Abstract
Neonatal diabetes, which can be transient or permanent, is defined as hyperglycemia that presents within the first month of life and requires insulin therapy. Transient neonatal diabetes mellitus has been associated with abnormalities of the paternally inherited copy of chromosome 6, including duplications of a portion of the long arm of chromosome 6 and uniparental disomy, implicating overexpression of an imprinted gene in this disorder. To date, all patients with transient neonatal diabetes mellitus and uniparental disomy have had complete paternal isodisomy. We describe a patient with neonatal diabetes, macroglossia, and craniofacial abnormalities, with partial paternal uniparental disomy of chromosome 6 involving the distal portion of 6q, from 6q24-qter. This observation demonstrates that mitotic recombination of chromosome 6 can also give rise to uniparental disomy and neonatal diabetes, a situation similar to that observed in Beckwith-Wiedemann syndrome, another imprinted disorder. This finding has clinical implications, since somatic mosaicism for uniparental disomy of chromosome 6 should also be considered in patients with transient neonatal diabetes mellitus.
Neonatal diabetes mellitus is a rare disorder, having an estimated incidence of ∼1 in 400,000 live births (von Muhlendahl and Herkenhoff 1995). Infants with this disorder present within the first month of life with hyperglycemia that lasts for ⩾2 wk and that requires insulin therapy (Fosel 1995; von Muhlendahl and Herkenhoff 1995; Shield et al. 1997). Other features include intrauterine growth retardation and, in rarer instances, macroglossia and umbilical and inguinal hernias (Gerrard and Chin 1962; Salerno et al. 1994; Battin et al. 1996; Shield et al. 1997). Neonatal diabetes can be transient or permanent. Transient neonatal diabetes mellitus (TNDM [MIM 601410]) accounts for more than half the patients with neonatal diabetes mellitus and requires exogenous insulin therapy to maintain euglycemia. TNDM usually resolves at a median age of 4 mo (Fosel 1995; von Muhlendahl and Herkenhoff 1995). An increased risk of developing diabetes, particularly type 2 diabetes, later in life has been observed in some patients with TNDM (Shield and Baum 1995; von Muhlendahl and Herkenhoff 1995). Macroglossia appears to be a feature more commonly associated with TNDM than with the permanent form of neonatal diabetes (Christian et al. 1999).
TNDM has been associated with abnormalities involving chromosome 6. To date, the genetic abnormalities identified in patients with TNDM include paternal uniparental disomy of chromosome 6 (UPD6pat) (Abramowicz et al. 1994; Temple et al. 1995; Whiteford et al. 1997; Gardner et al. 1998; Christian et al. 1999; Cave et al. 2000; Hermann et al. 2000; Marquis et al. 2000) and duplications involving the 6q22-q24 region of the paternally derived chromosome 6 (Temple et al. 1996; Arthur et al. 1997; Gardner et al. 1999; Cave et al. 2000). These findings have suggested the involvement of an imprinted gene(s) in this disorder expressed only from the paternal chromosome and that increased expression, either by paternal UPD or duplication, results in the diabetes phenotype. This hypothesis is supported by the absence of the diabetic phenotype in instances of maternal UPD of chromosome 6 (van den Berg-Loonen et al. 1996; Spiro et al. 1999). UPD is generally associated with meiotic nondisjunction events followed by trisomy or monosomy “rescue” (Spence et al. 1988; Nicholls 1991). Most instances of UPD are thought to occur because of maternal meiosis I nondisjunction, associated with advanced maternal age; paternal UPD is thought to arise from a postzygotic duplication of a monosomic conception (Ledbetter and Engel 1995). To date, all instances of UPD6pat in patients with TNDM have been isodisomic (i.e., two copies of chromosome 6 are identical) and complete (i.e., involving the entire chromosome 6), which is consistent with a postzygotic duplication of the paternally derived chromosome 6. More recently, methylation changes of an imprinted locus in 6q24 have also been implicated in TNDM (Gardner et al. 2000).
