Abstract
Steroidogenic factor 1 (NR5A1/SF-1) plays an essential role in the development of the hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes, controlling expression of their many important genes. The recent description of a 46,XY patient bearing a mutation in the NR5A1 gene, causing male pseudohermaphroditism and adrenal failure, demonstrated the crucial role of SF-1 in male gonadal differentiation. The role of SF-1 in human ovarian development was, until now, unknown. We describe a phenotypically and genotypically normal girl, with signs and symptoms of adrenal insufficiency and no apparent defect in ovarian maturation, bearing a heterozygote G→T transversion in exon 4 of the NR5A1 gene that leads to the missense R255L in the SF-1 protein. The exchange does not interfere with protein translation and stability. Consistent with the clinical picture, R255L is transcriptionally inactive and has no dominant-negative activity. The inability of the mutant (MUT) NR5A1/SF-1 to bind canonical DNA sequences might offer a possible explanation for the failure of the mutant protein to transactivate target genes. This is the first report of a mutation in the NR5A1 gene in a genotypically female patient, and it suggests that NR5A1/SF-1 is not necessary for female gonadal development, confirming the crucial role of NR5A1/SF-1 in adrenal gland formation in both sexes.
Steroidogenic factor 1 (SF-1), an orphan nuclear receptor alternatively designated “adrenal 4-binding protein,” now known as “nuclear receptor subfamily 5 group A member 1” (NR5A1 [MIM 184757]), was initially described as an important regulator of the tissue-specific expression of the cytochrome P450 steroid hydroxylases (Lala et al. 1992), 3-@β-hydroxysteroid dehydrogenase deficiency (MIM 201810), and steroidogenic acute response protein (STAR [MIM 600617]). Analyses of Ftz-F1 knockout mice showed that Sf-1 is essential for normal development of the adrenals and gonads (Luo et al. 1994). Since its characterization, several lines of research have demonstrated that SF-1 regulates the expression of both classes of hormones essential for male phenotypic differentiation—androgens and anti-Müllerian hormone (AMH [MIM 600957]). Apart from the adrenal gland and hypothalamus, Sf-1 is expressed in both male and female mouse embryos from very early stages of gonadogenesis, when the mesoderm condenses to form the urogenital ridge. With the onset of testicular differentiation, the levels of Ftz-F1 transcripts increase in both functional compartments of the testes—the interstitial region (androgen-producing Leydig cells) and the testicular cords (AMH-producing Sertoli cells). In contrast, Ftz-F1 transcripts in ovaries decrease in coincidence with sexual differentiation, suggesting that the persistent expression of Sf-1 may inhibit female sexual differentiation.
To examine the role of the gene in intact mice, Luo et al. (1994) used targeted disruption of the Ftz-F1 gene. Despite normal survival in utero, all Ftz-F1 null animals died by postnatal day 8; these animals lacked adrenal glands and gonads and were severely deficient in corticosterone, supporting adrenocortical insufficiency as the probable cause of death. Male and female Ftz-F1 null mice had female internal genitalia, despite complete gonadal agenesis. These studies established that the Ftz-F1 gene is essential for sexual differentiation and for formation of the primary steroidogenic tissues.
In humans, the importance of NR5A1/SF-1 in male gonadal differentiation has been confirmed by the description of a 46,XY patient affected by adrenal insufficiency and male pseudohermaphroditism (Achermann et al. 1999). Since no mutation was described in 46,XX patients, the role of NR5A1/SF-1 in human ovarian development was, until now, not known. In this report, we describe the first example of a female patient deficient in NR5A1/SF-1.
A phenotypically normal girl presented at age 14 mo with adrenal insufficiency and seizures after having had otitis and tonsillitis 1 wk earlier. The diagnosis of adrenocortical insufficiency was based on the clinical picture of severe slackness, muscular hypotonia, and the typical laboratory results of decreased sodium (104 mmol/liter; normal range 135–145), increased potassium (8.0 mmol/liter; normal range 3.5–4.8), elevated ACTH (2200 pg/ml; normal range 10–50 pg/ml), and inadequately low cortisol in plasma (165 nmol/liter) and urine (free urinary cortisol, 17.6 μg/m2/24 h; normal values 20–100 μg/m2/24 h) cortisol. All urinary steroid hormone metabolites were undetectable, excluding congenital adrenal hyperplasia caused by an enzyme defect of the steroid hormone pathway. Because cholesterol was absent in the urine, a diagnosis of STAR deficiency was unlikely. The personal history of the first year of life of the girl was uneventful, as was the family history with respect to adrenal insufficiency.
