Abstract
MicroRNAs (miRNAs) repress translation of target mRNAs by interaction with partially mismatched sequences in their 3′ UTR. The mechanism by which they act on translation has remained largely obscure. We examined the translation of mRNAs containing four partially mismatched miRNA-binding sites in the 3′ UTR in HeLa cells cotransfected with a cognate miRNA. The mRNAs were prepared by in vitro transcription and were engineered to employ different modes of translation initiation. We find that the 5′ cap structure and the 3′ poly(A) tail are each necessary but not sufficient for full miRNA-mediated repression of mRNA translation. Replacing the cap structure with an internal ribosome entry site from either the cricket paralysis virus or the encephalomyocarditis virus impairs miRNA-mediated repression. Collectively, these results demonstrate that miRNAs interfere with the initiation step of translation and implicate the cap-binding protein eukaryotic initiation factor 4E as a molecular target.
Keywords: mRNA translation, cap structure, processing bodies, mRNA decay, short interfering RNA
MicroRNAs (miRNAs) are ≈22-nucleotide RNAs derived from larger hairpin-forming precursors; they assemble into RNA-protein complexes and function as guide molecules to effect posttranscriptional regulation of gene expression. Hundreds of miRNAs have been identified in metazoa and plants, and many are phylogenetically conserved (1-5). Investigation of a small number of individual miRNAs and their targets indicates that they have a role in a variety of cellular and developmental pathways (1, 2, 4, 6-8). Bioinformatic analyses predict multiple mRNA targets for each miRNA, suggesting that substantial portions of the transcriptome are under the combinatorial control of cellular miRNA networks (9-13).
In a model that has come to be widely accepted, binding of a miRNA to an mRNA either triggers mRNA cleavage and decay or leads to inhibition of translation, predominantly without degrading the mRNA (1-4, 14). Endonucleolytic cleavage of the mRNA requires near perfect complementarity between miRNA and target but also the presence of a catalytically competent Argonaute core subunit in the miRNA-protein complex. For instance, of the four characterized human Ago proteins, only hAgo2 appears to be competent for catalytic cleavage of the mRNA (15, 16). In plants, miRNA-target interactions are often within the coding region and nearly perfectly complementary, which triggers mRNA cleavage. By contrast, animal miRNA/target duplexes generally are interrupted by gaps and mismatches and occur in the 3′UTR of mRNAs. Pioneering genetic studies in Caenorhabditis elegans identified two miRNAs, lin-4 and let-7, as regulators of developmental timing (4, 17). lin-4 acts on lin-14 and lin-28, and let-7 on lin-41, hbl-1, and let-60 (7); both do so by interaction with partially mismatched target sequences in the 3′ UTRs of the regulated mRNAs. Because the levels of lin-14 and lin-28 mRNA were only marginally affected by lin-4 regulation in the original studies (18, 19), it was concluded that the miRNA in some way inhibited mRNA translation. Experiments with engineered miRNA/mRNA pairings have since further supported and characterized the relationship among base complementarity, mRNA cleavage, and translational repression as outlined above (20-25). Furthermore, miRNAs can also regulate mRNA expression in other ways, as evidenced by a role for miR-16 in mRNA turnover mediated by an AU-rich element (26) or a likely role for miR-122 in facilitating hepatitis C virus replication (27).
Mechanisms to control translation frequently affect the initiation step, although regulation of later stages such as elongation, termination, or the release of the stable polypeptide has also been reported (28-30). Native complexes involving C. elegans lin-14 and lin-28 mRNAs were found to sediment into the polysomal region of density gradients, even when isolated from larval stages where protein expression is repressed by the miRNAs (18, 19). EDTA sensitivity of these complexes and the observation that they disassembled when added to a heterologous translation reaction led to the expectation that miRNAs affect a step after translation initiation (1, 2, 4, 30). Recently, it was shown that mRNAs that are translationally repressed by miRNAs collect within or adjacent to processing (P-) bodies (25, 31, 32). P-bodies are large cytoplasmic aggregates that serve as sites of mRNA degradation. They are also known to contain translationally masked mRNA, and a general block of translation elongation in cells leads to disassembly of P-bodies (33, 34), whereas a block in translation initiation results in increased P-body formation (34). The detailed mechanism by which miRNAs trigger sequestration of their mRNA targets in the P-bodies is not known. The spatial proximity of the repressed mRNA with sites of general mRNA degradation suggests that miRNA effects on the stability of a partially complementary target mRNA may be more pronounced or common than previously anticipated. A recent report (35) further highlights this issue by presenting evidence that, in contrast to previous reports (18, 19), lin-4 or let-7-mediated regulation of lin-14, lin-28, and lin-41 mRNAs in C. elegans leads to significant decreases in mRNA level by a pathway requiring a 5′ to 3′ exonuclease activity.
