Abstract
Polycystic liver disease (PCLD) is characterized by the growth of fluid-filled cysts of biliary epithelial origin in the liver. Although the disease is often asymptomatic, it can, when severe, lead to complications requiring surgical therapy. PCLD is most often associated with autosomal dominant polycystic kidney disease (ADPKD); however, families with an isolated polycystic liver phenotype without kidney involvement have been described. The clinical presentation and histological features of polycystic liver disease in the presence or absence of ADPKD are indistinguishable, raising the possibility that the pathogenetic mechanisms in the diseases are interrelated. We ascertained two large families with polycystic liver disease without kidney cysts and performed a genomewide scan for genetic linkage. A causative gene, PCLD, was mapped to chromosome 19p13.2-13.1, with a maximum LOD score of 10.3. Haplotype analysis refined the PCLD interval to 12.5 cM flanked by D19S586/D19S583 and D19S593/D19S579. The discovery of genetic linkage will facilitate diagnosis and study of this underdiagnosed disease entity. Identification of PCLD will be instrumental to an understanding of the pathogenesis of cyst formation in the liver in isolated PCLD and in ADPKD.
Polycystic liver disease (PCLD [MIM 174050]) is one of the hepatobiliary fibropolycystic diseases characterized by an overgrowth of biliary epithelium and supportive connective tissue (Torres 1996). The clinical entity of PCLD in autosomal dominant polycystic kidney disease (ADPKD [MIM 173900 and MIM 173910]) has been well studied. There is an age-dependent increase of hepatic cyst prevalence, from 20% of patients in the 3d decade of life to 75% by the 7th decade of life (Milutinovic et al. 1980; Grunfeld et al. 1985; Gabow et al. 1990). At any stage, women are more likely than men to have more and larger cysts (Grunfeld et al. 1985; Gabow et al. 1990), and nulliparous women who have never used estrogens are less likely to have cysts than are those who have been pregnant and/or used hormones (Gabow et al. 1990).
Autosomal dominant PCLD also occurs independently of ADPKD. Although likely an overestimate of the prevalence of isolated PCLD, the frequency of polycystic kidneys in old autopsy or surgical series of PCLD has been reported to be on the order of 50%–60% (Comfort et al. 1952; Melnick 1955). Nevertheless, families with PCLD either without renal cysts or with only a few renal cysts have been described (Sotaniemi et al. 1979), and a large retrospective study of medicolegal autopsies in Finland also has suggested that PCLD exists as an entity separate from ADPKD (Karhunen and Tenhu 1986). Most recently, several reports have demonstrated that PCLD is a unique entity, genetically distinct from PKD1 and PKD2, the genes responsible for ADPKD (Pirson et al. 1996; Iglesias et al. 1999). In the present study, we examined two large kindreds segregating PCLD as a dominant trait (fig. 1). Ascertainment required that at least one member of each family have both symptomatic PCLD treated by surgery and neither kidney involvement nor a family history of ADPKD. We used these families in a genomewide scan for linkage and established the existence of a locus for PCLD, on chromosome 19p13.2-13.1.
Figure 1.
