Abstract
Primary microcephaly is thought to result from genetic defects of the developmental program that generates large brain hemispheres in humans. Autosomal recessive inheritance is likely in most familial cases, and four loci were recently mapped by homozygosity. We report homozygosity mapping of a new locus, MCPH5, with a maximum multipoint LOD score of 3.51 at marker D1S1723, in a family of Turkish origin. The minimal critical region spans 11.4 cM between markers D1S384 and D1S2655, at 1q25-q32, and encompasses the cytogenetic breakpoints of chromosomal aberrations previously reported in unrelated patients with microcephaly.
Microcephaly is a condition in which the head circumference is less than three standard deviations (SDs) below the age-related mean. Primary microcephaly (MIM 251200) is diagnosed after exclusion of (1) craniosynostosis, (2) microcephaly occurring as part of a malformation syndrome (e.g., Down syndrome), and (3) known causes of secondary microcephaly (e.g., perinatal hypoxia) (Ross and Frias 1977). Mental retardation ranges from moderate to severe in primary microcephaly, but other neurological deficits are typically absent. It is a rare disorder, with an incidence of 1/30,000–1/250,000 (Van den Bosch 1959, and references therein). Inheritance in most familial cases has been consistent with an autosomal recessive trait, and parental consanguinity has often been reported (Cowie 1960).
Four loci were recently mapped by homozygosity (Lander and Botstein 1987) in several families with primary microcephaly: MCPH1, at 8p (Jackson et al. 1998); MCPH2, at 19q (Roberts et al. 1999); MCPH3, at 9q (Moynihan et al. 2000); and MCPH4, at 15q (Jamieson et al. 1999). We report the homozygosity mapping of a new locus for primary microcephaly, MCPH5, to an 11.4-cM region of chromosome 1q25-32, in a family not described elsewhere.
The proband in the present study was the third child from a sibship of eight, whose parents, both healthy and normocephalic, were born in two small Turkish villages 10 km apart. The parents were probably consanguineous, although not closer than second cousins. Microcephaly was noted at birth in the four affected siblings. The pregnancies and deliveries had been uneventful. No associated malformations were present. Motor development was within normal limits, and the medical histories were unremarkable. In childhood and adolescence, the head circumferences were 6–8 SDs below the means for age and sex. There were no episodes of seizures. Results of neurological examinations were normal except for moderate to severe mental retardation, with cognitive skills affected more severely than social skills. A standard lymphocyte karyotype (400 bands) and extensive metabolic work-ups were normal in the proband, and the levels of maternal blood glucose and phenylalanine were strictly normal. Brain imaging was not performed. The proband and eldest brother are shown in figure 1. The latter is now married to a distantly consanguineous, normocephalic spouse and is the father of two healthy boys.
Figure 1.
Facial features of the patients. The proband is shown at birth (upper left) and at age 21 years (upper right), and the eldest brother is shown at age 27 years (lower left and lower right).
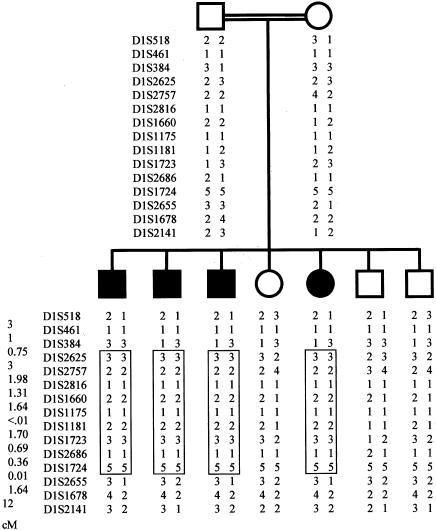
Polymorphic markers of candidate loci MCPH1, MCPH2, MCPH3, and MCPH4 gave no results in favor of linkage in this family. We then started a genomewide screen using 358 microsatellite markers from the Cooperative Human Linkage Center human screening set, Weber version 9 (Research Genetics). A DNA pooling approach was used in an initial screen, and only one microsatellite marker, D1S1660, was found homozygous in the DNA pool from affected siblings and heterozygous in the DNA pool from unaffected siblings and parents. Individual DNA analyses with additional, closely spaced markers (<2 cM) in the region identified a genomic segment in which all informative markers were homozygous in the affected siblings but heterozygous in the parents, indicating identity by descent and homozygosity for a common haplotype (fig. 2). Marker order was obtained from the Cooperative Human Linkage Center map, GeneMap'99, and the Center for Medical Genetics (Marshfield) map. Allele frequencies for each polymorphic marker in the candidate region were evaluated from genotyping of 30 unrelated individuals from the same ethnic population. Multipoint linkage analysis with the MAPMAKER/HOMOZ algorithm software (Kruglyak et al. 1995) assuming a fully penetrant disease with an allele frequency of .002, the parents being second cousins with two common ancestors, provided a maximum LOD score of 3.51 at marker D1S1723. According to the parents, consanguinity might be a few meioses more distant than second cousins, which would not alter the significance of the LOD score (e.g., 3.70 for third cousins). Heterozygosity in affected siblings at D1S384 and D1S2655 indicated a minimal critical region of 11.4 cM (fig. 2). This locus was named “MCPH5” by the Human Gene Nomenclature Database.
