Abstract
Hypohidrotic ectodermal dysplasia (HED), a congenital disorder of teeth, hair, and eccrine sweat glands, is usually inherited as an X-linked recessive trait, although rarer autosomal dominant and recessive forms exist. We have studied males from four families with HED and immunodeficiency (HED-ID), in which the disorder segregates as an X-linked recessive trait. Affected males manifest dysgammaglobulinemia and, despite therapy, have significant morbidity and mortality from recurrent infections. Recently, mutations in IKK-gamma (NEMO) have been shown to cause familial incontinentia pigmenti (IP). Unlike HED-ID, IP affects females and, with few exceptions, causes male prenatal lethality. IKK-gamma is required for the activation of the transcription factor known as “nuclear factor kappa B” and plays an important role in T and B cell function. We hypothesize that “milder” mutations at this locus may cause HED-ID. In all four families, sequence analysis reveals exon 10 mutations affecting the carboxy-terminal end of the IKK-gamma protein, a domain believed to connect the IKK signalsome complex to upstream activators. The findings define a new X-linked recessive immunodeficiency syndrome, distinct from other types of HED and immunodeficiency syndromes. The data provide further evidence that the development of ectodermal appendages is mediated through a tumor necrosis factor/tumor necrosis factor receptor–like signaling pathway, with the IKK signalsome complex playing a significant role.
The molecular basis of the ectodermal dysplasias, a group of inherited disorders involving absence or dysplasia of the ectodermal appendages, is steadily being discovered. The hypohidrotic or anhidrotic forms display abnormal development of eccrine sweat glands, teeth, and hair (Freire-Maia and Pinheiro 1988). Hypohidrotic ectodermal dysplasia (HED) is usually inherited as an X-linked recessive trait (ED1 [MIM 305100]), although rarer, autosomal dominant (ED3 [MIM 129490]) and autosomal recessive (MIM 224900) forms exist. Ectodysplasin-A, the product of the X-linked locus, is a member of the tumor necrosis factor (TNF) ligand superfamily (Monreal et al. 1998; Ezer et al. 1999), whereas the protein product of one of the autosomal forms is a member of the TNF-receptor (TNFR) superfamily (Headon and Overbeek 1999; Monreal et al. 1999). Recently, another disorder classified as an ectodermal dysplasia, familial incontinentia pigmenti (IP) (IP2 [MIM 308310]), has been shown to be due to mutations in IKK-gamma (NEMO) (Smahi et al. 2000). IKK-gamma is required for the activation, and subsequent translocation to the nucleus, of the transcription factor NF-κB, where NF-κB activates multiple target genes (Rothwarf et al. 1998; Yamaoka et al. 1998). Members of the TNF cytokine family are among multiple intra- and extracellular stimuli that can activate NF-κB. NF-κB is sequestered in the cytoplasm by I-κB, an inhibitory molecule that, on activation of the pathway, is phosphorylated and degraded by a multiprotein kinase complex, of which IKK-gamma is an essential regulatory component (Israel 2000). The importance of IKK-gamma for normal T and B cell development is supported by data from IKK-gamma knockout mice (Makris et al. 2000; Rudolph et al. 2000; Schmidt-Supprian et al. 2000), as well as by the findings of immunodeficiency in a rare surviving male patient with a mutation within IKK-gamma (Smahi et al. 2000). We have encountered the uncommon combination of HED and immunodeficiency (HED-ID) in males from four unrelated families. Although there have been rare reports of significant immunodeficiency in males with putative X-linked HED (Abinun et al. 1996; Schweizer et al. 1999), we have seen no immunodeficiency in males from families with known mutations at the ED1 (Xq13.1) locus (Clarke et al. 1987; Monreal et al. 1998). In addition, defects of the eccrine sweat glands, teeth, or hair are not present in males with known X-linked immunodeficiency syndromes (Conley 1994; Puck 1994; Smart and Ochs 1997). This has led us to hypothesize that HED-ID is a distinct X-linked recessive disorder. Even though the common IKK-gamma mutations seen in IP are lethal for males in utero, we hypothesize that mutations that preserve some IKK-gamma function may be responsible for HED-ID.