We have identified a patient with neonatal diabetes and macroglossia, who has only partial UPD6pat involving the distal portion of 6q. This finding is, to our knowledge, the first reported instance of a patient with neonatal diabetes mellitus and partial UPD6pat. As partial UPD can arise only because of a somatic crossover event, this finding indicates that UPD6 in neonatal diabetes can also arise by mitotic recombination. The limitation of UPD to the distal portion of 6q is consistent with the finding of 6q22-q24 duplications in patients with TNDM and further supports the localization of an imprinted gene(s) to this region of chromosome 6.
The patient, a Hispanic male infant whose parents are nonconsanguineous, was born at 32 wk gestation. He had a brother with myoclonic seizures and severe mental retardation, for whom an extensive metabolic evaluation revealed nothing. No one in the family was reported to have had diabetes mellitus. The patient's length was 43 cm, his weight 1.79 kg (both 75th percentile), and his head circumference 30 cm (50th percentile). He had extreme macroglossia, a prominent occiput, lambdoidal ridging, small fontanel, shallow orbits, prominent nose, dysmorphic ears, gingival and labial hypertrophy, high palate, micrognathia, and a patent ductus arteriosus. Overall, subcutaneous tissue was decreased. Serum glucose was 452 mg/dl on day 2 of life and remained markedly elevated, despite treatment with a continuous insulin infusion. His respiratory status required very aggressive support. He also had intermittent hypotension, requiring treatment with pressor agents. On day 10, a bronze tinge to the skin was noted. Serum iron was low, at 27 μg/dl (normal 42–135), the ferritin level was 834 ng/ml (normal 15–400), and transferrin was <100 mg/dl (normal 200–400). On day 12, he was pale and cyanotic, with complete opacification of his lungs. Despite aggressive therapy, he died on day 14 from Pseudomonas aeruginosa sepsis. He had a normal cortisol level of 12.9 μg/dl and an insulin level of 9 μU/ml (normal 5–25), obtained immediately prior to death, while he was still receiving the insulin infusion. On postmortem examination, gastric ulcers, laryngeal erosions, multiple necrotizing lung abscesses, and vasculitis were detected. Hyaline membrane disease and the patent ductus arteriosis were also confirmed. No histologic abnormalities of either the exocrine or endocrine pancreas were detected. Despite the suspected hemochromatosis, there was no evidence of excess iron storage. However, there was hemosiderosis of the liver, assumed to be secondary to repeated blood transfusions.
Because of the marked hyperglycemia and macroglossia observed in this patient, microsatellite analysis for UPD6 was performed. DNA was extracted from the peripheral blood samples of the patient and both parents, by means of the Puregene kit from Gentra systems, according to the manufacturer’s recommendations. For the initial analysis, seven microsatellite markers that map to chromosome 6 were used. Five of the seven markers (D6S1713, D6S389, D6S1613, D6S1639, and D6S435) showed normal biparental inheritance; however, the two most distal 6q markers used (D6S311 and D6S305), which map to 6q25.1 and 6q26, respectively, showed absence of a maternal allele and presence of a single paternal allele, in the patient’s blood sample (data not shown). These results are consistent with a partial isodisomy of the paternally derived chromosome 6, restricted to the distal 6q region. Chromosome analysis showed a normal male karyotype, 46,XY. In order to rule out a submicroscopic deletion of the distal 6q region of the maternally derived chromosome 6, FISH analysis was performed using a probe, PAC 196I5, which maps within 300 bp of the telomere of chromosome 6q (Knight et al. 2000). The probe was directly labeled with SpectrumGreen-dUTP (Vysis), using a standard nick-translation reaction. Slide preparation, probe preparation, and hybridization were completed using methods described elsewhere (Chong et al. 1997). Two hybridization signals were observed, indicating that there was no deletion of the 6q telomeric region (data not shown).
