Abstract
Neuronal remodeling is a fundamental process by which the brain responds to environmental influences, e.g., during stress. In the hippocampus, chronic stress causes retraction of dendrites in CA3 pyramidal neurons. We have recently identified the glycoprotein M6a as a stress-responsive gene in the hippocampal formation. This gene is down-regulated in the hippocampus of both socially and physically stressed animals, and this effect can be reversed by antidepressant treatment. In the present work, we analyzed the biological function of the M6a protein. Immunohistochemistry showed that the M6a protein is abundant in all hippocampal subregions, and subcellular analysis in primary hippocampal neurons revealed its presence in membrane protrusions (filopodia/spines). Transfection experiments revealed that M6a overexpression induces neurite formation and increases filopodia density in hippocampal neurons. M6a knockdown with small interference RNA methodology showed that M6a low-expressing neurons display decreased filopodia number and a lower density of synaptophysin clusters. Taken together, our findings indicate that M6a plays an important role in neurite/filopodium outgrowth and synapse formation. Therefore, reduced M6a expression might be responsible for the morphological alterations found in the hippocampus of chronically stressed animals. Potential mechanisms that might explain the biological effects of M6a are discussed.
Keywords: chronic stress, hippocampus
The adult nervous system can be strongly influenced by sensory input from the outside environment. For example, prolonged exposure to adverse situations can have severe consequences for the brain, conferring susceptibility to certain psychiatric disorders. Indeed, chronic stress is one of the main factors known to trigger depression in humans (1). One of the most extensively studied regions in the brain is the hippocampal formation, which possesses a remarkable degree of plasticity and is particularly sensitive to stress. Studies in rodents and tree shrews demonstrated that chronic stress can cause alterations in neuronal processes. Stressed animals show morphological changes in CA3 pyramidal neurons, characterized by a reduction of apical dendritic branching and total dendritic length (2, 3). In addition, stressed rats also display a marked retraction of thorny excrescences and reduced synaptic density (4-6). Interestingly, antidepressant treatment can block these stress effects (7). Although there has been important progress regarding the understanding of the stress response and antidepressant action, the molecular pathways underlying these plastic alterations still remain largely unknown.
By using subtractive hybridization libraries, we have recently identified the gene encoding the glycoprotein M6a as a stress-responsive gene. Expression levels for M6a are decreased in hippocampal tissue of tree shrews subjected to chronic psychosocial stress, and this down-regulation is prevented by chronic administration of the antidepressant clomipramine (8). Moreover, mice exposed to chronic restraint stress also show a reduction in hippocampal M6a transcript levels, and this effect is counteracted by treatment with the antidepressant tianeptine (9). M6a is a transmembrane protein that belongs to the myelin proteolipid protein (PLP) family. PLP constitutes the most abundant protein of the CNS myelin, and it has been shown to be involved in signaling through integrins in oligodendrocytes (10). Although PLP and its splice variant DM20 are glial proteins, M6a is found only in neurons (11). Despite M6a identification years ago (12) and its prominent expression in the CNS, little is known about its biological function. A previous study reported that overexpression of M6a in rat pheochromocytoma PC12 cells results in increased responsiveness to nerve growth factor-induced differentiation (13). In the present study, we first examined endogenous expression of M6a and then analyzed the morphological effects of the overexpression and knockdown of M6a in hippocampal neurons as well as in cell lines. Our findings indicate that M6a plays an important role in neurite outgrowth and filopodium/spine formation, and it might also be involved in synapse formation. These results suggest that this protein could, at least in part, be responsible for the plastic alterations found in the hippocampus of stress/antidepressant-treated animals.
Materials and Methods
In Situ Hybridization. In situ hybridization experiments were performed as described (14). Briefly, frozen brains from adult male Wistar rats (2 months old; from Harlan Winkelmann, Borchen, Germany) were cut on a cryostat. Sections (10 μm) were thaw-mounted, dried at room temperature, fixed in 4% buffered paraformaldehyde, rinsed in PBS, dehydrated, air dried, and frozen at -80°C. Riboprobes were generated with either T7 or SP6 polymerase (Promega) for the antisense and sense probes, respectively, in the presence of 9.25 MBq of 33P-UTP [ICN; specific activity 3,000 Ci/mmol (1 Ci = 37 GBq)]. Hybridization was performed for 18 h at 43°C. Sections were subsequently washed and exposed to Bio-Max MR film (Amersham Pharmacia) for 4 days at 4°C.