Figure 2.
Haplotypes in the kindred with microcephaly. The region of homozygosity is boxed. Distances between markers are shown in centimorgans, lower left. The distance between D1S384 and D1S2655 is 11.4 cM.
The development of large brain hemispheres is an almost human-specific process for which current animal models are poorly suited. Primary microcephaly is thought to result from genetic defects of this developmental program, which requires a balance of neuronal proliferation, migration, and apoptosis during brain development (Vaccarino et al. 1999). Our results represent strong evidence for the presence of a new gene implicated in primary microcephaly, MCPH5, at 1q25-q32. Interestingly, two unrelated patients have been reported with nonsyndromic microcephaly and a chromosomal abnormality in the 1q25-32 region—one with a 1q25-32 deletion (M. A. Ferguson-Smith, personal communication, July 9, 1981 [as cited in OMIM, MIM 251200]) and one with a balanced translocation involving a 1q31-q32.1 breakpoint (Bourrouillou et al. 1978; Perez-Castillo et al. 1984). The relevance of this region is further supported by a statistically significant association between deletions of bands 1q21-25 and 1q32 and microcephaly in patients with multiple congenital malformations (Brewer et al. 1998).
Acknowledgments
This work was supported by a grant from the Fonds A & J Forton/Fondation Roi Baudouin, Belgium. We thank C. Hubert for technical assistance in genotyping, C. Govaerts and J. Richelle for informatics, P. Vanderhaeghen for useful discussion, and G. Vassart for constant support and advice.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/ (for order and distances of markers on chromosome 15)
- Cooperative Human Linkage Center, http://lpg.nci.nih.gov/CHLC/ (for microsatellite markers used for the genome screen)
- GeneMap'99, http://www.ncbi.nlm.nih.gov/genemap99/ (for order and distances of markers on chromosome 1)
- Human Gene Nomenclature Database, HUGO Gene Nomenclature Committee, http://www.gene.ucl.ac.uk/nomenclature/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for microcephaly [MIM 251200])
References
- Bourrouillou G, Colombies P, Blanc P (1978) Trisomie 1q secondaire à une translocation réciproque maternelle. CR Soc Biol (Paris) 172:359–362 [PubMed] [Google Scholar]
- Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick D (1998) A chromosomal deletion map of human malformations. Am J Hum Genet 63:1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie V (1960) The genetics and sub-classification of microcephaly. J Ment Defic Res 4:42–47 [DOI] [PubMed] [Google Scholar]
- Jackson AP, McHale DP, Campbell DA, Jafri H, Rashid Y, Mannan J, Karbani G, Corry P, Levene MI, Mueller RF, Markham AF, Lench NJ, Woods CG (1998) Primary autosomal recessive microcephaly (MCPH1) maps to chromosome 8p22-pter. Am J Hum Genet 63:541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CR, Govaerts C, Abramowicz MJ (1999) Primary autosomal recessive microcephaly: homozygosity mapping of MCPH4 to chromosome 15. Am J Hum Genet 65:1465–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Lander ES (1995) Rapid multipoint linkage analysis of recessive traits in nuclear families, including homozygosity mapping. Am J Hum Genet 56:519–527 [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed] [Google Scholar]
- Moynihan L, Jackson AP, Roberts E, Karbani G, Lewis I, Corry P, Turner G, Mueller RF, Lench NJ, Woods CG (2000) A third novel locus for primary autosomal recessive microcephaly maps to chromosome 9q34. Am J Hum Genet 66:724–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Castillo A, Martin-Lucas MA, Abrisqueta JA (1984) Is a gene for microcephaly located on chromosome 1? Hum Genet 67:230–232 [DOI] [PubMed] [Google Scholar]
- Roberts E, Jackson AP, Carradice AC, Deeble VJ, Mannan J, Rashid Y, Jafri H, McHale DP, Markham AF, Lench NJ, Woods CG (1999) The second locus for autosomal recessive primary microcephaly (MCPH2) maps to chromosome 19q13.1-13.2. Eur J Hum Genet 7:815–820 [DOI] [PubMed] [Google Scholar]
- Ross JJ, Frias JL (1977) Microcephaly. In: Vinken PJ, Bruyn GW (eds) Congenital malformations of the brain and skull. Vol. 30, Handbook of clinical neurology, Elsevier Holland Biomedical Press, Amsterdam, pp 507–524 [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin D, Wyland JJ, Hung YTE (1999) Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci 2:246–253 [DOI] [PubMed] [Google Scholar]
- Van den Bosch J (1959) Microcephaly in the Netherlands: a clinical and genetical study. Ann Hum Genet 23:91–116 [DOI] [PubMed] [Google Scholar]