An additional five microsatellite markers (D6S980, D6S1599, D6S1719, D6S264, and D6S281), which map distal to 6q26 and extend to the 6q telomeric region, were analyzed. For all five markers tested, the patient was found to have inherited only a single paternal allele. These results, together with the normal cytogenetic and FISH analysis in this patient, confirmed the presence of partial UPD6pat and indicated that the UPD extended to the end of the long arm of chromosome 6. To more precisely localize the position of the somatic crossover event that defines the boundary of the partial UPD in this patient, five additional microsatellite markers, which map between D6S435 and D6S311 (the markers found to flank the somatic recombination event in the initial screen), were used to study the patient. These markers were D6S262, D6S292, D6S1009, D6S314, and D6S1003. The somatic recombination event was localized between markers D6S292 and D6S1009, which map to chromosome band 6q23.2 and 6q24.1, respectively, according to the Genome Database. The genetic map positions of the markers used are based on the genetic maps of the Center for Medical Genetics and of Généthon (Dib et al. 1996), as well as the chromosome 6 radiation-hybrid map of The Sanger Centre. The results of the microsatellite analysis are presented in table 1. All the markers that showed UPD showed a single paternal allele, indicative of paternal isodisomy for 6q24-qter. UPD analysis of the patient’s brother with developmental delay, seizures, and severe neurologic disease of unknown etiology showed normal biparental inheritance of chromosome 6.
Table 1.
Analysis of Results of Microsatellite Markers
| Locusa(pter→qter) | Father | Patient | Mother | Result |
| D6S1713 | 1,3 | 1,4 | 2,4 | Biparental |
| D6S389 | 1,3 | 1,4 | 2,4 | Biparental |
| D6S1613 | 3,4 | 1,4 | 1,2 | Biparental |
| D6S1639 | 2,3 | 1,3 | 1,1 | Biparental |
| D6S435 | 2,3 | 1,3 | 1,1 | Biparental |
| D6S262 | 1,2 | 1,1 | 1,1 | Uninformative |
| D6S292 | 1,3 | 2,3 | 2,2 | Biparental |
| D6S1009 | 2,2 | 2,2 | 1,1 | Paternal UPD |
| D6S314 | 2,3 | 2,2 | 1,1 | Paternal UPD |
| D6S1003 | 1,3 | 3,3 | 2,2 | Paternal UPD |
| D6S311 | 1,2 | 2,2 | 3,3 | Paternal UPD |
| D6S305 | 2,3 | 2,2 | 1,4 | Paternal UPD |
| D6S980 | 1,1 | 1,1 | 2,3 | Paternal UPD |
| D6S1599 | 1,4 | 4,4 | 2,3 | Paternal UPD |
| D6S1719 | 3,4 | 3,3 | 1,2 | Paternal UPD |
| D6S264 | 3,3 | 3,3 | 1,2 | Paternal UPD |
| D6S281 | 1,4 | 1,1 | 2,3 | Paternal UPD |
The markers are arranged in order from the distal end of the short arm to the distal end of the long arm of chromosome 6.
Our results demonstrate the presence of partial UPD6pat, involving only the distal portion of 6q, in a patient with neonatal diabetes and macroglossia. This patient is, to our knowledge, the first such identified case. Since our patient died on day 14 of sepsis, we do not know whether the diabetes in this patient would have been transient or permanent. However, we speculate that it would have been transient, since chromosome 6 abnormalities have been associated with TNDM rather than with the permanent form of neonatal diabetes (Christian et al. 1999; Hermann et al. 2000). Chromosome 6 duplications and UPD6pat appear to be responsible for approximately two-thirds of TNDM patients (Gardner et al. 1999; Temple et al. 1999). More recently, submicroscopic duplications and methylation mutations of the 6q24 region have been found in patients with TNDM, implying that perhaps more subtle changes of chromosome 6 may be involved in the remaining cases (Gardner et al. 2000). Our findings imply that mitotic recombination and partial UPD, possibly associated with mosaicism, also account for a proportion of patients with neonatal diabetes mellitus.