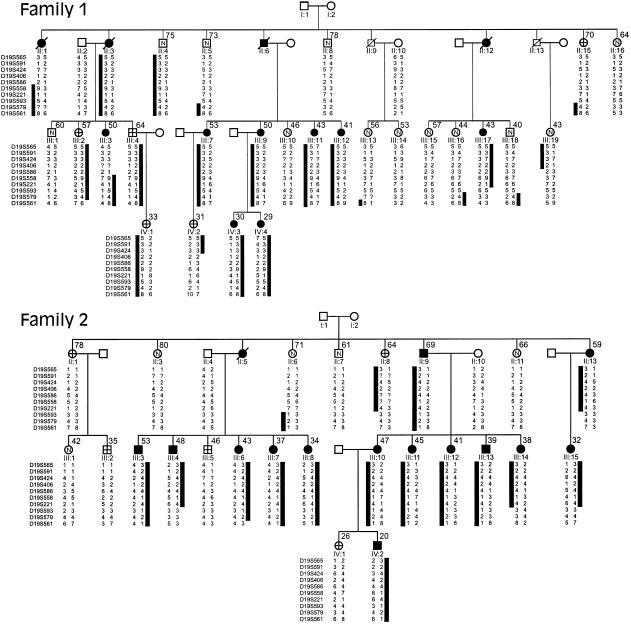
Pedigrees of families 1 and 2, which have isolated PCLD, and associated chromosome 19p haplotypes. The black bars denote the haplotypes at informative markers segregating with the disease phenotype. Black symbols denote affected individuals; white symbols containing an “N” denote unaffected individuals; white symbols containing cross hairs denote individual whose affection status is unknown; white symbols not containing any of the aforementioned items denote individuals not at risk. The numbers to the upper right of the symbols are the ages (in years) at ultrasound examination. The marker order is given to the left of each generation in the pedigrees; intermarker genetic distances are shown in figure 3. Question marks denote unknown alleles. Individuals II:5, III:16, III:17, and III:18 in family 1 and individuals II:6, II:13, III:4, and III:14 in family 2 define the centromeric boundary of the disease interval at D19S593/579. Individuals II:1 and III:19 in family 1 define the telomeric boundary at D19S406; III:3 also shows recombination between PCLD and D19S586.
The participants were studied at the Mayo Clinic General Clinical Research Center. Informed consent was obtained. All subjects underwent a medical history interview, physical examination, serum biochemical determinations and complete cell count, standard magnetic-resonance head imaging, magnetic-resonance angiography of the circle of Willis (Huston et al. 1996), ultrasonography of liver and kidneys, and echocardiography, including 2D and 2D-guided M-mode echocardiography and Doppler ultrasound with color-flow imaging (Nishimura et al. 1985; Marks et al. 1989). In some patients, computed tomographies of the abdomen, previously obtained for clinical indications, also were available for review. All clinical evaluations were done without knowledge of linkage status. There is no known relationship between the two kindreds.
The clinical criteria used to diagnose PCLD were a history consistent with autosomal dominant inheritance and the presence of either any hepatic cysts, in subjects <40 years old, or at least four hepatic cysts, in individuals >40 years old (fig. 2). No members of either kindred fulfilled criteria for the diagnosis of autosomal dominant polycystic kidney disease (Bear et al. 1992; Ravine et al. 1994). Individuals >40 years old who had no liver cysts were considered as unaffected. Individuals >40 years old who had one to three hepatic cysts were designated as having an “indeterminate” phenotype, since there is an age-dependent occurrence of simple liver cysts in the general population (Gaines and Sampson 1989; Caremani et al. 1993). Individuals <40 years old who had no cysts also were considered as having an indeterminate phenotype—an extrapolation from ADPKD, in which the liver-cystic disease generally lags behind the kidney cysts and where, in the absence of kidney cysts, the age of 30 years is used as the cutoff for exclusion of disease (Bear et al. 1992; Ravine et al. 1994). Student's t-test and Fischer's exact test were used to compare the clinical parameters of the affected and unaffected individuals. The P values reported are two-tailed, and .05 was used as the criterion of statistical significance.
Figure 2.
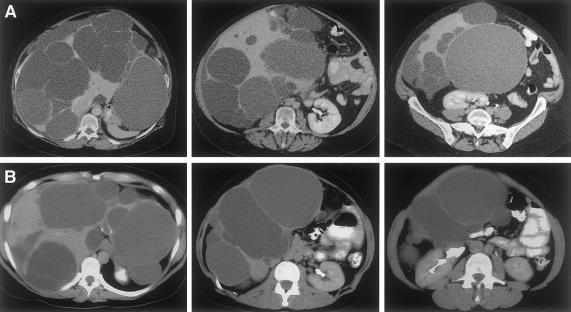
Computerized tomograms of the abdomen, illustrating extensive PCLD in the absence of renal cysts in (A panels) individual III:7 in family 1 and (B panels) individual III:11 in family 2.