We set out to determine what stage of translation (initiation/postinitiation) is affected by a miRNA. We provide evidence that a miRNA can affect translation initiation by inhibiting the roles of the mRNA cap structure and poly(A) tail, in the absence of accelerated mRNA decay. Our results furthermore indicate that within the initiation mechanism, the function of the cap-binding protein eukaryotic initiation factor (eIF) 4E is a molecular target.
Materials and Methods
DNA Constructs and miRNAs. The plasmids pRL-TK-4 sites or -0 sites, were gifts from J. Doench and P. A. Sharp (Massachusetts Institute of Technology, Cambridge, MA) (20, 21). Inserts for the plasmids pCrPVIG-RL-TK-4 sites and pEMCV-RL-TK-4 sites were created by PCR and ligated into the NheI and BamHI sites of pRL-TK-4 sites as detailed in Supporting Experimental Procedures, which is published as supporting information on the PNAS web site. The CXCR4 miRNA duplex was as described in refs. 20 and 21; the siCXCR4 duplex varied in the central bulge region to create perfect base-pairing matches with the targets. Sequences of the sense and antisense strands of the let-7 miRNA duplex were UGAGGUAGUA GGUUGUAUAG U and UAUCCAACCU ACUACCUCAG U, respectively. Synthetic RNAs (Dharmacon Research) were deprotected and annealed according to the manufacturer's instructions.
Synthesis of mRNA Transcripts. Plasmids pRL-TK-4 sites or -0 sites, pEMCV-RL-TK-4 sites, pCrPVIG-RL-TK-4 sites, or pT3luc (36) were linearized with BamHI, purified by agarose gel electrophoresis and the QiaexII kit (Qiagen), and served as templates for in vitro transcription reactions by using either the T7 or T3 MEGAscript kit (Ambion). All transcripts were capped, either with m7G(5′)ppp(5′)G or A(5′)ppp(5′)G (New England Biolabs). If required, the Poly(A) Tailing Kit (Ambion) was used to add a ≥150 nucleotide poly(A) tail to transcripts. mRNAs were purified with the MEGAclear kit (Ambion), and concentrations were estimated by A260. mRNA quality was inspected by denaturing agarose gel electrophoresis and by using the RNA 6000 Nano labchip kit on an Agilent 2100 bioanalyzer.
Cell Culture, mRNA Transfections, and Dual Luciferase Assays. HeLa cells were maintained in DMEM with 5% FCS, supplemented with glutamine and penicillin/streptomycin. Eight hours before transfection, cells were seeded in a 24-well plate. mRNA transfections were performed in triplicate at ≈60-70% confluency by adding a preincubated (30 min, room temperature) transfection solution (200 μl) and 300 μl of Opti-MEM I (Invitrogen) to each well. Unless otherwise stated, the 200 μl of transfection solution (in Opti-MEM I) contained 1 μg of Lipofectamine 2000, (Invitrogen), 20 ng of Renilla luciferase (R-luc)-encoding mRNA, 80 ng of F-luc-encoding mRNA (control), and miRNA at 2 nM, and cells were harvested 16 h after transfection. The Dual-Luciferase Reporter Assay system (Promega) was used for cell lysis (90 μl of Passive Lysis Buffer per well) and luciferase assays, in which 50 μl of each substrate was added to 45 μl of cell lysate and measured in a BMG FLUOstar Optima plate reader (BMG Labtech). After background subtraction, R-luc measurements were normalized against the corresponding F-luc value to correct for transfection efficiency before further data processing as detailed in the figure legends. The levels of “raw” R-luc activity varied systematically in different experimental contexts but were well above background levels measured with extracts from cells that had been mock transfected with a transfection mix containing only the F-luc-encoding control mRNA. Approximate levels of arbitrary light units (ALU) seen in typical transfections without a miRNA were as follows: mock transfection, ≈102 ALU; CrPVwt, A-cap, or A-cap&tail, ≈105 ALU; encephalomyocarditis virus (EMCV) and EMCV&tail, ≈106 ALU; cap and cap&tail, ≈107 ALU.