Immunohistochemistry with Rat Hippocampal Sections. Animals were transcardially perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.2); brains were removed and immediately frozen in liquid nitrogen. Coronal cryosections (40 μm) were washed in 0.5% Triton X-100 in PBS, immersed for 1 h at room temperature (RT) in blocking solution (PBS containing 5% normal rabbit serum and 0.5% Triton X-100), incubated 48 h at 4°C with monoclonal anti-M6a rat IgG (Medical and Biological Laboratories, Tokyo, 1/1,000), and washed again. Sections were then incubated in blocking solution (5% normal rat serum and 0.5% Triton X-100 in PBS) for 1 h at RT, treated with secondary antibody (biotin-conjugated rabbit anti-rat IgG, 1/400) for 4 h at RT, then washed. The sections were then incubated with streptavidin-horseradish peroxidase (1/200) for 2 h at RT, washed, and stained with diaminobenzidine according to the manufacturer's instructions (DAB-Kit, Vector Laboratories). Sections were mounted with Eukitt mounting medium (Bodo Schmidt, Göttingen) and inspected with a light microscope (Axiophot 2, Zeiss).
Hippocampal Cultures and Cell Lines. Dissociated neuronal cultures were prepared from hippocampi of embryonic day 19 Wistar rats, as described (15). Briefly, tissue was treated with 0.25% trypsin in Hanks' solution for 15 min at 37°C. A single-cell solution was prepared in MEM containing 2 mM glutamine, 10 μM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin (MEM1X) with 10% (vol/vol) horse serum. Cells were seeded on plates or coverslips coated with 0.1 mg/ml poly(l-lysine) hydrobromide (Sigma) and 20 μg/μl laminin (GIBCO) at a density of 20,000 cells/cm2. After 2 h, medium was changed to MEM/N2 (MEM1X with 1 g/liter ovalbumin and B27 serum-free supplements from GIBCO). Mouse neuroblastoma N2a and COS-7 cells were cultured in DMEM with 10% (vol/vol) FBS, penicillin, and streptomycin. For rat pheochromocytoma PC12 cells, the above medium was supplemented with 5% horse serum and cells were plated in poly(l-lysine)-coated plates.
Immunocytochemistry and Membrane Labeling of Cultured Neurons. Neurons were fixed in 4% paraformaldehyde/4% sucrose in PBS for 20 min at room temperature, followed by permeabilization with 0.1% Triton X-100 in PBS for 2 min. Cultures were blocked with 2% BSA in PBS for 1-2 h followed by incubation with the primary antibody in 2% BSA at 4°C overnight and with secondary antibodies at 37°C for 1 h. Primary antibodies were: monoclonal anti-M6a rat IgG 1/250 (Medical and Biological Laboratories), monoclonal anti-α-tubulin mouse IgG 1/2,000 (Sigma), monoclonal anti-Tau-1 mouse IgG (kindly provided by L. I. Binder, Northwestern University, Chicago), monoclonal anti-MAP2 mouse IgG 1/200 (Sigma), anti-spinophilin rabbit antiserum 1/500 (USBiological, Swampscott, MA), and anti-synaptophysin rabbit antiserum 1/500 (Synaptic Systems, Göttingen, Germany). Secondary antibodies were conjugated to Alexa Fluor 488 1/1,000, Alexa Fluor 594 1/300, Alexa Fluor 350 1/200 (Molecular Probes), or rhodamine 1/2,000 (Pierce). F-actin was stained with rhodamine phalloidin 1/1,000 (Molecular Probes), together with secondary antibody incubation. To visualize membrane protrusions (filopodia/spines), neurons were labeled with the lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) (Molecular Probes) 1/1,000 in PBS at 37°C for 5 min, washed with PBS, and then fixed and mounted. Only neurons completely stained with identifiable filopodia were used for quantification. Coverslips were mounted in FluorSave Reagent (Calbiochem). Fluorescent images were acquired by using a Nikon E600 microscope equipped with epifluorescence illumination (Nikon) or a confocal laser scanning microscope LSM5 Pascal (Zeiss) with a ×63 lens or a ×100 oil-immersion lens.
Plasmid and Small Interference RNA (siRNA) Transfections. For transfection, three plasmids were used: one encoding enhanced GFP (EGFP-C1; Clontech), a GFP::M6a fusion protein containing the coding region of M6a fused in frame to the C terminus of GFP in the same vector and plasmid pIRES2-EGFP (Clontech) containing the coding region of M6a. Double-strand siRNA oligonucleotides were purchased from Dharmacon Research (Lafayette, CO). The sequences were designed in a region specific for M6a, not overlapping with other related genes (M6a target sequences 5′-AAG GAU GUG UGA AUC UAC UGA-3′ or 5′-AAC GGC UGC UUU CUU UGU CUA-3′ for first- and second-siRNA experiment). Control siRNA consisted of a mix of 21-bp RNA double strand from the GFP coding sequence. For transfection, either 3 μg of DNA or 40 pmol of siRNA was mixed with 1 μl of lipofectamine 2000 (Invitrogen) and added to each well (in a 24 wells per plate format), following the manufacturer's instructions (media were changed 4-6 h later).
Real-Time Quantitative PCR. Real-time PCR reactions were performed by using SYBR green PCR Core Reagents (PE Biosystems), as described (8). Briefly, cells were homogenized in Trizol reagent (Life Technologies, Rockville, MD), and total RNA was isolated. cDNA samples were synthesized from poly(A)+ RNA purified with PolyATract mRNA Isolation System (Promega). Total mRNA was transcribed with Superscript II (Life Technologies) enzyme, and PCRs were carried out in a GeneAmp 5700 Sequence Detection system (Applied Biosystems).