Genomic DNA from individual family members was prepared from whole blood, by use of the PureGene kit (Gentra Systems). One DNA sample from family 1 (from individual II:1) was extracted from paraffin-embedded tissue (Ex-Wax; Oncor). The genome scan utilized the Marshfield (version 8a) human genome–scanning set of polymorphic markers (Center for Medical Genetics, Marshfield Medical Research Foundation). Fluorescent marker analysis was performed by an ABI 377 Prism sequencer at the Human Genome Program core at the Albert Einstein College of Medicine. The allele data were analyzed sequentially by the GENESCAN and GENOTYPER programs (ABI). Allele calls by GENOTYPER were checked manually for accuracy. The marker data were then checked for Mendelian inheritance within a pedigree, by GENEBASE (ABI), as well as by inspection after they were entered into CYRILLIC (Cherwell Scientific). Two-point linkage analysis used MLINK, and multilocus analysis used the LINKMAP program of LINKAGE (version 5.1) (Lathrop et al. 1985), both in the FASTLINK implementation (version 4.1p) (Cottingham et al. 1993; Schäffer et al. 1994). The disease-allele frequency in the population was set at .0002, the phenocopy rate was set at the same frequency, and the heterozygous disease penetrance was assumed to be .95. Marker-allele frequencies were set at 1/n, where n is the number of alleles observed. After linkage was established, further genetic analysis to confirm and refine the interval used backbone markers and genetic distances from the Marshfield sex-averaged linkage map, accessed through The Genome Database. Haplotypes were assigned on the basis of the principle of minimization of intermarker recombination.
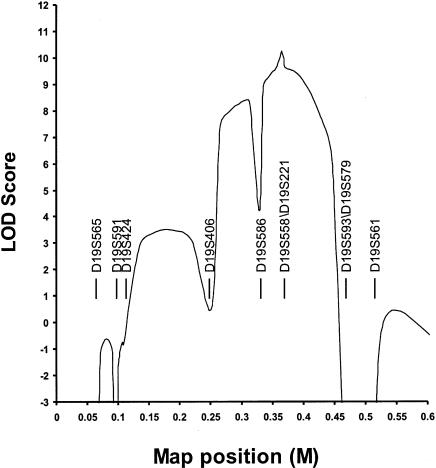
We confirmed that neither kindred segregated a gene for ADPKD, by excluding genetic linkage to both 16p13.3 (PKD1) and 4q21-23 (PKD2) (data not shown). Power analysis demonstrated that, in each family, linkage could be independently established, with a LOD score >3.0 (data not shown). On the basis of the expectation that the rate of false-positive diagnosis (i.e., at least four liver cysts in unaffected individuals) is less than the rate of false-negative diagnosis, we chose to use family 2 in the initial genome scan. Genetic linkage to chromosome 19 was suggested when D19S591 gave a LOD score >3.0 at recombination fraction (θ) 0. To further evaluate this finding, both kindreds were analyzed with markers throughout 19p (figs. 1 and 3). Families 1 and 2 independently support linkage to 19p13.2-13.1, with peak two-point LOD scores of 4.54 and 5.14, respectively, at D19S558 (θ=0). The combined two-point data are given in table 1. Multipoint analysis suggests that the most likely location for the disease locus is centered around D19S558 and D19S221 (maximum LOD score 10.3; see fig. 4). The 3-LOD-support confidence interval spans a region of ∼20 cM within the interval bounded by D19S406 and D19S593/D19S579. Inspection of the predicted haplotypes (fig. 1) reveals that there are no obligate recombinants between the disease and either D19S558 or D19S221. Obligate recombinants are observed with all other makers in the analysis. Individual II:4 in family 1 has the entire affected haplotype yet is clinically unaffected at age 75 years, suggesting that PCLD is not completely penetrant.
Figure 3.