RNA Analysis. HeLa cells were transfected in six-well plates by scaling up the 24-well protocol by a factor of 5. Total RNA was collected by using TRIzol (Invitrogen).
Dual-probe ribonuclease protection assays (RPAs) were performed on 1 or 5 μg of total RNA with the RPA III kit (Ambion; digestion was with RNaseT1 only after 1 in 10 dilution). Probes for R-luc and F-luc mRNA were transcribed by T7 RNA polymerase in the presence of α-32P-UTP (Amersham Pharmacia Biosciences) by using the Riboprobe In Vitro Transcription System (Promega). Reactions were resolved by denaturing PAGE. Construction and linearization of plasmid templates was as detailed in Supporting Experimental Procedures.
For RT-PCR analysis, 1 μg of total RNA was reverse transcribed with AMV reverse transcriptase (Promega) by using oligo dT priming. PCR was performed by using primers that spanned the CXCR4 target sites in R-luc-4 sites mRNA or a region of the F-luc control transcript (see Supporting Experimental Procedures). Dilutions of cDNA and cycle conditions were chosen so that PCR products were analyzed in the subsaturating range of the amplification process. Products were resolved on 1.5% agarose gels stained with SYBR Green.
Gels were analyzed by phosphorimaging or fluorescence scan with a FLA-5100 imager and multigauge software (Fujifilm).
Results
We used a system in which reporter mRNAs are synthesized in vitro and cotransfected with a miRNA into HeLa cells. It is based on a method in which a synthetic RNA duplex (CXCR4) is cotransfected with a plasmid (pRL-TK-4 sites) that expresses a Renilla-luciferase mRNA containing four imperfectly matching binding sites for the CXCR4 miRNA in its 3′UTR (R-luc-4 sites). CXCR4 induces robust and specific repression of R-luc protein expression (≈12-fold) without loss of the target mRNA, indicating that this system faithfully recreates miRNA-mediated translational control (20, 21). We used a direct mRNA transfection approach because it allows precise control over features contained in the R-luc mRNA. We in vitro transcribed R-luc-4 sites mRNA from pRL-TK-4 sites (Fig. 1A), prepared an unrelated firefly luciferase (F-luc) mRNA (36) as a transfection control, and cotransfected them into HeLa cells together with CXCR4 miRNA. To ensure efficient translation, and to closely mimic the situation in plasmid-transfected cells, both transcripts were made with a physiological 5′ cap structure [m7G(5′)ppp(5′)G] and a 3′ poly(A) tail. Variation of mRNA amount, time of cell harvest (see Fig. 3A; see also Fig. 5, which is published as supporting information on the PNAS web site) and miRNA concentration (Fig. 2D), defined conditions that optimized sensitivity and extent of repression. The CXCR4 miRNA induces ≈5.5-fold repression of the R-luc-4 sites mRNA (Fig. 1B). The reduced magnitude of repression compared to the plasmid-based system may reflect a time lag of active miRNA-protein complex formation relative to the onset of translation of the transfected R-luc-4 site mRNA (Fig. 5B). Importantly, repression of transfected mRNA highly depends on the miRNA and its targets in the mRNA 3′ UTR: a control miRNA (C. elegans let-7) did not repress the R-luc-4 sites mRNA, and neither miRNA repressed an mRNA without the 3′ UTR target sites (R-luc-0 sites, Fig. 1 A and B).