Quantification and Statistical Data Analysis. The number of primary neurites (stained with anti-α-tubulin antibodies) per cell was counted in 40-50 cells per group from three independent experiments. Filopodium/spine density (number of protrusions stained with DiI per 20-μm dendrite length measured within 50 μm from the soma) was quantified in 65-75 neurites of different neurons per group for overexpression cultures from three independent experiments and in 75-95 neurons per group for siRNA cultures from two independent experiments. The number of immunopositive synaptophysin puncta was counted in 20-μm neurite length measured from the neuronal body in 55-60 different neurons per group from two independent experiments. For cell lines, the percentage of cells showing filopodial protrusions was calculated from 200, 110, and 130 cells per group from multiple experiments for PC12, N2a, and COS-7, respectively. We first verified whether values for each experiment were normally distributed with a Shapiro-Wilk W test (95% confidence interval). Group means were then analyzed for overall statistical significance by using Student's t test or the nonparametric Mann-Whitney U test, two-tailed. Calculations were performed with the software analyse-it for Microsoft excel.
Results
M6a Transcripts Localize to Cell Bodies, and M6a Protein Is Targeted to Neuronal Processes. We first analyzed M6a expression in rat hippocampal tissue. In situ hybridization showed M6a mRNA expression in two types of hippocampal neurons: granule cells of the dentate gyrus and pyramidal neurons in CA1 and CA3 (Fig. 1A). Hybridization signals were confined specifically to regions containing granule and pyramidal cell bodies and were not detected elsewhere in the hippocampus. In contrast, the immunohistochemical detection of M6a revealed no expression in the somata of granule and pyramidal neurons. Instead, a strong immunoreactivity was found in the area of the mossy fiber terminals and in the hilus (Fig. 1B). Furthermore, a laminated pattern of strong immunoreactivity was detected in the molecular layer of the dentate gyrus, a region comprised predominantly of dendrites from granule cells of the dentate gyrus. Within CA1, stratum oriens and stratum radiatum were moderately labeled by the M6a antibody. Therefore, the immunocytochemical detection of M6a in the rat hippocampal formation indicates that the protein is targeted to the processes (axons and dendrites) of granule and pyramidal neurons.
Fig. 1.
Adult rat hippocampus. M6a mRNA localizes to somata of granule and pyramidal neurons, whereas M6a protein is detected in neuronal processes. M6a mRNA expression in the hippocampus as determined by in situ hybridization (A) was found to be localized to the granule cell layer (gcl) of the dentate gyrus and pyramidal neuron cell bodies (stratum pyramidale, pyr) of the CA fields (CA1, CA3). (Scale bar, 1 mm.) In contrast, immunocytochemical detection of M6a protein (B) revealed a distinct absence of M6a expression within the granule cell layer and cell bodies of pyramidal neurons (pyr). M6a expression was rather detected in regions of relatively dense synaptic contact, including the hilus (h), granule cell mossy fiber terminals (mf), and the molecular layer (ml) of the dentate gyrus. Moderate staining was also evident in the stratum oriens (or) and stratum radiatum (rad). (Scale bar, 0.5 mm.)
M6a Protein in Primary Hippocampal Neurons Is Localized to Plasma Membrane/Filopodia. To investigate the possible biological roles of M6a and the cellular consequences of M6a differential expression, we used primary cultures of embryonic rat hippocampal neurons. We first analyzed the subcellular localization of endogenous M6a protein by staining the cells with the monoclonal M6a antibody. These experiments revealed localization of M6a protein in the plasma membrane, along the neurites and in filopodia, structures that were also visualized with the F-actin marker phalloidin (Fig. 2 A and B). Primary cultures were transfected with an eukaryotic expression vector that encodes a fusion protein consisting of GFP and M6a. The GFP::M6a fusion protein was found to be targeted to the plasma membrane and processes of the cells, both dendrites and axons (costained with antibodies against MAP2 and Tau-1, respectively) and was enriched in filopodia/spines (Fig. 2C). A punctuate M6a pattern was observed, although high-expression spots only partially colocalize with either pre- or postsynaptic markers such as synaptophysin and spinophilin, respectively (see Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 2.
Localization in cultured neurons: both M6a endogenous protein and GFP::M6a fusion protein are present in membrane and filopodia. (A) Immunocytochemical staining of cultured neurons revealed that M6a protein (green) is localized to somatic membrane, along processes and filopodia (B). Staining with the actin marker phalloidin (red) revealed filopodial structures. (C) Hippocampal cultures were transfected with GFP::M6a construct and overexpressed M6a (green) is also sorted to processes, both to dendrites (a) and axons (b) as confirmed with the respective protein markers MAP2 and Tau-1 (red). Fusion protein is restricted to plasma membrane, as shown in a single-frame picture (0.37 μm slice) of the soma (c) and highly enriched in filopodia (d). (Scale bars, 10 μm.)