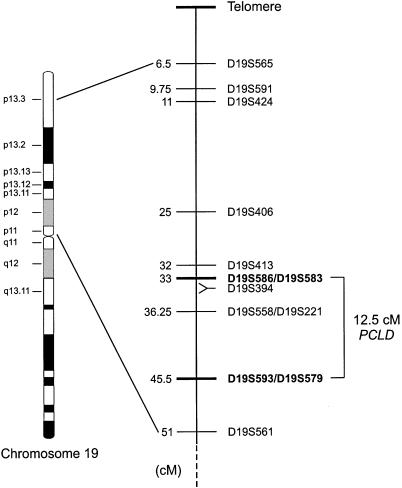
Genetic map of markers used in this study. Distances (in cM) were obtained from the Marshfield sex-averaged map. D19S394 is not genetically mapped but, rather, on the basis of the Chromosome 19–p Arm Metric Physical Map (Lawrence Livermore Laboratory) maps 1 Mb centromeric to D19S586p. Markers defining the PCLD genetic interval are in boldface.
Table 1.
Two-Point LOD Scores, between PCLD and Chromosome 19 Markers
|
LOD Score at θ = |
|||||||
| Marker | .00 | .01 | .05 | .10 | .20 | .30 | .40 |
| D19S565 | 4.33 | 4.28 | 4.08 | 3.78 | 2.99 | 1.97 | .80 |
| D19S591 | −.35 | 2.75 | 3.88 | 3.98 | 3.33 | 2.19 | .83 |
| D19S424 | 5.63 | 5.58 | 5.27 | 4.76 | 3.55 | 2.21 | .86 |
| D19S406 | 1.37 | 1.37 | 1.35 | 1.28 | 1.00 | .60 | .20 |
| D19S586 | 2.89 | 4.30 | 4.56 | 4.31 | 3.40 | 2.22 | .88 |
| D19S558 | 9.68 | 9.58 | 9.06 | 8.28 | 6.44 | 4.28 | 1.83 |
| D19S221 | 7.05 | 6.99 | 6.63 | 6.05 | 4.62 | 2.93 | 1.12 |
| D19S593 | −.97 | −.77 | .62 | 1.25 | 1.4 | .99 | .37 |
| D19S579 | −9.18 | −2.75 | −.15 | 1.06 | 1.67 | 1.35 | .58 |
| D19S561 | −13.62 | −6.31 | −2.11 | −.29 | .95 | .96 | .45 |
Figure 4.
Multipoint analysis with 10 chromosome 19 markers. The maximum LOD score is obtained with D19S221/D19S558, and the 3-LOD support interval is between D19S406 and D19S593/D19S579.
The centromeric boundary for the PCLD interval occurs at D19S593/D19S579. This is supported by the multipoint analysis and the apparent recombinant haplotypes in individuals II:5, III:16, III:17, and III:18 in family 1 and individuals II:6, II:13, III:4, and III:14 in family 2 (fig. 1). There are three apparent recombination events, all occurring in family 1, between the disease locus and telomeric markers. Results of study of individuals II:1, III:3, and III:19 define the telomeric recombinatorial boundary as being at D19S406, and the 3-LOD-support interval is consistent with this boundary. Also, individual III:3, who is affected with six liver cysts at age 50 years, further shows apparent recombination between D19S586 and PCLD. D19S586 was not informative in II:1 and III:19.
We examined family 1, using additional markers—D19S413, D19S583, and D19S394 (fig. 3). D19S413 is not recombined with the disease haplotype in the affected individual, II:1, whereas unaffected individual III:19 has the normal D19S413 allele (data not shown). In both individuals, the recombination defining the telomeric boundary of the PCLD genetic interval occurs in the region between D19S406 and D19S413. However, in individual III:3, D19S586 and D19S583 are informative and recombine with the disease locus whereas D19S394 does not, placing the recombination in the interval between these loci. This analysis suggests that the telomeric recombinatorial boundary for PCLD is defined at D19S586/D19S583, refining the location of the PCLD gene to a 12.5-cM interval between D19S586/D19S583 and D19S593/D19S573 (fig. 3).