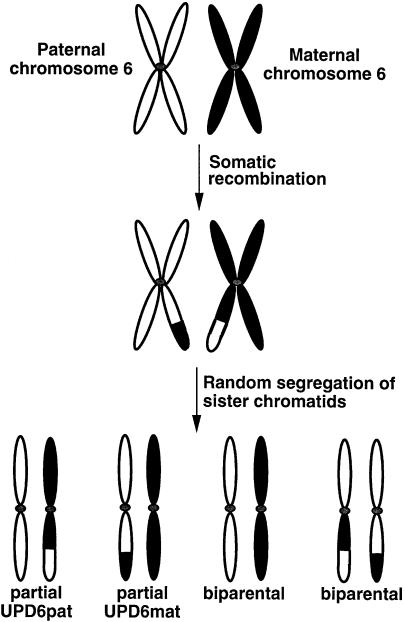
Only a postzygotic mitotic recombination event can account for a partial UPD finding. Somatic crossover between the two copies of chromosome 6 at mitosis, followed by random segregation of sister chromatids in the zygote, is likely to have given rise to the partial UPD6 observed in this patient. Somatic recombination and random segregation can produce different cell types, as illustrated in figure 1. This mechanism can result in somatic mosaicism, the extent of which will depend on which stage the postzygotic recombinational event occurred. Somatic recombination at a very early stage, followed by preferential death of a particular cell type, may be the cause of the lack of mosaicism observed in this patient. It is also possible that a low level of mosaicism in this patient may have been undetected. Additionally, the study of other tissues in this patient may have revealed some mosaicism; however, only lymphocytes were available for study.
Figure 1.
Somatic recombination resulting in paternal isodisomy for the distal portion of 6q. Mitotic recombination and random segregation of sister chromatids can result in other cell populations with maternal isodisomy and biparental inheritance of chromosome 6. The extent of mosaicism will depend on the stage at which the recombinational event occurs.
The observation of partial UPD6 in neonatal diabetes is similar to the situation of Beckwith-Wiedemann syndrome (BWS), another imprinting disorder associated with overgrowth, umbilical anomalies, macroglossia, and, conversely to UPD6, transient neonatal hypoglycemia. In BWS, partial paternal UPD of chromosome 11, paternal duplications of 11p, and gene mutations that affect imprinting have been observed (reviewed by Li et al. 1998). Somatic mosaicism has been observed in a number of patients with BWS who have either partial or total UPD11pat (Henry et al. 1993; Bischoff et al. 1995; Catchpoole et al. 1997). The presence of somatic mosaicism, as well as the different genetic mechanisms that give rise to BWS, are thought to be the cause of the highly variable phenotype seen in this disorder (Henry et al. 1993; Catchpoole et al. 1997). The observation of partial UPD6pat in a child with neonatal diabetes suggests that somatic mosaicism may be present in some cases of TNDM as well. The presence of complete UPD6, duplications of chromosome 6, 6q24 methylation mutations, and now partial UPD6 may contribute to the variability in phenotype that is seen in patients with TNDM, as is the case with BWS.
One other patient with partial UPD6pat has been reported (Lopez-Gutierrez et al. 1998), but the UPD involved only the short arm of chromosome 6. The phenotype of the patient was notable for congenital adrenal hypoplasia caused by homozygosity for a paternally transmitted point mutation in the steroid 21-hydroxylase gene at 6p21.3. However, this patient did not have neonatal diabetes, consistent with the UPD not extending to the long arm of chromosome 6. In contrast, the extent of the paternal UPD in our patient is consistent with and overlaps the area of paternal duplications observed in patients with TNDM (Gardner et al. 1999; Cave et al. 2000). The inclusion of the 6q24 region in the UPD observed in this patient supports the localization of an imprinted gene(s) involved in neonatal diabetes to this region of chromosome 6 (Gardner et al. 1999, 2000; Cave et al. 2000). A candidate gene for TNDM, ZAC/PLAGL1, which maps to 6q24 and appears to be imprinted, has recently been described (Kamiya et al. 2000). Other loci implicated in insulin-dependent diabetes mellitus (IDDM) have been localized to the long arm of chromosome 6 by linkage analysis (Luo et al. 1995, 1996; Davies et al. 1996; Delepine et al. 1997), and it may be speculated that the genes at one or more of these loci may also play a role in TNDM.