M6a Overexpression Increases Neurite Number and Filopodium/Spine Density. As a first step toward establishing the function of M6a, we performed a gain-of-function approach by overexpressing M6a in hippocampal neurons. Immunocytochemical staining with antibodies against M6a showed higher immunoreactivity in GFP::M6a-transfected than in nontransfected cells (not shown). We then examined the effect of M6a overexpression on the morphology of the neurons. Cells were transfected with GFP::M6a or with GFP (control) constructs 1 day after plating and fixed 2-3 days later. Cultures were stained with antibodies against α-tubulin (Fig. 3A), and the number of primary neurites per cell was determined for both experimental groups. Fig. 3B shows that GFP::M6a-transfected neurons have a significantly higher number of neurites as compared with control cells. Therefore, M6a overexpression promotes the formation of primary neurites in neurons.
Fig. 3.
M6a overexpression induces neurite outgrowth and increases filopodium/spine density. Neurons were transfected 1 day after plating with either GFP (control, A) or GFP::M6a constructs (A′), fixed, and stained with antibodies against α-tubulin 2-3 days later. Primary neurites per cell were quantified and mean values +SEM of 40-50 cells per group from three independent experiments are shown in B. Cultures from 7 days in vitro were transfected with GFP::M6a and stained with the membrane dye DiI 2-3 days later. (C and D) Control. (C′ and D′) M6a overexpression. Filopodium/spine density (number of protrusions per 20-μm dendrite length) as shown in D and D′ was quantified and mean +SEM of 65-75 neurons per group from three independent experiments are shown in E as a percentage of control. **, P < 0.001, Mann-Whitney U test, two-tailed. (Scale bars, 10 μm.)
We then analyzed the morphological effects of exogenous M6a in neurons from cultures of 9-10 days, a stage in which active synapses begin to appear (16). At this time in vitro, cultured neurons contain both spine- and filopodium-like structures. Initially, cells have numerous transient and dynamic filopodia, whereas later dendritic protrusions form mostly stable spines. These observations led to the proposal that filopodia are the precursors of spines (17). Because at the stage of ≈10 days in culture it is difficult to distinguish between filopodia and spines based on fluorescence microscope investigation, we categorized every dendritic protrusion from 1 to 5 μm in length as filopodium/spine. Cultured cells were transfected at day 7 and fixed 2-3 days later after membrane staining with DiI. Quantification of filopodium/spine density (number of protrusions in 20-μm dendrite length) revealed that M6a-transfected neurons showed a dramatic increase in the number of membrane protrusions compared with control cells (Fig. 3 C-E). The distribution of the M6a-induced protrusions was uniform along neurites within 50 μm from the cell body, because no difference in filopodium/spine density was observed between segments at 0 and 30 μm from the soma (9.8 ± 2.5 and 9.3 ± 2.8 filopodia, respectively; n = 52, P = 0.49, Mann-Whitney U test). Thus, high M6a levels induce the formation of filopodia/spines in cultured neurons at the time of onset of synaptogenesis. However, viability of GFP::M6a-expressing cells was compromised ≈1 week after transfection, indicating that M6a overexpression may be deleterious in the long run.
We next performed a characterization of M6a-induced membrane protrusions in cultures of 9-10 days by using several well known markers. Results showed that these structures develop from dendrites that are stained with antibodies against MAP2 (Fig. 4A), contain an F-actin cytoskeleton (Fig. 4B), and show labeling for spinophilin (Fig. 4C). Presynaptic axons (stained with antibodies against Tau-1 in Fig. 4D) contact and run along these membrane protrusions. It has been established that synapse formation for dissociated neurons in culture strongly correlates with focal accumulations of structures labeled with antibodies against synaptic vesicle proteins such as synaptophysin (16). Therefore, synaptophysin-labeled clusters over passing axons that contact M6a-induced filopodia/spines (Fig. 4 E and E′) presumably indicate synaptic sites.
Fig. 4.
Characterization of membrane protrusions induced by M6a overexpression. Neurons between 7 and 9 days in vitro were transfected with GFP::M6a, and fixed 1 or 2 days later. M6a-induced protrusions (green) are observed in dendrites stained with MAP2 (A), are rich in F-actin (B), and contain spinophilin clusters (C). Passing axons, stained with Tau-1, appear to make contact with M6a-promoted protrusions (D). Synaptophysin clusters are observed in contact sites between axons and dendritic protrusions of M6a overexpressing neurons (E). E′ shows higher magnifications of the boxes indicated in E; the arrow points out an example of presynaptic synaptophysin clusters over dendritic filopodia. (Scale bars, 10 μm.)