A total of 49 at-risk individuals underwent clinical evaluations for this study; 47 had a complete evaluation, and 2 underwent only ultrasonography of the liver and kidneys. In addition, DNA was available from two clinically affected individuals who were deceased at the time of the study. Of the 49 participants, 22 were affected by PCLD, 17 were not affected, and 10 had indeterminate clinical diagnoses. All individuals with at least four liver cysts at any age carried the disease haplotype, suggesting that this criterion is adequate for differentiation between most of the individuals in the population who are affected and those who have a few isolated liver cysts. Of the 10 at-risk individuals with indeterminate clinical diagnoses, 3 (III:2, III:4, and IV:1, all from family 1) carry the affected disease haplotype (fig. 1). III:2, a woman, and III:4, a man, have one to three liver cysts at an advanced age, whereas IV:1, another woman, has no cysts at age 33 years. Individual II:15 in family 1 and individual II:8 in family 2 are classified as unknown, with one to three liver cysts at an advanced age. They both carry a portion of the disease haplotype but have a recombination within in the PCLD critical interval, which, at the time of the study, obviated genetic diagnosis. Of the remaining five individuals with an “unknown” clinical diagnosis, all are predicted to be unaffected, on the basis of genetic analysis; three of these individuals (individual IV:2 in family 1 and individuals III:2 and IV:1 in family 2) are classified as unknown solely on the basis of an age of <40 years and the absence of cysts, and the two others (individuals II:1 and III:5 in family 2) have one to three cysts at an advanced age. Finally, as discussed above, the disease is not penetrant in individual II:4 in family 1, at age 75 years.
The group of 46 patients who underwent evaluations at the Mayo Clinic General Clinical Research Center and for whom a genetic diagnosis could be established comprised 26 genetically affected individuals and 20 genetically unaffected individuals. Abnormal mitral valve leaflets were observed more frequently in the group with PCLD (35% vs. 5%; P=.028). The prevalence of intracranial aneurysms (one in each group) was low and not different between affected and unaffected individuals. No difference in the prevalence of either renal cysts or hypertension was detected. The severity of PCLD was graded from 0 to 6, depending on the number of cysts detected by ultrasound (0 = 0 cysts, 1= 1–3 cysts, 2 = 4–6 cysts, 3 = 7–10 cysts, 4 = 11–15 cysts, 5 = 16–20 cysts, and 6 = >20 cysts). PCLD was more severe in affected women than in affected men (4.4±2.3 vs. 1.8±1.2; P=.009). The more severe liver cystic disease in women and the higher prevalence of mitral valve leaflet abnormalities in affected individuals reinforce the similarities between the phenotypes of isolated PCLD and ADPKD and raise the possibility that PCLD, like ADPKD, is a systemic disorder.
The 12.5-cM PCLD interval is too large to be practical for positional cloning even as the genomic sequencing for the region is being completed. Further refinement of the recombinatorial boundaries will be necessary before saturation mutation detection of expressed sequences becomes feasible. However, a positional candidate approach based on the current understanding of the disease pathogenesis could identify PCLD. In this regard, no putative proteins with sequence similarity to PKD1 or PKD2 were identified in the interval by BLAST searches of the available genomic sequence dynamically translated in six reading frames. PKD1 is thought to function as a cell-surface receptor active in cell-cell or cell-matrix contact, and PKD2 is a putative calcium-channel protein whose activity may be interdependent with that of PKD1 (Emmons and Somlo 1999). Genes expressed in the liver that have potential functions related to the function of PKD1 and PKD2 may serve as candidates.