Under treatment with hydrocortisone (7.5 mg/d) and fludrocortisone (0.1 mg/d), the clinical signs normalized within 2 d. At age 27 mo, growth and psychomotoric development were completely normal. Additional evaluation showed a normal female chromosomal pattern (46,XX) and normal LH and FSH serum levels (e.g., 0.5 and 2.8 U/liter, respectively) at ages 14 and 27 mo. Pelvic ultrasonography, besides failing to reveal the adrenals, showed a normal infantile uterus (2.6 cm) and infantile ovaries of normal size and structure (maximal diameter 1.2 cm). Magnetic resonance imaging (MRI) also confirmed the presence of ovaries normal in location. To further characterize the function of the ovaries, we performed inhibin A and B assays (Oxford Bio-innovation). The inhibin levels were low but normal for age (inhibin A: 3,1 pg/ml; inhibin B: 4.5 pg/ml) (Sehested et al. 2000) Therefore, there is no evidence of any abnormality of the reproductive system. Direct sequencing of PCR fragments amplified from genomic DNA of the patient revealed the presence of a heterozygote G→T transversion in exon 4 of the NR5A1 gene, leading to the missense R255L in the hinge region of NR5A1/SF-1 protein (fig. 1a–c). To search for evidence of mosaicism, we obtained genomic DNA from hair roots (Dracopoli et al. 2000).
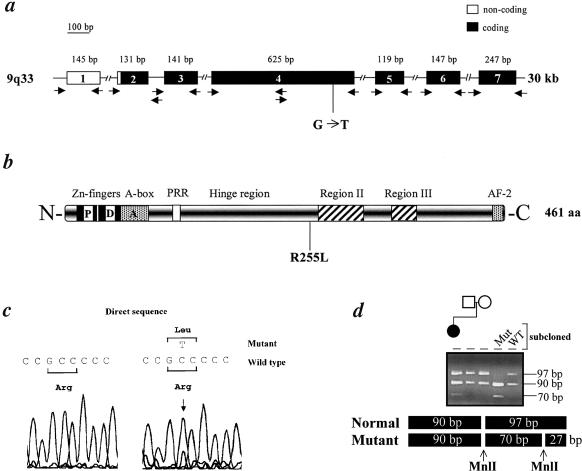
Figure 1.
NR5A1 gene (a) and protein (b). Blackened boxes represent the coding exons; unblackened boxes, the noncoding exons. The sizes (in base pairs) of the PCR products are also indicated. The oligonucleotides used for genomic DNA amplification are depicted by arrows. The position of the transversion in exon 4 and the correspondent R255L missense mutation in the hinge region of the NR5A1/SF-1 protein are sketched. P box = proximal box; D box = distal box; A box = FTZ-F1 box; PRR = proline-rich region; regions II and III = domains conserved among NR5A1/SF-1 proteins with unclear function; AF-2 = activation function domain. c, DNA sequences. Chromatograms obtained by direct sequencing of PCR products showing the presence of the heterozygote G→T substitution in exon 4, not present in healthy individuals (WT, representative example from 30, 60 alleles). d, Restriction endonuclease analysis of a 626-bp exon 4 PCR product. The rearrangement introduces a novel MnlI (CCTC[N]7) restriction site that generates two additional bands of 27 and 70 bp in the patient, leaving another 90-bp band intact. The presence of the transversion in the heterozygote state was confirmed by restriction analysis in cloned and amplified DNA.