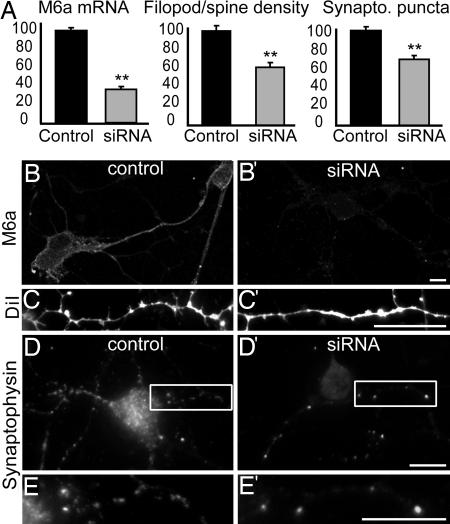
M6a Knockdown Impairs Filopodium/Spine Formation and Reduces the Number of Synaptic Clusters. To further demonstrate the involvement of M6a in filopodium/spine formation, a loss-of-function experiment was performed. We used siRNA to deplete endogenous M6a. A single siRNA administration was enough to lower down M6a mRNA levels to ≈40%, measured with real-time RT-PCR 72 h after siRNA delivery. Control cells were transfected with an unrelated sequence (Fig. 5A). To maintain low M6a expression levels during 9-10 days, we treated cell cultures every 2-3 days with siRNA. The amount of M6a protein was clearly reduced in these neurons, as determined by immunocytochemical staining with antiM6a antibodies (Fig. 5 B and B′). Subsequently, morphological consequences of siRNA administration were analyzed. Neither M6a nor control siRNA-treated cells showed any obvious alteration in the development and survival through 9-10 days in culture (Fig. 7, which is published as supporting information on the PNAS web site). However, membrane labeling with DiI and quantification of protrusions on the dendrites in a blind analysis revealed a marked decrease in filopodium/spine number in M6a low expressing neurons, demonstrating that low M6a levels result in reduced filopodium density (Fig. 5 A, C, and C′). In addition, a second experiment by using a different M6a target sequence for siRNA showed essentially the same results: filopodium density for M6a siRNA treated cells was 68 ± 7% of control (P < 0.005, n = 70, Mann-Whitney U test, two-tailed).
Fig. 5.
RNA interference reduces M6a mRNA and protein levels, resulting in a lower number of filopodia/spines and synaptophysin immunoreactive clusters. (A) Cultures from 9-10 days in vitro were siRNA-treated with M6a or control sequences. M6a siRNA-treated cultures showed lower M6a transcript levels (measured with real-time RT-PCR, values normalized with the gene β-actin), lower filopodium/spine density (number of protrusions per 20-μm dendritic length, quantified in 75-95 neurons per group from two independent experiments), and lower number of synaptophysin immunopositive puncta (counted in a 20-μm fragment of dendrites measured from the neuronal body for 55-60 cells per group from two independent experiments). Mean values +SEM are expressed as a percentage of control. Representative pictures of control (B-E) and M6a siRNA-treated cells (B′-E′) stained with the antiM6a antibody (B and B′), membrane-labeled with DiI (C and C′) or immunostained with the antibody against synaptophysin (D and E). E and E′ are higher magnifications of the areas indicated in D and D′. Significant differences: **, P < 0.0001, Mann-Whitney U test, two-tailed. (Scale bars, 10 μm.)
Several studies have demonstrated that dendritic filopodia are capable of participating in synapses (17, 18), and filopodia formation has been suggested to play a permissive role in synaptogenesis (19). Therefore, we analyzed whether M6a down-regulation affects synaptic density. We determined the number of synaptophysin fluorescent puncta per 20-μm dendritic length in control and M6a siRNA-treated neurons after 9 days in vitro. Results showed that neurons with reduced M6a protein expression display significantly lower density of presynaptic clusters over their dendrites than control cells (Fig. 5 A, D, D′, E, and E′). Thus, M6a down-regulation causes not only decreased filopodium density but also reduced synaptic density in hippocampal neurons.
M6a Overexpression Induces Filopodium Formation in Neuronal and Nonneuronal Cell Lines. To analyze whether the relationship between M6a expression and formation of membrane protrusions is restricted to primary hippocampal neurons, we analyzed different cell lines. The neuronal cells PC12 and N2a cells show high filopodium density along the somata and processes after differentiation. However, among undifferentiated cells, there are cells with or without filopodia. Overexpression of M6a increased the percentage of undifferentiated cells with filopodia 1- to 2-fold in both cell lines (Fig. 8, which is published as supporting information on the PNAS web site). For the nonneuronal COS-7 cells, quantification of filopodia-bearing cells revealed that M6a overexpression promotes filopodium formation also in these cells (Fig. 8). Visualization of GFP::M6a in transfected cells showed that the fusion protein was localized to the membrane and highly enriched in filopodia. Therefore, exogenous expression of M6a can promote filopodium formation in diverse cellular systems besides primary hippocampal neurons. Overexpression of M6a without the GFP tag resulted in similar filopodia outgrowth effect (Fig. 9, which is published as supporting information on the PNAS web site).