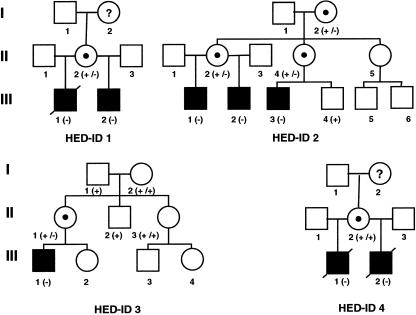
Families with HED-ID were identified at clinical centers and were recruited into a research study approved by the institutional review board of Oregon Health Sciences University. Consent was obtained for the use of clinical information, relevant family history, photographs, and DNA samples. Family histories and clinical data were provided by the collaborating centers. The proband of family 1 (III-1; see fig. 1) presented, during the 1st year of life, with recurrent infections and had repeated hospitalization for pneumonia and bacterial infections of both bone and soft tissues. Immunoglobulin levels at age 10 mo showed abnormally low levels of IgG (<200 mg/dl [normal range 294–1,069 mg/dl]), low-to-normal levels of IgA, and elevated levels of IgM (1,100 mg/dl [normal range 41–149 mg/dl]). The remainder of his immunological evaluation was essentially normal, and he was begun on intravenous gammaglobulin (IVIG) therapy. An inability to sweat had been noted since infancy, requiring lifelong cooling measures. Dental examination at age 12 years showed absence of seven teeth from his secondary dentition, on dental radiographs, as well as conical-shaped maxillary lateral incisors. The patient had periorbital wrinkling of the skin but normal scalp hair. Despite intensive treatment, he developed bronchiectasis with pulmonary insufficiency, and he died at age 17 years, after bilateral lung transplantation. The proband's younger maternal half-brother (III-2; see fig. 2a) was studied during infancy and was found to have a similar dysgammaglobulinemia, with low levels of IgG and elevated IgM levels. He was begun on IVIG but still experienced hospitalizations because of recurrent bacterial infections. He had conical-shaped incisors, hypodontia (missing four of his permanent teeth), and normal scalp hair, and, because inadequate sweating, he required special cooling measures. The mother of these two patients (II-2) had a normal results on examination and an unremarkable family history.
Figure 1.
Pedigrees of families with HED-ID. Black squares denote affected males; circles containing dots denote carrier females. (−) = Mutant allele; (+) = wild-type allele.
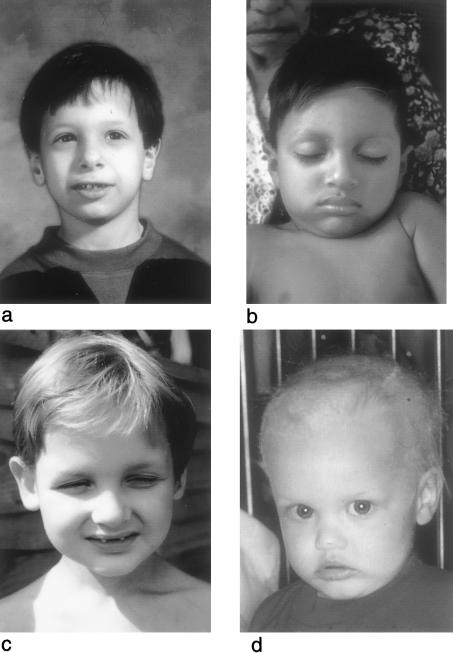
Figure 2.
Affected males from each family: HED-ID 1 (a), HED-ID 2 (b), HED-ID 3 (c), and HED-ID 4 (d). Note the hypodontia and conical-shaped teeth and the periorbital wrinkling and darkening in panels a and c. Scalp hair of the affected individuals is variable, with a normal appearance in panels a and b, mild thinning in panel c, and sparse thin hair in panel d.