The results of mutational analysis of this DNA were identical to those in leukocytes. Although we cannot exclude that another rearrangement is present in a different region of the gene, no mutation was found in the other exons, in the intron-exon boundaries, or in 500 bp upstream of the start site of transcription. Although not located in one of the conserved regions, arginine 255 is conserved in the human (National Center for Biotechnology Information accession number NP004950), bovine (National Center for Biotechnology Information accession number AAB23574), rat (National Center for Biotechnology Information accession number BAA07722]), and mouse (National Center for Biotechnology Information accession number S65876) NR5A1/SF-1 proteins. The rearrangement introduces a novel MnlI restriction site that is absent in the parents of the patient (fig. 1d) and in 30 normal controls (60 alleles, not shown) and, therefore, represents a de novo phenomenon. Since de novo mutations (germline or somatic) represent a rare event, we had to confirm parentage via amplification of microsatellites (D9S1798, D9S1821, and D9S159 [Généthon] [Dib et al. 1996], not shown). The absence of pathognomonic cholesterol metabolites in urine and the lack of rearrangements in STAR cDNA (performed as described in the study by Biason-Lauber et al. [2000]; National Center for Biotechnology Information accession number NM000349) allowed us to exclude lipoid adrenal hyperplasia (not shown).
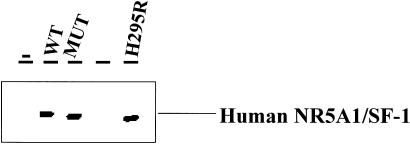
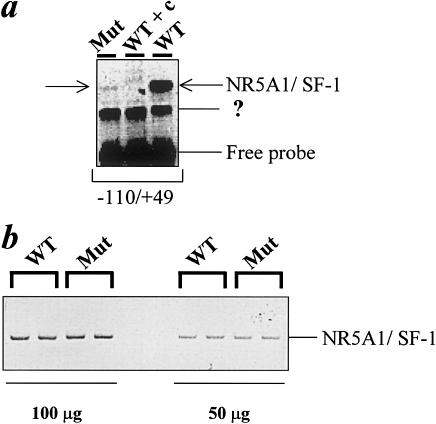
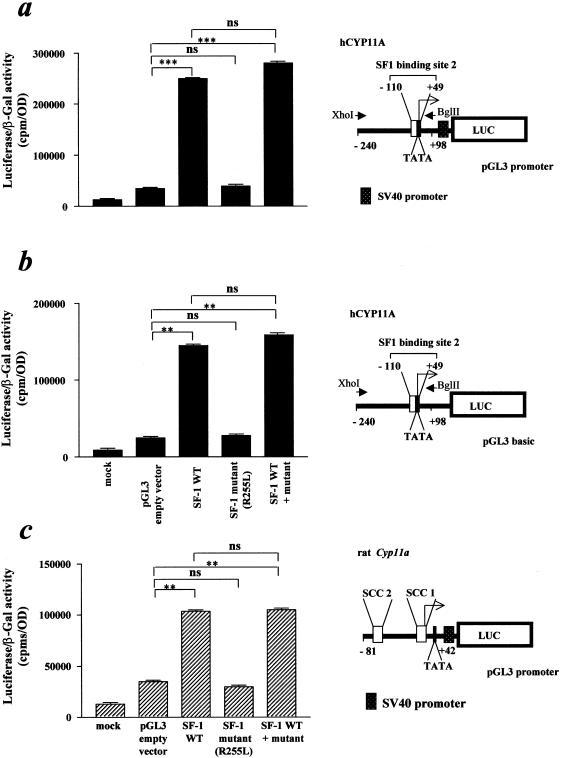
The mutation in the NR5A1 gene of our patient was recreated by site-directed mutagenesis and was used for functional studies in 293 human embryonic kidney cells. This rearrangement does not interfere with protein translation and stability, as demonstrated by the western blot analysis (fig. 2). The MUT NR5A1/SF-1 does not bind properly to the known responsive element of the human CYP11A promoter (−110/+49 [Monté et al. 1998]; fig. 3) (MIM 118485 [National Center for Biotechnology Information accession number M604212]). Consistent with the clinical picture, the R255L NR5A1/SF-1 mutant does not transactivate two known responsive sequences (human CYP11A promoter, binding site 1: −1679 to −1620 not shown; binding site 2: −110 to +49; fig. 4a and b) cloned upstream from a luciferase reporter gene. Similar results were obtained by means of another target promoter—that is, the rat Cyp11a promoter (National Center for Biotechnology Information accession number J005511), −81/+42 [Clemens et al. 1994]); (fig. 4c). The MUT NR5A1 does not show any dominant-negative activity when transiently coexpressed with wild-type (WT) protein (fig. 4). To establish whether the MUT NR5A1/SF-1 has the same differential activity in adrenal and ovarian cells in vitro as appears to be the case in vivo in our patient, we performed similar functional studies in human adrenal adenocarcinoma cells NCI-H295R and ovarian NIH-OvCar3 cells. Transfection of different amounts (1, 3, and 10 mg) of empty vector—WT and MUT NR5A1/SF-1 or a combination of the two—did not overcome the endogenous expression of NR5A1, preventing us from finding differences in transactivation of the reporter (not shown).