Fig. 1.
miRNAs target the initiation step of translation. HeLa cells were cotransfected with an R-luc mRNA, the F-luc control mRNA, and (where indicated) a synthetic miRNA. For each R-luc mRNA, transfections were performed with specific CXCR4 miRNA, with let-7 control miRNA, and without miRNA. R-luc activity from each transfection was normalized to the corresponding F-luc measurement. (A) The four R-luc test mRNAs. R-luc-4 sites and -0 sites are m7G(5′)ppp(5′)G-capped and polyadenylated and either contain or lack four target sites for miRNA CXCR4 (black squares). Two further variants of the R-luc-4 sites mRNA carried the wild-type CrPV intergenic IRES or an inactive mutant (indicated by the asterisks, ref. 37). The CrPV IRES mRNAs carry an A-cap and are not polyadenylated. (B) Repression by CXCR4 miRNA. Repression was calculated by dividing the normalized R-luc activity without miRNA by the normalized R-luc activity in the presence of miRNA. Bars represent averaged results mostly from three to five independent triplicate experiments (filled bars, CXCR4; open bars, let-7). Error bars indicate standard deviation where appropriate. (C) Activity of the CrPV IRES. Bars represent normalized R-luc activity with mutant or wild-type CrPV IRES mRNA (no miRNA added; expression from the wild-type IRES is expressed as 1.0). R-luc expression driven by the wild-type CrPV IRES was inefficient but still ≈3 orders of magnitude above background readings from mock-transfected controls. Averaged results from two independent triplicate experiments are shown.
Fig. 3.
The miRNA/target interaction does not trigger accelerated mRNA decay. (A) Multiple aliquots of HeLa cells were cotransfected with R-luc-4 sites and F-luc control mRNAs, either with (▪) or without CXCR4 miRNA (•), and harvested at different times. R-luc expression was not normalized to F-luc. Five time series of this kind were each scaled to the total level of recovered R-luc protein and then averaged. Error bars represent standard deviation. The functional half-life of an mRNA in cells is defined as the time required for half-maximal accumulation of R-luc activity (43). (B) HeLa cells were cotransfected with an R-luc-4 sites mRNA, CXCR4 miRNA (as specified above the lanes), and the F-luc control mRNA (lanes 4-8). Total RNA was isolated 7 h after transfection, and 1-μg aliquots were subjected to dual-probe ribonuclease protection assays. Undigested probe mix (lane 1), a model reaction with input R-luc and F-luc mRNAs (marker, lane 2), and total RNA from untransfected HeLa cells (lane 3) are shown for comparison. Positions of probes and protected fragments are indicated on the right. Different “exposures” of the same gel are shown in the top and bottom half of the insert. The box below lists the R-luc/F-luc band intensity ratios relative to the value in lane 7. Each assay was also performed with 5 μg of total RNA to ascertain conditions of probe excess (data not shown). (C) Cells were cotransfected and total RNA was isolated as in B, followed by RT-PCR analyses (see Materials and Methods) with primer pairs specific for either R-luc-4 sites mRNA (across the miRNA target sites) or the F-luc control mRNA. Because the PCR primers anneal to sites on either side of the miRNA targets, cleavage induced by the miRNA should reduce or abolish the R-luc PCR product. PCR products were analyzed after 15, 18, 21, and 24 cycles to demonstrate subsaturation of the PCR amplification (the panel only shows PCR products after 18 cycles). miRNA-mediated R-luc repression in this experiment was ≈4.5-fold (data not shown). Equivalent results were obtained with RNA isolated 16 h after transfection (data not shown) and in three independent transfection experiments.
Fig. 2.