The proband of family 2 (III-1; see fig. 2b) presented, at age 28 mo, after developing pneumococcal meningitis and pneumonia. His history was remarkable for recurrent cellulitis, soft-tissue abscesses, and sepsis since age 2 wk. Initial laboratory evaluation included a normal lymphocyte phenotype, except for increased circulating B cells (33%), IgG 1,030 mg/dl with normal levels of IgG subclasses, elevated levels of IgA (401–771 mg/dl), and normal levels of both IgM (45 mg/dl) and IgE (54 IU/ml). Karyotype, neutrophil oxidative burst, complement (CH50), properidin level, and antibody response to immunization with tetanus toxoid were normal. However, isohemagglutinin titers and specific antibody production to pneumococcal polysaccharide were absent. In vitro lymphocyte proliferative responses were poor initially but subsequently normalized. Despite administration of IVIG, the patient developed pneumococcal sepsis, recurrent pneumonia, and giardiasis. He was noted to have conical teeth, decreased sweating, normal scalp hair, and dry skin with some areas of hypopigmentation. Because of the family history, the proband’s maternal half-brother (III-2) was evaluated at age 8 mo, and initial evaluation revealed increased circulating B cells (36%), elevated levels of both IgG (1,090 mg/dl) and IgA (668 mg/dl), normal levels of IgM (70 mg/dl), and undetectable IgE. Monthly IVIG therapy was begun, but the patient subsequently developed recurrent pneumococcal meningitis, multiple pneumonias, cellulitis, and a soft-tissue abscess. At age 6.5 years, isohemagglutinin levels and results of lymphocyte phenotyping were normal. The patient had fine sparse hair, dry skin, decreased sweating, conical teeth, and no evidence of osteopetrosis. The mother of these two patients (II-2) has conical teeth and dry skin, as well as elevated levels of IgA (498 mg/dl). A maternal male cousin (III-3) presented, at age 12 mo, with clinical and laboratory findings similar to those of his affected cousins. CD40 ligand and CD69 expression on stimulated T cells were normal. This cousin's mother (II-4) has conical teeth, some variable hyperpigmented lesions of the skin, and elevated levels of IgA (542 mg/dl).
In family 3, the proband (III-1) was first admitted to the hospital at age 8 mo, with viral meningitis, with subsequent admissions for pneumococcal meningitis. At age 20 mo, his IgA (3.1 g/liter [normal range 0.3–1.3 g/liter]) and IgM (7.94 g/liter [normal range 0.5–2.2 g/liter]) levels were elevated, and both his IgG levels and IgG2 (0.25 g/liter [normal range 0.3–2.9 g/liter]) levels were low. He failed to form antibodies in response to pneumococcal vaccine and was begun on IVIG therapy. Sparse hair, inability to sweat, conical teeth, and absence of 10 primary teeth were noted. Radiographs of his permanent dentition revealed only 15 teeth, some of which were hypoplastic. Despite therapy, he has had chronic lung infections with resultant bronchiectasis and pulmonary insufficiency. There is no other family history of immunodeficiency, but his mother has only 25 permanent teeth and a large patch of skin with neither hair nor sweating response. The proband of family 4 (III-1; see fig. 1) presented, at age 2 d, with Pseudomonas bacteremia and, because of bacterial infections, was hospitalized multiple times during the 1st year of life. A thymic shadow was noted on x-ray, as was an increased bone density consistent with osteopetrosis. Laboratory studies at age 4 mo age demonstrated low levels of both IgG (143 mg/dl [normal range 280–750 mg/dl]) and IgA (8 mg/dl [normal range 6–50 mg/dl]) but high levels of IgM (221-868 mg/dl [normal range 15–70 mg/dl). Normal numbers of B, T, and NK cells were present, and lymphocyte-proliferation studies showed a good response to mitogens. Karyotype, nitroblue tetrazolium testing, and C3, C4, and CH50 levels were normal. He was noted to have reduced sweating, thin sparse scalp hair and eyebrows, and mild frontal bossing (fig. 2d). His dentition was abnormal, and, at age 20 mo, only two widely spaced conical upper incisors had erupted. Dental x-rays revealed only two conically shaped upper incisors, and either absence or extreme hypoplasia of all four lower incisors. The patient was treated with IVIG therapy but developed diffuse mycobacterial granulomatosis and died at age 2 years. The proband's younger maternal half-brother (III-2) presented, at age 3 mo, with bacterial sepsis and meningitis. Immunoglobulin levels were abnormal, with low levels of both IgG (154 mg/dl) and IgA (15 mg/dl) but with elevated levels of IgM (251–888 mg/dl). Lymphocyte proliferation and results of phenotyping were essentially normal, as were CD40 ligand expression, nitroblue tetrazolium testing, and levels of properdin, adenosine deaminase, and nucleoside phosphorylase. The patient had osteopetrosis, sparse scalp hair, and only two conical-shaped maxillary central incisors. Similar to his brother, he had very low amounts of sweat on a quantitative sweat test. He developed pneumocystis carinii pneumonia and disseminated mycobacterium avium intracellulare infection and died at age 33 mo. The mother of these two patients had normal dentition, scalp and body hair, and breast development, and her family history was unremarkable for features of either ectodermal dysplasia or immunodeficiency.