Figure 2.
Expression of WT and MUT NR5A1/SF-1. Western blot analysis was performed on protein extract of 293 cells transiently transfected with an empty pCMV4 vector (−), the expression vector containing the WT NR5A1/SF-1 cDNA or the MUT NR5A1/SF-1 R255L cDNA, using lipofectamine reagent (Life Technologies). As positive control, protein extract from NCI-H295R human adrenal carcinoma cells was used (H295R). The antihuman NR5A1/SF-1 antibodies were used at 1:250 dilution and were purchased from Upstate Biotechnology. The R255L rearrangement does not interfere with protein translation or stability.
Figure 3.
a, DNA-binding capacity of human MUT NR5A1/SF-1. Nuclear extracts prepared from 293 cells transfected with WT in the absence or presence of a 10-fold excess cold competitor (WT + c) or MUT NR5A1/SF-1 underwent electrophoretic mobility-shift assay using phospholabeled probes corresponding to one of the two sites of the human CYP11A promoter (−110 to +49). Similar results were obtained by use of the other binding site (−1679/−1620) (not shown). A non-NR5A1/SF-1 protein binding to the −110/+49 sequence is also present in the reaction (?). b, Western blot analysis demonstrating that the differences in binding capacity are not due to variations in expression between WT and MUT NR5A1/SF-1. The protein extract duplicates were loaded in two different amounts (100 and 50 mg).
Figure 4.
Transcriptional activity of MUT NR5A1/SF-1. A luciferase reporter construct (10 mg) containing two human CYP11A promoter regions (a and b, blackened bars; binding site 2: −110 to +49; with or without SV40 promoter) and part of the rat Cyp11a promoter region (c, hatched bars; −81/+42) was transfected into 293 cells with WT, MUT NR5A1/SF-1, or a combination of the two (molar ratio 1:1). Mock transfected 293 cells serve as negative control, whereas the luciferase-containing empty vector (pGL3; Promega) represents background luciferase activity. The results were subjected to statistical analysis (t-test, paired). *** = P<.0001; ** = P<.001; ns = not significant. The data represent the mean ± SD of three independent experiments.
The recent description of a 46,XY patient bearing a mutation in the NR5A1 gene causing male pseudohermaphroditism and adrenal failure demonstrated the crucial role of SF-1 in male gonadal differentiation (Achermann et al. 1999). The MUT R255L NR5A1/SF-1 is unable to properly bind DNA, thus explaining the loss of transactivation potential of the R255L protein. The reason for this impaired DNA binding is at present unclear, since the mutation is not located in the classic Zn-fingers or in the A domain, and further experiments are necessary to clarify this point. The transcriptionally inactive MUT NR5A1/SF-1 has no dominant-negative activity when transiently coexpressed with WT protein. It is possible that the mutant protein fails to interact with other proteins involved in adrenal development, such as, for example, DAX-1 (MIM 300200).