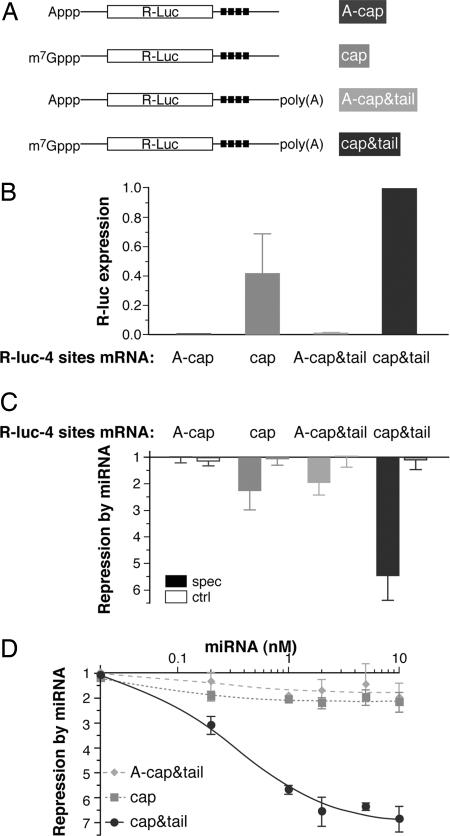
miRNA-mediated repression requires the 5′ cap structure and poly(A) tail. HeLa cells were cotransfected with mRNA and miRNAs as in Fig. 1. (A) Schematic of the R-luc-4 sites mRNAs. (B) Normalized R-luc activity from the four R-luc-4 sites mRNAs (no miRNA added; expression from the cap&tail mRNA is set to 1.0). Averaged results from two (A-cap) or five experiments are shown with standard deviation where appropriate. (C) Repression by a miRNA (calculated as in Fig. 1B). Averaged results from three to five experiments are shown with standard deviation (filled bars, CXCR4; open bars, let-7). (D) Repression of different R-luc-4 site mRNAs by CXCR4 miRNA is saturated at similar miRNA levels. The effect was measured as a function of miRNA concentration in the transfection mix and calculated as in Fig. 1B. Most data points represent averaged results from three experiments with standard deviation where appropriate (squares, cap; diamonds, tail; circles, cap&tail).
Expression of an mRNA Translated via Internal Ribosome Entry Is Not Repressed by a miRNA. Earlier studies have suggested that miRNAs affect translation at a step after initiation (18, 19). To test this possibility directly, we used the internal ribosome entry site (IRES) from the intergenic region of cricket paralysis virus (CrPV) (37-39). Canonical translation initiation requires many eIFs, as well as aminoacylated initiator tRNA (Met-tRNAimet), which recognizes the AUG start codon (28, 29). Translation driven by the CrPV IRES retains the canonical aspects of elongation and termination while replacing initiation with an entirely different process by initiating translation from an alanine codon without involvement of Met-tRNAimet or any eIFs (40, 41). Thus, if miRNA-mediated repression targets a step after initiation, it should still act on CrPV IRES-driven mRNA. We prepared variants of the R-luc-4 sites mRNA carrying the wild-type CrPV IRES, or an inactive mutant version (IGRmut14, ref. 37), in front of the R-luc reading frame (Fig. 1A). To ensure genuine CrPV IRES activity in the transfected cells, we prepared these mRNAs without a poly(A) tail and with a 5′ A-cap [A(5′)ppp(5′)G], which is inactive in translation but protects against accelerated decay (42). As expected, the efficiency of mRNA translation driven by the wild-type CrPV IRES was well below cap-dependent translation (≈2%, data not shown), but it was still 50 times greater than that of the IGRmut14 control (Fig. 1C), indicating genuine IRES function. We find that wild-type CrPV-driven translation is completely insensitive to miRNA-mediated repression (Fig. 1B). This lack of repression points to the initiation phase of translation as the major target for miRNA-mediated control.