Mutation analyses of IKK-gamma were performed by PCR amplification of genomic DNA from males in each family, by use of previously published primer sets (Smahi et al. 2000) and Taq DNA Polymerase (Qiagen). PCR products were sequenced in both directions by an ABI 373 stretch sequencer (Applied Biosystems). Allele-specific oligonucleotides for each mutant and its corresponding wild-type sequence were hybridized against PCR products of exon 10 amplified from the genomic DNAs of family members and 30 unrelated unaffected females (60 X chromosomes), by previously published methods (Ferguson et al. 1998). RNA was extracted (Ambion) from a lymphoblastoid cell line established from an affected male from family 2. It was reversed transcribed by Moloney murine leukemia virus reverse transcriptase (New England Biolabs) and random hexamers. The DNA product was amplified by use of oligonucleotides F 5′-GCCTATCACCAGCTCTTCCA-3′ and R 5′-CCCGTGTGCATGGTAAGAG-3′, were heminested with R 5′-ACCAGCGGATCAACAGCTGAA-3′, and subsequently were sequenced in both directions.
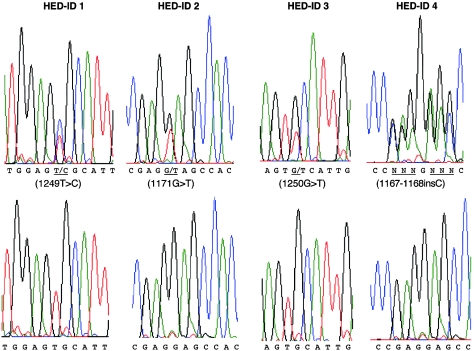
The IKK-gamma sequence from genomic DNA of affected males from all four families revealed single-base-pair variants within exon 10 (fig. 3). Exon 10 is the most 3′ exon of IKK-gamma, and the variants are all within the open reading frame (Jin and Jeang 1999). As has been noted elsewhere (Smahi et al. 2000), males hemizygous for mutations appear to be heterozygous on genomic sequence analysis, because of the presence of a highly conserved, nonexpressed, IKK-gamma pseudogene. The pseudogene contains only a duplication of exons 3–10, and our attempts at preferential amplification of the expressed sequence from genomic DNA were unsuccessful. As expected, sequences derived from cDNA isolated from a lymphoblastoid cell line from a male in family 2 showed only the variant T at nucleotide 1171 (data not shown). Variants cosegregated with the disorder in all multiplex families and occurred de novo in family 3 (fig. 1). Variants were not detected in the 60 unrelated control X chromosomes. Mutations in families 2 and 4 produce a nonsense mutation at codon 391 (E391X) and a frameshift at codon 390, respectively, within the carboxy-terminal end of the 419-amino-acid protein. The frameshift is the result of an insertion of a cytosine (1167–1168insC) within a wild-type run of seven cytosines and introduces novel amino acids at codons 390–393, as well as a premature stop at codon 394. Both mutations truncate the protein and delete a putative zinc-finger domain (amino acids 397–417) (Smahi et al. 2000). A western blot of an extract from a lymphoblastoid cell line derived from family 2 demonstrates that the protein is expressed (data not shown). The variants in families 1 (C417R) and 3 (C417F) are nonconserved missense mutations within the putative zinc-finger domain and alter a common cysteine residue to an arginine and a phenylalanine, respectively.
Figure 3.
Relevant mutant (upper graphs) and wild-type (lower graphs) genomic sequence from each family. Note that affected hemizygous males appear to be heterozygous, because of a highly homologous IKK-gamma pseudogene.