There are several lines of evidence for the interaction of DAX-1 and NR5A1/SF-1 (for review, see Parker et al. 1999). The consequences of this putative interaction appear to be tissue dependent. In the adrenal cortex, NR5A1/SF-1 and DAX-1 may cooperate to activate the expression of target genes necessary for tissue-specific development and functions. The dependence of adrenal development and function on NR5A1/SF-1 is confirmed by the congenital adrenal insufficiency in our patient. In contrast, the action of NR5A1/SF-1 (and DAX-1) in gonadal cells seems to be antithetic. NR5A1/SF-1 is required for testes development and male sexual differentiation, as demonstrated by the Ftz-F1 knockout mouse model and the recent description of a 46,XY phenotypically female NR5A1/SF-1–deficient patient (Achermann et al. 1999). On the other hand, the expression of NR5A1/SF-1 in the ovaries is maintained up to the 15th wk of gestation (Hanley et al. 1999) and declines afterwards, at the beginning of embryonic female sexual differentiation. These findings suggested that NR5A1/SF-1 is essential for normal male gonadal differentiation but may not be crucial for, or even impair, ovarian development. This is consistent with the clinical presentation of our 46,XX NR5A1/SF-1 mutant patient who, although still prepubertal, showed no morphological or functional sign of ovarian failure, such as elevated gonadotropins, which occur, for example, in the case of prepubertal girls with Turner syndrome. It must be mentioned, however, that the NR5A1/SF-1 mutation might affect GnRH and/or FSH-LH release and, therefore, produce the same effect. It remains to be established whether NR5A1/SF-1 is necessary for the normal pubertal maturation of the ovaries. The haploinsufficiency of the two human patients deficient in NR5A1/SF-1 (Achermann et al. 1999 and the present report), in contrast with the Ftz-F1 knockout mouse model, could be due to the fact that the normal allele is not expressed at all. This is difficult to demonstrate, because, for ethical reasons, it is not possible to obtain NR5A1/SF-1–expressing tissues from this patient. For the same reasons, we cannot fully characterize a putative genetic mosaicism and the stage of embryonic development in which the de novo mutation arose. The presence of the mutation in the heterozygote state in tissues of different embryonic origin (leukocytes and hair roots) might suggest that the de novo event occurred before the germ layers separate at gastrulation, implying an ovarian involvement. On the other hand, it is appealing to hypothesize that NR5A1 represents a further example of a dosage-sensitive mechanism of sex differentiation in human males, similar to those already shown for DAX-1, WT1, and SOX9. Perhaps more significantly, our results demonstrate that NR5A1, analogous to DAX-1, is not an ovary-determining gene in humans; the results also indicate the necessity of further studies in the search for genes responsible for ovarian determination and differentiation. Our findings provide insights into the role of NR5A1/SF-1 human ovarian development and suggest that NR5A1/SF-1 mutations might be the molecular basis of non–X-linked congenital adrenal insufficiency.
Acknowledgments
The authors are grateful to Dr. M. E. Lauber, Professor M. Zachmann, Dr. T. Torresani, Dr. B. Schäfer, and Dr. B. Thöny, for helpful discussion of the work; and to Professor C. W. Heizmann, for his continuous support. The present work was supported by The Swiss National Science Foundation grant no. 32-052724.97.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Généthon, http://www.genethon.fr
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for NR5A1 [MIM 184757], 3-@β-hydroxysteroid dehydrogenase deficiency [MIM 201810], STAR [600617], AMH [MIM 600957], and DAX-1 [MIM 300200])
References
- Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL (1999) A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- Biason-Lauber A, Zachmann M, Schoenle EJ (2000) Effect of leptin on CYP17 enzymatic activities in human adrenal cells: new insight in the onset of adrenarche. Endocrinology 141:1446–1454 [DOI] [PubMed] [Google Scholar]
- Clemens JW, Lala DS, Parker KL, Richards JS (1994) Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in the rat granulosa cells. Endocrinology 134:1499–1508 [DOI] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Dracopoli NC, Haines JL, Korf BR, Morton CC, Seidman CE, Seidman JG, Smith DR (eds) (2000) Current protocols in human genetics. Vol 3. John Wiley & Sons, New York [Google Scholar]
- Hanley NA, Ball SG, Clement-Jones M, Hagan DM, Strachan T, Linsday S, Robson S, Ostrer H, Parker Kl, Wilson DI (1999) Expression of steroidogenic factor 1 and Wilms’ tumour 1 during early human gonadal development and sex determination. Mech Dev 87:175–180 [DOI] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL (1992) Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homologue of fushi tarazu-factor I. Mol Endocrinol 6:1249–1258 [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Monté D, DeWitte F, Hum DW (1998) Regulation of the human P450scc gene by steroidogenic factor 1 is mediated by CBP/p300. J Biol Chem 273:4585–4591 [DOI] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP, Schedl A (1999) Genes essential for early events in gonadal development. Cell Mol Life Sci 55:831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehested A, Juul A, Andersson AM, Holm Petersen J, Kold Jensen T, Müller J, Skakkebaek NE (2000) Serum inhibin A and inhibin B in healthy prepubertal, pubertal, and adolescent girls and adult women: relation to age, stage of puberty, menstrual cycle, follicle-stimulating hormone, luteinizing hormone, and estradiol levels. J Clin Endocrinol Metab 85:1634–1640 [DOI] [PubMed] [Google Scholar]