Discussion
We recently found that chronic stress, which causes retraction of dendrites in hippocampal neurons, simultaneously induces a decrease in hippocampal mRNA levels for the neuron-specific membrane protein M6a (8, 9). We now provide evidence that differential expression of M6a is biologically relevant, because M6a is critically involved in promoting neuronal processes. The number of both neurites and filopodium/spine-like protrusions in hippocampal neurons as well as in different cell lines is modulated by levels of M6a expression. In addition, synaptic cluster density is also affected by M6a expression in hippocampal neurons. These results lead to the conclusion that reduced expression of M6a could contribute to the neuroplastic changes in hippocampal neurons of chronically stressed animals.
M6a Protein Is Targeted to the Neurites in Neurons of the Adult Rat Hippocampus. The present data show that M6a protein in hippocampal neurons is targeted to the processes distal to the somata. This coincides with the observation that in sections from the rat hippocampus, the M6a immunoreactivity is present in mossy fiber terminals and in the hilus, which is innervated by granule cell axon collaterals, suggesting that translated M6a protein is transported anterogradely to distal portions of the granule cell axons (20). Strong M6a immunoreactivity was detected in the inner third of the dentate gyrus molecular layer, a region thought to be innervated solely by the hilus (21). However, no in situ hybridization signal was detected in the hilus. Therefore, it remains unclear which neuronal projections give rise to the strong M6a immunoreactivity detected in the inner third of the molecular layer.
M6a Is Involved in Neurite Outgrowth and Filopodium/Spine Dynamics and Could Modulate Synaptic Formation. Previous reports have suggested that M6a protein could play a role in neurite outgrowth/extension, although this hypothesis arose from indirect evidence using antibodies against M6a in cultured cerebellar neurons (12) and on PC12 cells (13). Here we show that overexpression of M6a in developing hippocampal neurons stimulates the formation of neurites. Based on these results, it is possible that this protein might be relevant for hippocampal development.
Neurons in the stage of synaptogenesis expressing high levels of M6a showed enhanced filopodium/spine density along their processes, whereas neurons expressing low levels of M6a displayed reduced number of these protrusions. These complementary results suggest a critical role for M6a in filopodium/spine turnover. Dendritic filopodia are highly active during development, and the number of such motile protrusions is inversely correlated with the appearance of more stable dendritic spines and synapses (17, 22, 23). These observations have led to the proposal that dendritic filopodia initiate synapse formation via their extension toward the axon terminals and subsequent stabilization of the resulting contacts (19). In agreement with this model, we found reduced synaptophysin immunoreactivity and lower synaptic cluster density in cultures with decreased M6a levels.
Possible Mechanisms of M6a-Induced Neurite and Filopodium Outgrowth. Despite the fact that PLP family proteins have been studied for >20 years, there is still a lack of information regarding their interaction with other cellular components or signaling pathways. The M6a gene encodes a 278-aa protein that contains four putative transmembrane domains with both N and C termini facing the cytosol. One of the two extracellular loops has two potential N-linked glycosylation sites, and within the intracellular domains, there are two putative phosporylation sites for protein kinase C and one site for casein kinase 2. The presumptive conformation of M6a protein suggests that its extracellular domains could interact with external ligands, which is also supported by the fact that incubation of live cultured neurons with antibodies against M6a interferes with neurite extension (12). M6a might also be interacting with other membrane proteins, as was found, for example, for the related protein PLP, which was shown to form a complex with integrins in oligodendrocytes (10). A previous work (13) has suggested that M6a could act as a Ca2+ channel in PC12 cells. If this is the case, changes in M6a levels could result in different intracellular calcium ion concentrations leading to the activation of molecular pathways downstream to this second messenger. Alterations in [Ca2+]i have been shown to be involved in the regulation of neurite extension and filopodium/spine activity (24, 25), indicating that M6a could be acting through this pathway. The putative phosporylation sites in the cytosolic domains may provide a means for M6a regulation and could be relevant for intracellular signaling. In support of this view, treatment of PC12 cells with PKC inhibitors has been shown to abolish M6a effect on nerve growth factor-primed increment of Ca2+ (13).
The growth of dendrites, filopodia, and spines is shaped by cell adhesion interactions and the protrusive forces of actin polymerization (26). Several studies have shown that proteins known to affect actin cytoskeletal structure and dynamics can alter dendrite morphology, spine density, and synapse formation (27-31). Although our results suggest that M6a is not directly linked to the actin cytoskeleton (see Fig. 10, which is published as supporting information), it would be interesting to explore the possibility that M6a interacts with other protein(s) involved in the dynamics of actin assembly. That M6a promotes filopodium formation in other systems, including nonneuronal COS-7 cells (this work), suggests that M6a effects on membrane protrusions might be acting through common signaling processes that can be found in diverse cell types. In this sense, the Rho family of small GTPases (Rac, Cdc42, and Rho) has been demonstrated to play critical roles in the regulation of the cytoskeleton required for filopodium or neurite extension and retraction in a wide variety of cells (32, 33). However, no activation or inhibition of Rac1, Cdc42, or RhoA by M6a could be detected in N2a and COS-7 transfected cells (results not shown).