The most recent Chromosome 19–p Arm Metric Physical Map (Lawrence Livermore Laboratory) lists 28 genes in the interval between D19S586 and D19S593/D19S579. Mutations in the voltage-dependent calcium channel gene, CACNA1A, which maps to the interval, already have been associated with spinocerebellar ataxias (Jodice et al. 1997), and mutations in NOTCH3 cause cerebral autosomal dominant arteriopathy with subcotical infarcts and leukoencephalopathy (Joutel et al. 1996). Since PKD2 may function as an intracellular calcium channel (Cai et al. 1999), calreticulin (gene CALR), which codes for a calcium-binding luminal endoplasmic-reticulum chaperone involved in intracellular calcium homeostasis and cell adhesion (Michalak et al. 1998), is considered a candidate. The receptor-associated Janus kinase (gene TYK2) has been implicated in growth hormone–receptor signaling and acute-phase response in the liver (Kim and Baumann 1997; Hellgren et al. 1999) and may also be a candidate if it is active in biliary epithelial cells. The transcription factor coded by NFI-X is expressed in liver (Chaudhry et al. 1997), and its pleiotropic functions may include hormone-responsive gene expression (reviewed in Gronostajski 2000). ICAM-1 and ICAM-3, the genes for intercellular adhesion molecules that are members of the immunoglobulin superfamily, map to this region, but no clear function in biliary epithelia has been attributed to these adhesion molecules. The remainder of the known genes in this region either have reported tissue distributions or putative functions (if known) that do not suggest them as strong positional candidates. At present, the most prudent course toward identification of PCLD remains a combination of refinement of the genetic interval and continued gene identification based on the genomic sequence of the region. The identification of PCLD will have an impact on the understanding of liver-cyst pathogenesis and may point the way toward nonsurgical therapy for patients affected with symptomatic liver disease.
Acknowledgments
We thank the family members for their generous participation in this study. We thank the Human Genome Program at the Albert Einstein College of Medicine and, in particular, Raju Kucherlapati and George Grills, for supporting our genome scan. We thank Ali Gharavi and Rick Lifton for helpful discussions, Carl James for programming support, and Patricia Urban for help with patient recruitment. This work was supported by National Institutes of Health grants DK51041 (to S.S. and V.E.T.) and GM29177 (to C.T.F.), Mayo Clinic General Clinical Research Center grant M01-RR00585, and Yale Liver Center Training Grant T32 DK07356 (to A.L.). A.L. and S.S. are members of the Yale Center for the Study of Polycystic Kidney Disease (grant P50 DK57328).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Chromosome 19–p Arm Metric Physical Map, http://greengenes.llnl.gov/genome-bin/loadmap?region=mp
- Genome Database, The, http://www.gdb.org (for Marshfield sex-averaged linkage map)
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/sets/Combo8Frames.htm
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for ADPKD [MIM 173900 and MIM 173910] and PCLD [MIM 174050])
References
- Bear JC, Parfrey PS, Morgan JM, Martin CJ, Cramer BC (1992) Autosomal dominant polycystic kidney disease: new information for genetic counseling. Am J Med Genet 43:548–553 [DOI] [PubMed] [Google Scholar]
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S (1999) Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274:28557–28565 [DOI] [PubMed] [Google Scholar]
- Caremani M, Vincenti A, Benci A, Sassoli S, Tacconi D (1993) Ecographic epidemiology of non-parasitic hepatic cysts. J Clin Ultrasound 21:115–118 [DOI] [PubMed] [Google Scholar]
- Chaudhry AZ, Lyons GE, Gronostajski RM (1997) Expression patterns of the four nuclear factor I genes during mouse embryogenesis indicate a potential role in development. Dev Dyn 208:313–325 [DOI] [PubMed] [Google Scholar]
- Comfort MW, Gray HK, Dahlin DC, Whitesell FB (1952) Polycystic disease of the liver: a study of 24 cases. Gastroenterology 20:60–78 [PubMed] [Google Scholar]