To date, all patients with TNDM and UPD6pat have had intrauterine growth retardation (Abramowicz et al. 1994; Temple et al. 1995; Whiteford et al. 1997; Gardner et al. 1998; Christian et al. 1999; Cave et al. 2000; Hermann et al. 2000; Marquis et al. 2000). Even though our patient’s growth parameters, including his weight, were in the normal range, he did have an apparent marked decrease in subcutaneous tissue. From the extent of UPD present in our patient, however, we might speculate that at least some gene(s) responsible for growth retardation maps proximal to 6q24. Macroglossia has been specifically mentioned in a proportion of TNDM patients with UPD6pat (Christian et al. 1999; Cave et al. 2000; Hermann et al. 2000). From the extent of UPD in our patient, we can speculate that the locus responsible for macroglossia maps distal to 6q24. The mechanism is more likely to be related to imprinting than to rare recessive genes unmasked by UPD, since macroglossia and intrauterine growth retardation are specific findings in more than one of these patients. It should be noted that, analogously, macroglossia is quite variably associated with BWS. Thus, it is likely that macroglossia associated with UPD6 is also influenced by other genetic and environmental factors.
Our patient also had several dysmorphic features that have not been associated with TNDM. Two other patients with neonatal diabetes and abnormalities of chromosome 6, with death in infancy, have been described in the literature (Pivnick et al. 1990; Abramowicz et al. 1994). For both these patients, the genetic basis for the severity in phenotype was determined and included a reduction to homozygosity for a MUT gene mutation (Abramowicz et al. 1994) and the presence of an unbalanced chromosome translocation involving chromosome 6 (Pivnick et al. 1990). Another example of a patient with an unbalanced chromosome translocation involving chromosome 6 and death in infancy has been reported; however, neonatal diabetes was not mentioned with regard to this patient (Franchino et al. 1987). No karyotypic abnormalities were observed in our patient that would account for the severity of the phenotype. Although it is possible that the patient inherited a recessive mutation, which became reduced to homozygosity, from the father, no other specific syndrome could be assigned to the patient.
Intriguing observations in the patient were the iron, ferritin, and transferrin indices, which were suggestive of hereditary hemochromatosis. As the HFE gene for hereditary hemochromatosis maps to chromosome 6 at 6p21.3, we had initially speculated a total UPD6pat in this patient resulting in a reduction to homozygosity of a mutation in the HFE gene inherited from the father. However, the UPD was found not to extend to the short arm of chromosome 6, and analysis of the HFE gene for the common C282Y mutation and the H63D variant, performed as described elsewhere (Feder et al. 1996), showed that the patient had inherited only one allele of the H63D variant, which is not sufficient for the manifestation of hereditary hemochromatosis. Both parents were found to be carriers of the H63D allele, and the parental origin of the H63D allele in the patient could not easily be determined. Thus, the elevated ferritin level may have been a nonspecific acute-phase reactant, rather than a primary abnormality. It is also interesting to note that the pancreas in our subject appeared to be normal on postmortem examination. Unfortunately, stains specific for pancreatic beta cells were not performed in our patient, so we cannot be certain if there was selective agenesis of these cells, as was observed in the subject reported by Abramowicz et al. (1994).
In summary, we describe a patient with neonatal diabetes, macroglossia, and other complications, including neonatal death due to sepsis, who had a partial UPD6pat involving distal 6q from 6q24-qter. This is the first description of a patient with neonatal diabetes, macroglossia, and partial UPD6pat. This observation further reinforces the complexity of the genetic basis of neonatal diabetes and emphasizes the importance of using polymorphic markers that map distal to the 6q24 region for molecular diagnosis of UPD6 in neonatal diabetes.