The molecular and clinical findings in these families establish HED-ID as a distinct X-linked recessive disorder. The phenotype and genetics (recessive vs. dominant) of HED-ID differ significantly from those of familial IP. Even though they are allelic disorders, it may be more clinically useful to distinguish the phenotypes and, thus, to refer to affected males as having HED-ID rather than IP. The clinical findings of hypohidrosis and abnormal dentition are similar to those seen in other forms of HED (Clarke et al. 1987; Munoz et al. 1997), although the involvement of the scalp hair is more variable. These findings explain the previous reports that imply that serious immunodeficiency may be a complication of the more common X-linked form of HED (ED1) (Abinun et al. 1996; Schweizer et al. 1999). However, immunodeficiency is absent even in males with no ectodysplasin-A protein, the product of the ED1 locus (Thomas et al. 1993; Monreal et al. 1998). HED-ID is also distinct from other X-linked immunodeficiencies, even though the immunoglobulin levels in families 1 and 4 originally suggested a diagnosis of hyper-IgM syndrome (MIM 308230). However, CD40 ligand expression, measured in families 2 and 4, was normal. Males from all four families presented, during the first 2 years of life, with life-threatening infections, continued to have recurrent infections despite IVIG therapy, and, because of recurrent pulmonary infections and bronchiectasis, have had significant morbidity and mortality. It is noteworthy that a male with features of HED-ID has recently been reported to have also undergone bilateral lung transplantation (Smythe et al. 2000). Although there is variability, in the clinical and laboratory findings, among the four families, all patients had either normal or increased numbers of B cells and had normal T cell numbers and proliferation. Dysgammaglobulinemia with normal to low levels of IgG and with elevated levels of either IgA or IgM was a consistent finding. Delayed or absent production of isohemagglutinins, as well as an inability to form specific antibody to Streptococcus pneumoniae, are other common features. Previous reports in the literature have described a few males with HED-ID who are also characterized by both an impaired ability to respond to polysaccharide-type antigens and either low or absent isohemagglutinins (Abinun et al. 1996; Schweizer et al. 1999). The clinical findings in family 4 were the most severe, with death occurring by age 3 years. The findings in this family, including osteopetrosis, are similar to those in a rare surviving male reported with “IP” and a mutation (X420W) in the IKK-gamma stop codon (Smahi et al. 2000). Heterozygous females had no clinical signs of immunodeficiency, although IgA levels were elevated in two females in family 2. Female carriers had variable manifestations, ranging from normal dentition to minor degrees of hypodontia and conical teeth. Two females had areas of spotty hyperpigmentation of the skin, but none reported earlier inflammatory vesicles of the skin.
It is apparent from these data that different mutations within IKK-gamma can produce markedly different phenotypes in hemizygous males and heterozygous females. Mutations that produce a complete loss of IKK-gamma function result in a phenotype of IP, an X-linked dominant trait with affected males dying in utero, and heterozygous females having variable defects of skin, hair, teeth, brain, and eye (Landy and Donnai 1993). A common genomic rearrangement, deletion of exons 4–10, accounts for 80% of new mutations in classical IP (Smahi et al. 2000). Heterozygous females display nonrandom X inactivation and thus have surviving T and B cells expressing only the wild-type IKK-gamma allele (Parrish et al. 1996). Therefore, immunodeficiency is not part of their phenotype. Murine knockout experiments confirm that complete loss of IKK-gamma function is embryonic lethal in hemizygous males and produces a phenotype in heterozygous females that is similar to IP (Makris et al. 2000; Rudolph et al. 2000; Schmidt-Supprian et al. 2000). Evidence that IKK-gamma is essential for T and B cell viability can be seen in heterozygous knockout females (Ikk-gamma +/−) of age 9 d, who have an extremely small thymus with destruction of cortical lymphocytes as the result of increased apoptosis (Makris et al. 2000). Furthermore, chimeric mice made from IKK-gamma–deficient embryonic stem cells (−/−) have no detectable embryonic stem cell–derived (−/−) B or T cells (Schmidt-Supprian et al. 2000). In contrast with classical IP, mutations