. Rev. 49:7-15. [PubMed] [Google Scholar]
- 3.Dega, H., J. Robert, P. Bonnafous, V. Jarlier, J. Grosset. 2000. Activity of several antimicrobials on Mycobacterium ulcerans infection in mice. Antimicrob. Agents Chemother. 44:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman, W. H., and A. G. Karlson. 1957. Mycobacterium ulcerans infections: response to chemotherapy in mice. Am. Rev. Tuberc. 75:266-279. [DOI] [PubMed] [Google Scholar]
- 5.Fenner, F. 1956. Pathogenic behavior of Mycobacterium ulcerans and Mycobacterium balnei in mouse and developing chick embryo. Am. Rev. Tuberc. 73:650-673. [DOI] [PubMed] [Google Scholar]
- 6.George, K. M., L. P. Barker, D. M. Welty, and P. L. Small. 1998. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect. Immun. 66:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert, D. N. 2000. Aminoglycosides, p. 307-329. In G. L. Mandell, R. G. Douglas, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 8.Grosset, J. 1978. The sterilizing value of rifampicin and pyrazinamide in experimental short course chemotherapy. Tubercle 59:287-297. [PubMed] [Google Scholar]
- 9.Grosset, J., C. Truffot-Pernot, C. Lacroix, and B. Ji. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harboe, M. 1985. The immunology of leprosy, p. 53-87. In R. C. Hastings (ed.), Leprosy. Churchill Livingstone, New York, N.Y.
- 11.Hoeprich, P. D. 1994. Antibacterial chemotherapy, p. 247-274. In P. D. Hoeprich, M. C. Jordan, and A. R. Ronald (ed.), Infectious diseases, 5th ed. J. B. Lippincott, Philadelphia, Pa.
- 12.Jacobson, R. R. 1985. Treatment, p. 193-222. In R. C. Hastings (ed.), Leprosy. Churchill Livingstone, New York, N.Y.
- 13.Ji, B., E. G. Perani, and J. H. Grosset. 1991. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35:579-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalande, V., C. Truffot-Pernot, A. Paccaly-Moulin, J. Grosset, and B. Ji. 1993. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 37:407-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher, D., P. Chaulet, S. Spinaci, and A. Harries. 1997. Treatment of tuberculosis: guidelines for national programmes. World Health Organization, Geneva, Switzerland.
- 16.Pattyn, S. R., and J. Royackers. 1965. Traitement de l'infection expèrimentale de la souris par Mycobacterium ulcerans et Mycobacterium balnei. Ann. Soc. Belg. Med. Trop. 45:31-38. [PubMed] [Google Scholar]
- 17.Shepard, C. C. 1967. A kinetic method for the study of the activity of drugs against Mycobacterium leprae. Int. J. Lepr. 35:429-436. [Google Scholar]
- 18.Shepard, C. C., and D. H. Mc Rae. 1968. A method for counting acid-fast bacteria. Int. J. Lepr. 36:78-82. [PubMed] [Google Scholar]
- 19.Shepard, C. C. 1960. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepard, C. C. 1982. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50:96-101. [PubMed] [Google Scholar]
- 21.Van der Werf, T., W. T. A. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]