Acknowledgments
We would like to thank Dr. Susan Christian, for helpful discussions regarding the chromosome 6 genetic map, and Dr. Robert Rosenfield, for critical reading of the manuscript.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Center for Medical Genetics, http://research.marshfieldclinic.org/genetics/
- Généthon, http://www.genethon.fr
- Genome Database, http://www.gdb.org
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for TNDM [601410])
- Sanger Centre, The, http://www.sanger.ac.uk/ (for chromosome 6 radiation hybrid map)
References
- Abramowicz MJ, Andrien M, Dupont E, Dorchy H, Parma J, Duprez J, Ledley FD, Courtens W, Vamos E (1994) Isodisomy of chromosome 6 in a newborn with methylmalonic acidemia and agenesis of pancreatic beta cells causing diabetes mellitus. J Clin Invest 94:418–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur EI, Zlotogora J, Lerer I, Dagan J, Marks K, Abeliovich D (1997) Transient neonatal diabetes mellitus in a child with inv dup(6)(q22q23) of paternal origin. Eur J Hum Genet 5:417–419 [PubMed] [Google Scholar]
- Battin M, Yong C, Phang M, Daaboul J (1996) Transient neonatal diabetes mellitus and macroglossia. J Perinatol 16:288–291 [PubMed] [Google Scholar]
- Bischoff FZ, Feldman GL, McCaskill C, Subramanian S, Hughes MR, Shaffer LG (1995) Single cell analysis demonstrating somatic mosaicism involving 11p in a patient with paternal isodisomy and Beckwith-Wiedemann syndrome. Hum Mol Genet 4:395–399 [DOI] [PubMed] [Google Scholar]
- Catchpoole D, Lam WW, Valler D, Temple IK, Joyce JA, Reik W, Schofield PN, Maher ER (1997) Epigenetic modification and uniparental inheritance of H19 in Beckwith-Wiedemann syndrome. J Med Genet 34:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave H, Polak M, Drunat S, Denamur E, Czernichow P (2000) Refinement of the 6q chromosomal region implicated in transient neonatal diabetes. Diabetes 49:108–113 [DOI] [PubMed] [Google Scholar]
- Chong SS, Pack SD, Roschke AV, Tanigami A, Carrozzo R, Smith ACM, Dobyns WB, Ledbetter DH (1997) A revision of the lissencephaly and Miller-Dieker syndrome critical regions in chromosome 17p13.3. Hum Mol Genet 6:147–155 [DOI] [PubMed] [Google Scholar]
- Christian SL, Rich BH, Loebl C, Israel J, Vasa R, Kittikamron K, Spiro R, Rosenfield R, Ledbetter DH (1999) Significance of genetic testing for paternal uniparental disomy of chromosome 6 in neonatal diabetes mellitus. J Pediatr 134:42–46 [DOI] [PubMed] [Google Scholar]
- Davies JL, Cucca F, Goy JV, Atta ZA, Merriman ME, Wilson A, Barnett AH, Bain SC, Todd JA (1996) Saturation multipoint linkage mapping of chromosome 6q in type 1 diabetes. Hum Mol Genet 5:1071–1074 [DOI] [PubMed] [Google Scholar]
- Delepine M, Pociot F, Habita C, Hashimoto L, Froguel P, Rotter J, Cambon-Thomsen A, Deschamps I, Djoulah S, Weissenbach J, Nerup J, Lathrop M, Julier C (1997) Evidence of a non-MHC susceptibility locus in type I diabetes linked to HLA on chromosome 6. Am J Hum Genet 60:174–187 [PMC free article] [PubMed] [Google Scholar]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, et al (1996) A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 13:399–408 [DOI] [PubMed] [Google Scholar]
- Fosel S (1995) Transient and permanent neonatal diabetes. Eur J Pediatr 154:944–948 [DOI] [PubMed] [Google Scholar]