Potential Implication of M6a for Stress-Induced Remodeling of Neurons in the Hippocampus. Because stress is one of the main factors known to participate in the etiology of depression in humans, experimental paradigms that use chronic stress in animals are widely used to study this illness (34-36). Studies in humans showing there is a selective reduction in the volume of the hippocampal formation in depressive patients have led to the hypothesis that neuronal remodeling may play a role in the symptomatology and treatment of this psychiatric illness (36-38). In agreement with this view, changes in neurite remodeling induced by chronic stress can be restored with antidepressant treatment (39). Reduction in synaptic density has also been reported in the hippocampus of stressed animals (4, 5). Accordingly, M6a expression is down-regulated in the hippocampus of stressed animals, and this effect is reversed by chronic treatment with antidepressants (8, 9). By using siRNA technology, we could mimic in cultured hippocampal neurons the stress effect on M6a expression. These experiments demonstrated that a reduction in M6a expression to an extent comparable to that found in stressed animals is enough to produce physiological consequences in the cell. Indeed, decreased M6a mRNA and therefore protein amounts result in a reduction in filopodium/spine density and in the number of synaptic cluster. Thus, it is possible that differential expression of M6a could contribute to the morphological effects found in the hippocampus of stressed animals.
Dendritic spines in the adult brain are dynamic structures that can respond to various external stimuli such as memory training and stress (40, 41). Therefore, formation and elimination of dendritic spines appear to be mechanisms by which neuronal connections can be remodeled (42). The resulting cellular alterations are thought to influence brain functions, e.g., changes in spine dynamics have been proposed to play a role in psychiatric disorders (43). In the present study, we show that a gene whose expression levels are susceptible to stress conditions is involved in filopodium/spine formation. Taken together, our observations suggest that M6a is a good candidate gene to be considered in stress-related disorders such as depression.
Supplementary Material
Acknowledgments
We thank A. F. Schinder, M. Brocco, and all members of our laboratory for extensive discussion; L. Centanin for technical assistance; L. I. Binder for providing anti-Tau-1 antibody; and P. Wappner for confocal microscope. We also thank E. Fuchs for initial collaboration on this research line. The work of A.C.F. was supported in part by an International Research Scholars Grant from the Howard Hughes Medical Institute and by the National Research Council (CONICET, Buenos Aires). J.A. and M.E.F. are research fellows, and A.C.F is a researcher, from CONICET. The study was also supported by the Deutsche Forschungsgemeinschaft through the Deutsche Forschungsgemeinschaft Research Center Molecular Physiology of the Brain (CMPB), University of Göttingen.
Author contributions: J.A., M.E.F., B.C., G.F., and A.C.F. designed research; J.A., M.E.F., and B.C. performed research; J.A., M.E.F., B.C., G.F., and A.C.F. analyzed data; and J.A. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PLP, proteolipid protein; siRNA, small interference RNA; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine.
References
- 1.Kendler, K. S., Karkowski, L. M. & Prescott, C. A. (1999) Am. J. Psychiatry 156, 837-841. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe, Y., Gould, E. & McEwen, B. S. (1992) Brain Res. 588, 341-345. [DOI] [PubMed] [Google Scholar]
- 3.Magarinos, A. M., McEwen, B. S., Flugge, G. & Fuchs, E. (1996) J. Neurosci. 16, 3534-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandi, C., Davies, H. A., Cordero, M. I., Rodriguez, J. J., Popov, V. I. & Stewart, M. G. (2003) Eur. J. Neurosci. 17, 2447-2456. [DOI] [PubMed] [Google Scholar]
- 5.Sousa, N., Lukoyanov, N. V., Madeira, M. D., Almeida, O. F. & Paula-Barbosa, M. M. (2000) Neuroscience 97, 253-266. [DOI] [PubMed] [Google Scholar]
- 6.Stewart, M. G., Davies, H. A., Sandi, C., Kraev, I. V., Rogachevsky, V. V., Peddie, C. J., Rodriguez, J. J., Cordero, M. I., Donohue, H. S., Gabbott, P. L., et al. (2005) Neuroscience 131, 43-54. [DOI] [PubMed] [Google Scholar]
- 7.McEwen, B. S. (1999) Annu. Rev. Neurosci. 22, 105-122. [DOI] [PubMed] [Google Scholar]