Full miRNA-Mediated Repression Requires the 5′ Cap Structure and Poly(A) Tail. Canonical translation initiation is jointly promoted by the physiological cap structure and the poly(A) tail (43, 44); translational control mechanisms commonly affect, either directly or indirectly, the function of these mRNA end modifications in initiation (28-30). Prompted by the CrPV-IRES result (Fig. 1B), we asked whether miRNA repression targets the function of either the cap or the poly(A) tail. We prepared the R-luc-4 sites mRNA in four different versions (Fig. 2 A): with no poly(A) tail and either an A-cap or a physiological cap, or with a poly(A) tail and either an A-cap or a physiological cap. The four mRNA versions display the typical functional synergy between the physiological cap and the poly(A) tail in that combining both end modifications on the same mRNA molecule has a more than additive effect on R-luc translation (Fig. 2B; see also Fig. 3 for an analysis of mRNA stability). Importantly, this transcript set displays markedly different responses to the CXCR4 miRNA (Fig. 2C). Compared to the response of capped and polyadenylated mRNA (≈5.5-fold repression), translation driven either solely by the physiological cap or solely by the poly(A) tail was only partially responsive to the CXCR4 miRNA (≈2-fold repression). The low level of translation from the mRNA having neither a physiological cap nor a poly(A) tail (Fig. 2B) was completely resistant to miRNA addition (Fig. 2C). A weaker miRNA/target interaction might explain the lesser repression seen with the physiological cap only (“cap”) or the “A-cap&tail” R-luc-4 site mRNA (Fig. 2C). We addressed this possibility by increasing the concentration of cotransfected miRNA. We found that repression of each mRNA became saturated beyond 2 nM miRNA at its intrinsically different level (Fig. 2D), excluding differential miRNA/target affinity as an explanation for differences in repression level. We conclude that full miRNA-mediated repression requires both the cap structure and the poly(A) tail.
The CXCR4 miRNA/Target Interaction Primarily Leads to Repression of mRNA Translation. Previous studies with the CXCR4 miRNA/R-luc-4 site partially mismatched target pairing had demonstrated the absence of pronounced miRNA-mediated activation of mRNA degradation by using Northern blotting, RT-PCR, and RNase-protection assays (20, 21). Two types of accelerated decay are at least formally possible in our system. First, despite the mismatched pairing, the CXCR4 miRNA could still activate endonucleolytic cleavage of the target mRNA. Our observations are inconsistent with this mechanism. We find that variants of the R-luc-4 sites mRNAs differing only in their 5′ UTR sequence (CrPV-R-luc-4 sites in Fig. 1 and EMCV-R-luc-4 sites in Fig. 4) are completely unaffected by cotransfection of CXCR4 miRNA. By contrast, cotransfection of the EMCV-R-luc-4 sites mRNA, together with a synthetic RNA duplex with perfect complementarity to the target sites (a “short interfering” or siCXCR4), led to robust reduction of R-luc expression (≈6-fold; data not shown). These observations indicate that both target site accessibility and the potential for an siRNA-type degradative mechanism are not perturbed by the insertion of an IRES element in the 5′ UTR. Second, the CXCR4 miRNA could activate degradation of the target mRNA by other means, for instance by activating a pathway involving 5′ to 3′ exonucleolytic decay (35). We set out to independently assess this possibility for our RNA transfection assays by measuring the stability of the R-luc reporter mRNAs in relevant experimental conditions. We first determined the functional stability of the transfected R-luc-4 sites mRNA by following the accumulation of R-luc protein in transfected cells over time (selected time-course data are shown in Fig. 3A). Functional stability information, although somewhat indirect, has the advantage of relating specifically to the activity of the R-luc-4 sites mRNA population that entered the cells as functional molecules. We then directly assessed the physical stability of the reporter mRNAs by performing RNase protection assays (Fig. 3B) and semiquantitative RT-PCR (Fig. 3C). Based on our time-course measurements of R-luc protein accumulation (Fig. 3A) and miRNA-mediated repression (Fig. 5B), we chose to perform the physical stability analyses 7 h after transfection; at this time point, clear miRNA-mediated repression is observed, and appreciable levels of residual R-luc mRNA still remain in the cells. As observed in refs. 42 and 43, the physical and the functional stability of the transfected reporter mRNA did not vary appreciably with the addition or omission of the functional cap and poly(A) tail to the mRNA (Fig. 3B, lanes 3-6; see also Table 1, which is published as supporting information on the PNAS web site). We estimated functional half-lives of ≈4-5 h for all mRNAs tested (Table 1). Importantly, cotransfection of the CXCR4 miRNA with the capped and polyadenylated R-luc-4 sites mRNA had no detectable effect on the physical stability of the mRNA as measured by RNase protection (Fig. 3B, lanes 7 and 8) with a probe that protects a part of the R-luc coding region, or RT-PCR (Fig. 3C, lanes 3 and 4), which amplified a large portion of the coding region extending into the 3′ region of the R-luc-4 site mRNA and spanning the miRNA target sites. Analysis of the time-course data suggests a possible tendency toward a minor (≈1.15-fold) reduction in functional half-life of the cap&tail R-luc-4 sites mRNA when the miRNA is present (Fig. 3A). This small reduction is consistent with previous analyses in similar systems (20-25) and clearly insufficient to explain the ≈5.5-fold miRNA-mediated repression of R-luc protein expression from this mRNA. Collectively, these results exclude selective mRNA degradation as an explanation for the patterns of R-luc expression in our experiments, confirming that miRNA-mediated repression in our system acts primarily on translation.