- Franchino CJ, Beneck D, Greco MA, Wolman SR (1987) Partial trisomy 6q: case report with necropsy findings. J Med Genet 24:300–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RJ, Mackay DJ, Mungall AJ, Polychronakos C, Siebert R, Shield JP, Temple IK, Robinson DO (2000) An imprinted locus associated with transient neonatal diabetes mellitus. Hum Mol Genet 9:589–596 [DOI] [PubMed] [Google Scholar]
- Gardner RJ, Mungall AJ, Dunham I, Barber CK, Shield JPH, Temple IK, Robinson DO (1999) Localisation of a gene for transient neonatal diabetes mellitus to an 18.72 cR3000 (∼5.4Mb) interval on chromosome 6q. J Med Genet 36:192–196 [PMC free article] [PubMed] [Google Scholar]
- Gardner RJ, Robinson DO, Lamont L, Shield JP, Temple IK (1998) Paternal uniparental disomy of chromosome 6 and transient neonatal diabetes mellitus. Clin Genet 54:522–525 [DOI] [PubMed] [Google Scholar]
- Gerrard JW, Chin W (1962) The syndrome of transient diabetes. J Pediatr 61:89–93 [DOI] [PubMed] [Google Scholar]
- Henry I, Puech A, Riesewijk A, Ahnine L, Mannens M, Beldjord C, Bitoun P, Tournade MF, Landrieu P, Junien C (1993) Somatic mosaicism for partial paternal isodisomy in Wiedemann-Beckwith syndrome: a post-fertilization event. Eur J Hum Genet 1:19–29 [DOI] [PubMed] [Google Scholar]
- Hermann R, Laine AP, Johansson C, Niederland T, Tokarska L, Dziatkowiak H, Ilonen J, Soltesz G (2000) Transient but not permanent neonatal diabetes mellitus is associated with paternal uniparental isodisomy of chromosome 6. Pediatrics 105:49–52 [DOI] [PubMed] [Google Scholar]
- Kamiya M, Judson H, Okazaki Y, Kusakabe M, Muramatsu M, Takada S, Takagi N, Arima T, Wake N, Kamimura K, Satomura K, Hermann R, Bonthron D, Hayashizaki Y (2000) The cell cycle control gene ZAC/PLAGL1 is imprinted: a strong candidate gene for transient neonatal diabetes. Hum Mol Genet 9:453–460 [DOI] [PubMed] [Google Scholar]
- Knight SJ, Lese CM, Precht KS, Kuc J, Ning Y, Lucas S, Regan R, Brenan M, Nicod A, Lawrie NM, Cardy DL, Nguyen H, Hudson TJ, Riethman HC, Ledbetter DH, Flint J (2000) An optimized set of human telomere clones for studying telomere integrity and architecture. Am J Hum Genet 67:320–332 [DOI] [PMC free article] [PubMed]
- Ledbetter DH, Engel E (1995) Uniparental disomy in humans: development of an imprinting map and its implications for prenatal diagnosis. Hum Mol Genet 4:1757–1764 [DOI] [PubMed] [Google Scholar]
- Li M, Squire JA, Weksberg R (1998) Molecular genetics of Wiedemann-Beckwith syndrome. Am J Med Genet 79:253–259 [PubMed] [Google Scholar]
- Lopez-Gutierrez AU, Riba L, Ordonez-Sanchez ML, Ramirez-Jimenez S, Cerrillo-Hinojosa M, Tusie-Luna MT (1998) Uniparental disomy for chromosome 6 results in steroid 21-hydroxylase deficiency: evidence of different genetic mechanisms involved in the production of the disease. J Med Genet 35:1014–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DF, Bui MM, Muir A, Maclaren NK, Thomson G, She JX (1995) Affected-sib-pair mapping of a novel susceptibility gene to insulin-dependent diabetes mellitus (IDDM8) on chromosome 6q25-q27. Am J Hum Genet 57:911–919 [PMC free article] [PubMed] [Google Scholar]