- 8.Alfonso, J., Pollevick, G. D., Van Der Hart, M. G., Flugge, G., Fuchs, E. & Frasch, A. C. (2004) Eur. J. Neurosci. 19, 659-666. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso, J., Frick, L., Silberman, D., Palumbo, M., Genaro, A. & Frasch, A. C. (2005) Biol. Psychiatry, in press. [DOI] [PubMed]
- 10.Gudz, T. I., Schneider, T. E., Haas, T. A. & Macklin, W. B. (2002) J. Neurosci. 22, 7398-7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan, Y., Lagenaur, C. & Narayanan, V. (1993) Neuron 11, 423-431. [DOI] [PubMed] [Google Scholar]
- 12.Lagenaur, C., Kunemund, V., Fischer, G., Fushiki, S. & Schachner, M. (1992) J. Neurobiol. 23, 71-88. [DOI] [PubMed] [Google Scholar]
- 13.Mukobata, S., Hibino, T., Sugiyama, A., Urano, Y., Inatomi, A., Kanai, Y., Endo, H. & Tashiro, F. (2002) Biochem. Biophys. Res. Commun. 297, 722-728. [DOI] [PubMed] [Google Scholar]
- 14.Meyer, H., Palchaudhuri, M., Scheinin, M. & Flugge, G. (2000) Brain Res. 880, 147-158. [DOI] [PubMed] [Google Scholar]
- 15.Brocco, M., Pollevick, G. D. & Frasch, A. C. (2003) J. Neurosci. Res. 74, 744-753. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher, T. L., Cameron, P., De Camilli, P. & Banker, G. (1991) J. Neurosci. 11, 1617-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziv, N. E. & Smith, S. J. (1996) Neuron 17, 91-102. [DOI] [PubMed] [Google Scholar]
- 18.Fiala, J. C., Feinberg, M., Popov, V. & Harris, K. M. (1998) J. Neurosci. 18, 8900-8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goda, Y. & Davis, G. W. (2003) Neuron 40, 243-264. [DOI] [PubMed] [Google Scholar]
- 20.Roussel, G., Trifilieff, E., Lagenaur, C. & Nussbaum, J. L. (1998) J. Neurocytol. 27, 695-703. [DOI] [PubMed] [Google Scholar]
- 21.Swanson, L. W., Köhler, C. & Björklund, A. (1981) in Handbook of Chemical Neuroanatomy, eds. Björklund, A., Hökfelt, T. & Swanson, L. W. (Elsevier, Amsterdam), Vol. 5, pp. 125-227. [Google Scholar]
- 22.Dunaevsky, A., Tashiro, A., Majewska, A., Mason, C. & Yuste, R. (1999) Proc. Natl. Acad. Sci. USA 96, 13438-13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okabe, S., Miwa, A. & Okado, H. (2001) J. Neurosci. 21, 6105-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikonenko, I., Jourdain, P., Alberi, S., Toni, N. & Muller, D. (2002) Hippocampus 12, 585-591. [DOI] [PubMed] [Google Scholar]
- 25.Lau, P. M., Zucker, R. S. & Bentley, D. (1999) J. Cell Biol. 145, 1265-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo, L. (2002) Annu. Rev. Cell Dev. Biol. 18, 601-635. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama, A. Y. & Luo, L. (2000) Hippocampus 10, 582-586. [DOI] [PubMed] [Google Scholar]
- 28.Meng, Y., Zhang, Y., Tregoubov, V., Falls, D. L. & Jia, Z. (2003) Rev. Neurosci. 14, 233-240. [DOI] [PubMed] [Google Scholar]
- 29.Penzes, P., Beeser, A., Chernoff, J., Schiller, M. R., Eipper, B. A., Mains, R. E. & Huganir, R. L. (2003) Neuron 37, 263-274. [DOI] [PubMed] [Google Scholar]
- 30.Zito, K., Knott, G., Shepherd, G. M., Shenolikar, S. & Svoboda, K. (2004) Neuron 44, 321-334. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, W. & Benson, D. L. (2001) J. Neurosci. 21, 5169-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall, A. (1998) Science 279, 509-514. [DOI] [PubMed] [Google Scholar]
- 33.Luo, L. (2000) Nat. Rev. Neurosci. 1, 173-180. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs, E. & Flugge, G. (2002) Pharmacol. Biochem. Behav. 73, 247-258. [DOI] [PubMed] [Google Scholar]
- 35.Willner, P. (1997) Psychopharmacology 134, 319-329. [DOI] [PubMed] [Google Scholar]
- 36.McEwen, B. S. & Magarinos, A. M. (2001) Hum. Psychopharmacol. 16, S7-S19. [DOI] [PubMed] [Google Scholar]
- 37.Duman, R. S., Heninger, G. R. & Nestler, E. J. (1997) Arch. Gen. Psychiatry 54, 597-606. [DOI] [PubMed] [Google Scholar]
- 38.Castren, E. (2004) Curr. Opin. Pharmacol. 4, 58-64. [DOI] [PubMed] [Google Scholar]
- 39.McEwen, B. S. (2000) Biol. Psychiatry 48, 721-731. [DOI] [PubMed] [Google Scholar]
- 40.Shors, T. J., Chua, C. & Falduto, J. (2001) J. Neurosci. 21, 6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leuner, B., Falduto, J. & Shors, T. J. (2003) J. Neurosci. 23, 659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chklovskii, D. B., Mel, B. W. & Svoboda, K. (2004) Nature 431, 782-788. [DOI] [PubMed] [Google Scholar]
- 43.Halpain, S., Spencer, K. & Graber, S. (2004) Prog. Brain Res. 147, 29-37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.