within exon 10 of IKK-gamma produce an X-linked recessive disorder affecting only males. All four mutations within exon 10 either delete or alter a putative zinc-finger domain (amino acids 397–417) at the C-terminal end of the protein, and deletion of this domain is reported to result in a loss of function of NF-κB activation (Smahi et al. 2000). The C-terminal region of the IKK-gamma protein is believed to connect the IKK complex to its upstream activators (Rothwarf et al. 1998). Deletion of the carboxy-terminal end of IKK-gamma has been shown to markedly diminish, but not completely eliminate, stimulation of NF-κB by Tax, the product of the type 1 human leukemia virus (Harhaj and Sun 1999; Xiao et al. 2000). The mutations seen in HED-ID must preserve partial IKK-gamma function, since hemizygous males survive and have normal numbers of T and B cells, albeit with dysgammaglobulinemia and a defective ability to produce some specific antibodies. Additional undefined immunological defects likely exist, in addition to the dysgammaglobulinemia, since IVIG therapy is not protective against recurrent infections by S. pneumoniae. It remains to be determined whether the immunological defects are due solely to IKK-gamma’s role in regulation of the NF-κB pathway or whether other signaling pathways are involved. Defects in immunoglobulin isotype switching are seen in humans and mice deficient in CD40-mediated signaling. This signaling pathway is dependent on the activation of multiple transcription factors, including NF-κB (Pype et al. 2000). NF-κB is a heterodimeric transcription factor composed of various combinations of structurally related proteins, including p50, p65, and p52 (Kaufman and Fuchs 2000). In mice, separate targeted disruptions of p50 and p65 produce defects in lymphocyte development and function (Sha et al. 1995; Doi et al. 1997). Furthermore, a double p50/p52 knockout in mice blocks both B-cell and osteoclast maturation, the latter causing osteopetrosis (Franzoso et al. 1997). Targeted disruption of the receptor activator of NF-κB, a member of the TNFR superfamily, also causes osteopetrosis and B-cell defects in mice (Dougall et al. 1999). Finally, these data provide further evidence that the development of teeth and eccrine sweat glands is mediated by the IKK protein complex. The ectodermal defects in HED-ID are very similar to those seen in the X-linked and autosomal forms of HED and suggest that the protein products of these loci, members of the TNF/TNFR superfamily, may regulate upstream activators of the IKK signalsome complex.
Acknowledgments
This work was supported by National Institute of Dental and Craniofacial Research grant DE11311 (to J.Z.), a National Foundation for Ectodermal Dysplasias grant (to J.Z.), and National Institutes of Health grant M01 RR01271 (to the University of California, San Francisco, Pediatric Clinical Research Center). We would like to thank the families, as well as the physicians and dentists who assisted in gathering clinical information and samples. Specific thanks are due to Vicki Cox, and Barbara Pober, for their assistance in referring and evaluating the families.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for ED1 [MIM 305100], ED3 [MIM 129490], autosomal recessive HED [MIM 224900], IP2 [MIM 308310], and hyper-IgM syndrome [MIM 308230])
References
- Abinun M, Spickett G, Appleton AL, Flood T, Cant AJ (1996) Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. Eur J Pediatr 155:146–147 [DOI] [PubMed] [Google Scholar]
- Clarke A, Phillips DI, Brown R, Harper PS (1987) Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child 62:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley ME (1994) X-linked immunodeficiencies. Curr Opin Genet Dev 4:401–406 [DOI] [PubMed] [Google Scholar]
- Doi TS, Takahashi T, Taguchi O, Azuma T, Obata Y (1997) NF-kappa B RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J Exp Med 185:953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer S, Bayes M, Elomaa O, Schlessinger D, Kere J (1999) Ectodysplasin is a collagenous trimeric type II membrane protein with a tumor necrosis factor-like domain and co-localizes with cytoskeletal structures at lateral and apical surfaces of cells. Hum Mol Genet 8:2079–2086 [DOI] [PubMed] [Google Scholar]
- Ferguson BM, Thomas NS, Munoz F, Morgan D, Clarke A, Zonana J (1998) Scarcity of mutations detected in families with X linked hypohidrotic ectodermal dysplasia: diagnostic implications. J Med Genet 35:112–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev 11:3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-Maia N, Pinheiro M (1988) Ectodermal dysplasias—some recollections and a classification. Birth Defects 24:3–14 [PubMed] [Google Scholar]