- Cottingham RW Jr, Idury RM, Schäffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- Emmons S, Somlo S (1999) Mating, channels and kidney cysts. Nature 401:339–340 [DOI] [PubMed] [Google Scholar]
- Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT (1990) Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 11:1033–1037 [DOI] [PubMed] [Google Scholar]
- Gaines PA, Sampson MA (1989) The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol 62:335–337 [DOI] [PubMed] [Google Scholar]
- Gronostajski RM (2000) Roles of the NFI/CTF gene family in transcription and development. Gene 249:31–45 [DOI] [PubMed] [Google Scholar]
- Grunfeld JP, Albouze G, Jungers P, Landais P, Dana A, Droz D, Moynot A, Lafforgue B, Boursztyn E, Franco D (1985) Liver changes and complications in adult polycystic kidney disease. Adv Nephrol Necker Hosp 14:1–20 [PubMed] [Google Scholar]
- Hellgren G, Jansson JO, Carlsson LM, Carlsson B (1999) The growth hormone receptor associates with Jak1, Jak2 and Tyk2 in human liver. Growth Horm IGF Res 9:212–218 [DOI] [PubMed] [Google Scholar]
- Huston J, III, Torres VE, Wiebers DO, Schievink WI (1996) Follow-up of intracranial aneurysms in autosomal dominant polycystic kidney disease by magnetic resonance angiography. J Am Soc Nephrol 7:2135–2141 [DOI] [PubMed] [Google Scholar]
- Iglesias DM, Palmitano JA, Arrizurieta E, Kornblihtt AR, Herrera M, Bernath V, Martin RS (1999) Isolated polycystic liver disease not linked to polycystic kidney disease 1 and 2. Dig Dis Sci 44:385–388 [DOI] [PubMed] [Google Scholar]
- Jodice C, Mantuano E, Veneziano L, Trettel F, Sabbadini G, Calandriello L, Francia A, Spadaro M, Pierelli F, Salvi F, Ophoff RA, Frants RR, Frontali M (1997) Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet 6:1973–1978 [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E (1996) Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383:707–710 [DOI] [PubMed] [Google Scholar]
- Karhunen PJ, Tenhu M (1986) Adult polycystic liver and kidney diseases are separate entities. Clin Genet 30:29–37 [DOI] [PubMed] [Google Scholar]
- Kim H, Baumann H (1997) Transmembrane domain of gp130 contributes to intracellular signal transduction in hepatic cells. J Biol Chem 272:30741–30747 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1985) Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 37:482–498 [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Choong CY, Sanfilippo AJ, Ferre M, Weyman AE (1989) Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N Engl J Med 320:1031–1036 [DOI] [PubMed] [Google Scholar]
- Melnick PJ (1955) Polycystic liver: analysis of seventy cases. Arch Pathol 59:162–172 [PubMed] [Google Scholar]
- Michalak M, Mariani P, Opas M (1998) Calreticulin, a multifunctional Ca2+ binding chaperone of the endoplasmic reticulum. Biochem Cell Biol 76:779–785 [DOI] [PubMed] [Google Scholar]
- Milutinovic J, Fialkow PJ, Rudd TG, Agodoa LY, Phillips LA, Bryant JI (1980) Liver cysts in patients with autosomal dominant polycystic kidney disease. Am J Med 68:741–744 [DOI] [PubMed] [Google Scholar]
- Nishimura RA, McGoon MD, Shub C, Miller FA Jr, Ilstrup DM, Tajik AJ (1985) Echocardiographically documented mitral-valve prolapse: long- term follow-up of 237 patients. N Engl J Med 313:1305–1309 [DOI] [PubMed] [Google Scholar]
- Pirson Y, Lannoy N, Peters D, Geubel A, Gigot JF, Breuning M, Verellen-Dumoulin C (1996) Isolated polycystic liver disease as a distinct genetic disease, unlinked to polycystic kidney disease 1 and polycystic kidney disease 2. Hepatology 23:249–252 [DOI] [PubMed] [Google Scholar]
- Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM (1994) Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343:824–827 [DOI] [PubMed] [Google Scholar]
- Schäffer AA, Gupta SK, Shriram K, Cottingham RW Jr (1994) Avoiding re-computation in linkage analysis. Hum Hered 44:225–237 [DOI] [PubMed] [Google Scholar]
- Sotaniemi EA, Luoma PV, Jarvensivu PV, Sotaniemi KA (1979) Impairment of drug metabolism in polycystic non-parasitic liver disease. Br J Clin Pharmacol 8:331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE (1996) Polycystic liver disease. In: Watson ML, Torres VE (eds) Polycystic kidney disease. Oxford University Press, Oxford, pp 500–529 [Google Scholar]