- Luo DF, Buzzetti R, Rotter JI, Maclaren NK, Raffel LJ, Nistico L, Giovannini C, Pozzilli P, Thomson G, She JX (1996) Confirmation of three susceptibility genes to insulin dependent diabetes mellitus: IDDM4, IDDM5 and IDDM8. Hum Mol Genet 5:693–698 [DOI] [PubMed] [Google Scholar]
- Marquis E, Robert JJ, Benezech C, Junien C, Diatloff-Zito C (2000) Variable features of transient neonatal diabetes mellitus with paternal isodisomy of chromosome 6. Eur J Hum Genet 8:137–140 [DOI] [PubMed] [Google Scholar]
- Nicholls RD (1991) Uniparental disomy as the basis for an association of rare disorders [letter]. Am J Med Genet 41:273–274 [DOI] [PubMed] [Google Scholar]
- Pivnick EK, Qumsiyeh MB, Tharapel AT, Summitt JB, Wilroy RS (1990) Partial duplication of the long arm of chromosome 6: a clinically recognisable syndrome. J Med Genet 27:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno M, Gasparini N, Sandomenico ML, Franzese A, Tenore A (1994) Two interesting cases of transient neonatal diabetes mellitus. J Pediatr Endocrinol 7:47–52 [DOI] [PubMed] [Google Scholar]
- Shield JPH, Baum JD (1995) Transient neonatal diabetes and later onset diabetes: a case of inherited insulin resistance. Arch Dis Child 72:56–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield JPH, Gardner RJ, Wadsworth EJK, Whiteford ML, James JR, Robinson DO, Baum JD, Temple IK (1997) Aetiopathology and genetic basis of neonatal diabetes. Arch Dis Child Fetal Neonatal Ed 76:F39–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JE, Perciaccante RG, Greig GM, Willard HF, Ledbetter DH, Hejtmancik JF, Pollack MS, O’Brien WE, Beaudet AL (1988) Uniparental disomy as a mechanism for human genetic disease. Am J Hum Genet 42:217–226 [PMC free article] [PubMed] [Google Scholar]
- Spiro RP, Christian SL, Ledbetter DH, New MI, Wilson RC, Roizen N, Rosenfield RL (1999) Intrauterine growth retardation associated with maternal uniparental disomy for chromosome 6 unmasked by congenital adrenal hyperplasia. Pediatr Res 46:510–513 [DOI] [PubMed] [Google Scholar]
- Temple IK, Gardner RJ, Mackay DJG, Robinson DO, Valerio G, Franzese A, Barber JCK, Shield JPH (1999) Early onset non insulin dependent diabetes mellitus (NIDDM): a link with paternal duplication of 6q24 and transient neonatal diabetes mellitus (TNDM). Am J Hum Genet Suppl 65:A107 [Google Scholar]
- Temple IK, Gardner RJ, Robinson DO, Kibirige MS, Ferguson AW, Baum JD, Barber JCK, James RS, Shield JPH (1996) Further evidence for an imprinted gene for neonatal diabetes localised to chromosome 6q22-q23. Hum Mol Genet 5:1117–1121 [DOI] [PubMed] [Google Scholar]
- Temple IK, James RS, Crolla JA, Sitch FL, Jacobs PA, Howell WM, Betts P (1995) An imprinted gene(s) for diabetes? Nat Genet 9:110–112 [DOI] [PubMed] [Google Scholar]
- van den Berg-Loonen EM, Savelkoul P, van Hooff H, van Eede P, Riesewijk A, Geraedts J (1996) Uniparental maternal disomy 6 in a renal transplant patient. Hum Immunol 45:46–51 [DOI] [PubMed] [Google Scholar]
- von Muhlendahl KE, Herkenhoff H (1995) Long-term course of neonatal diabetes. N Engl J Med 333:704–708 [DOI] [PubMed] [Google Scholar]
- Whiteford ML, Narendra A, White MP, Cooke A, Wilkinson AG, Robertson KJ, Tolmie JL (1997) Paternal uniparental disomy for chromosome 6 causes transient neonatal diabetes. J Med Genet 34:167–168 [DOI] [PMC free article] [PubMed] [Google Scholar]