Fig. 4.
The EMCV IRES element impairs miRNA repression. HeLa cells were cotransfected with mRNA and miRNAs as in Figs. 1 and 2. (A) Schematic of the R-luc-4 sites mRNAs used in this experiment: They carry either the standard 5′ UTR or the EMCV IRES, either with or without a poly(A) tail; all are A-capped. (B) Normalized R-luc activity from the four versions of R-luc-4 sites mRNA (no miRNA added; expression from the EMCV&tail mRNA is set to 1.0). Averaged results from two (A-cap) to four experiments are shown with standard deviation where appropriate. (C) Repression by a miRNA (calculated as in Figs. 1B and 2C). Averaged results from three to five experiments are shown with standard deviation (filled bars, CXCR4; open bars, let-7).
miRNAs Impair the Function of eIF4E. To further investigate the molecular targets of miRNA-mediated repression within the initiation machinery, we used the special mode of initiation by the EMCV IRES (39, 40). The recruitment of eIF4G is a central step in early initiation that is jointly stimulated by the cap and poly(A) tail (28-30). The cap structure interacts with the cap-binding protein eIF4E, whereas the poly(A) tail is bound to the poly(A)-binding protein (PABP). eIF4E and PABP concurrently bind to eIF4G and recruit it to the mRNA. The EMCV IRES is still responsive to poly(A)-mediated stimulation via the eIF4G-PABP interaction, but it recruits eIF4G without employing eIF4E. Thus, it retains most features of canonical initiation but does not require eIF4E (39, 40, 42, 45). We prepared two R-luc-4 sites mRNAs carrying the EMCV IRES sequence; both were A-capped, one carried a poly(A) tail, and the other had no tail (Fig. 4A). These EMCV IRES mRNAs gave robust levels of R-luc expression (≈10% of the equivalent cap-driven mRNA, data not shown), and expression was enhanced by the poly(A) tail (Fig. 4B). This expression reflects genuine IRES activity, because levels are well above those observed with A-capped R-luc-4 sites mRNAs lacking the EMCV IRES. As observed with the CrPV IRES (Fig. 1C), translation driven solely by the EMCV IRES is completely resistant to miRNA repression (Fig. 4C). The presence of a poly(A) tail on EMCV&tail reinstates a partial repression by the CXCR4 miRNA, equivalent to that seen with the A-cap&tail transcript (Fig. 4C). The following conclusions can be drawn from this data. First, an overall comparison of the four mRNAs used here demonstrates that the level of miRNA-mediated repression (Fig. 4C) is not simply a function of overall translation efficiency of the targeted mRNA (Fig. 4B). Second, comparison of the two EMCV-driven mRNAs with their respective capped counterparts independently confirms that the cap structure is important for full miRNA-mediated repression of translation initiation. Third, resistance of the EMCV IRES-driven initiation mode to repression indicates that a miRNA impairs the recruitment and/or function of eIF4E.