- Harhaj EW, Sun SC (1999) IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem 274:22911–22914 [DOI] [PubMed] [Google Scholar]
- Headon DJ, Overbeek PA (1999) Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat Genet 22:370–374 [DOI] [PubMed] [Google Scholar]
- Israel A (2000) The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol 10:129–133 [DOI] [PubMed] [Google Scholar]
- Jin DY, Jeang KT (1999) Isolation of full-length cDNA and chromosomal localization of human NF-kappaB modulator NEMO to Xq28. J Biomed Sci 6:115–120 [DOI] [PubMed] [Google Scholar]
- Kaufman CK, Fuchs E (2000) It's got you covered: NF-kappaB in the epidermis. J Cell Biol 149:999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy SJ, Donnai D (1993) Incontinentia pigmenti (Bloch-Sulzberger syndrome). J Med Genet 30:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M (2000) Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell 5:969–979 [DOI] [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J (1999) Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet 22:366–369 [DOI] [PubMed] [Google Scholar]
- Monreal AW, Zonana J, Ferguson B (1998) Identification of a new splice form of the EDA1 gene permits detection of nearly all X-linked hypohidrotic ectodermal dysplasia mutations. Am J Hum Genet 63:380–389 (erratum: Am J Hum Genet 63:1253–1255 [1998]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz F, Lestringant G, Sybert V, Frydman M, Alswaini A, Frossard PM, Jorgenson R, Zonana J (1997) Definitive evidence for an autosomal recessive form of hypohidrotic ectodermal dysplasia clinically indistinguishable from the more common X-linked disorder. Am J Hum Genet 61:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JE, Scheuerle AE, Lewis RA, Levy ML, Nelson DL (1996) Selection against mutant alleles in blood leukocytes is a consistent feature in incontinentia pigmenti type 2. Hum Mol Genet 5:1777–1783 [DOI] [PubMed] [Google Scholar]
- Puck JM (1994) Molecular and genetic basis of X-linked immunodeficiency disorders. J Clin Immunol 14:81–89 [DOI] [PubMed] [Google Scholar]
- Pype S, Declercq W, Ibrahimi A, Michiels C, Van Rietschoten JG, Dewulf N, de Boer M, Vandenabeele P, Huylebroeck D, Remacle JE (2000) TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem 275:18586–18593 [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Zandi E, Natoli G, Karin M (1998) IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297–300 [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW (2000) Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev 14:854–862 [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Israel A, Rajewsky K, Pasparakis M (2000) NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell 5:981–992 [DOI] [PubMed] [Google Scholar]
- Schweizer P, Kalhoff H, Horneff G, Wahn V, Diekmann L (1999) Polysaccharide specific humoral immunodeficiency in ectodermal dysplasia: case report of a boy with two affected brothers (in German). Klin Pädiatr 211:459–461 [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D (1995) Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80:321–330 [DOI] [PubMed] [Google Scholar]
- Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, Israel A, et al (2000) Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti: The International Incontinentia Pigmenti (IP) Consortium. Nature 405:466–472 [DOI] [PubMed] [Google Scholar]
- Smart BA, Ochs HD (1997) The molecular basis and treatment of primary immunodeficiency disorders. Curr Opin Pediatr 9:570–576 [DOI] [PubMed] [Google Scholar]
- Smythe WR, Bridges ND, Gaynor JW, Nicolson S, Clark BJ, Spray TL (2000) Bilateral sequential lung transplant for ectodermal dysplasia. Ann Thorac Surg 70:654–656 [DOI] [PubMed] [Google Scholar]
- Thomas NST, Chelly J, Zonana J, Davies KJP, Morgan S, Gault J, Rack KA, Buckle VJ, Brockdorff N, Clarke A, Monaco A (1993) Characterisation of molecular DNA rearrangements within the Xq12-q13.1 region, in three patients with X-linked hypohidrotic ectodermal dysplasia (EDA). Hum Mol Genet 2:1679–1685 [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC (2000) Domain-specific interaction with IKKgamma is an essential step in tax-mediated activation of IKK. J Biol Chem 275 (electronically published; print version pending) [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Israel A (1998) Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231–1240 [DOI] [PubMed] [Google Scholar]