Discussion
In recent years, miRNAs have emerged as a major new class of highly conserved gene expression regulators. In animals, miRNAs are thought to predominantly act by repressing translation of their mRNA targets. In a quest for an experimental system that would allow a detailed study of this repressive mechanism, we examined the sensitivity of canonical and IRES-driven translation in HeLa cells cotransfected with suitably engineered reporter mRNAs and a miRNA. Using the CrPV IRES (which essentially bypasses all of the translation initiation apparatus) as an experimental tool, we observe that miRNAs target the initiation phase of translation (Fig. 1). The cap structure and the poly(A) tail have central roles in early initiation (28-30, 43, 44), and we find that both are required for full miRNA-mediated repression (Fig. 2). Based on three independent measures of mRNA stability, we can exclude accelerated mRNA decay as an explanation of our findings (Fig. 3). Finally, our experiments with the EMCV IRES (which selectively dispenses with the requirement for eIF4E during initiation) demonstrate that the miRNA mechanism blocks a very early stage of initiation and identifies eIF4E as a molecular target (Fig. 4). Interference with the binding of eIF4E to the cap structure of the mRNA, or a block of its function once bound to the cap, are both plausible explanations for the miRNA effect. After the initial submission of this manuscript, another paper reported broadly similar findings, namely that a miRNA blocks the initiation phase of translation, possibly by interfering with the cap recognition step (25). Using comparable approaches in HeLa cells transfected with luciferase reporter constructs, these authors assessed the effects of targeting the endogenous let-7 miRNA to three partially mismatched target sites in the reporter 3′ UTR. Alternatively, a hAgo2 fusion protein was tethered to multiple sites within the reporter mRNA 3′ UTR. In both cases, they found a clear withdrawal of the repressed mRNAs from the polysomal region in density gradient analyses and an insensitivity of cap-independent translation to the repressive mechanism.
The features of miRNA-mediated translational repression that we have uncovered here are reminiscent of established paradigms of translational control. In Xenopus oocytes, the cytoplasmic polyadenylation control element-binding protein (CPEB) recruits the eIF4E-binding protein Maskin to maternal mRNAs and blocks translation by establishing a repressive interaction between the 3′ UTR-bound repressor complex and cap-bound eIF4E. The Drosophila protein Cup functions in an analogous manner in the developmental control of oskar and nanos mRNA (46). In the same organism, Bicoid-mediated repression of caudal mRNA was shown to function through recruitment of the repressive cap-binding protein 4E-HP to displace eIF4E (47). Involvement of the poly(A) tail as demonstrated here is another feature that miRNA repression has in common with at least some of the translational control paradigms outlined above. CPEB-bound dormant mRNAs have short poly(A) tails. During oocyte maturation, phosphorylation of CPEB stimulates poly(A) tail elongation leading to PABP-binding, recruitment of eIF4G, displacement of Maskin from eIF4E, and activation of translation (46). In addition to its role in eIF4G recruitment, PABP also acts during the 60S joining step of initiation (48) and, through an interaction with the eukaryotic release factor 3 (28), may mediate ribosome recycling on circular polysomes (29). Our demonstration that the full effect of miRNAs requires also the poly(A) tail indicates that miRNAs may target either a joint function of the cap structure and the poly(A) tail (i.e., eIF4G recruitment) or a separate function of the tail in translational initiation as outlined above.
Our results should be seen in conjunction with emerging information on the dynamic composition and function of P-bodies. The proximity of the repressed mRNA to sites of general mRNA degradation and the recent reanalysis of miRNA effects during larval development in C. elegans (35) raise questions about the relative contributions of mRNA decay and blocked translation to the repressive mechanism. At present, there is no simple technical explanation for the discrepancies between the different studies in C. elegans, and there is no evidence in the recent study that mRNA degradation elicited by the endogenous let-7 and lin-4 miRNAs involves mRNA cleavage by an siRNA-type mechanism (35). To unify these conflicting reports, we favor a “hybrid model” of miRNA action (25, 31), whereby a block of translation initiation as described here represses protein expression per se and leads to sequestration and reinforced silencing of the targeted mRNA in a P-body. In some systems, this sequestration may eventually lead to degradation of the silenced mRNA within the P-body environment. We have shown here that in our experimental system, similar to related systems (20-25), the miRNA primarily affects mRNA translation. Furthermore, our data suggest the existence of a cap- or eIF4E-interacting factor that mediates the miRNA effects. This idea may relate to a recent study demonstrating that eIF4E and the eIF4E-transporter (4E-T) protein function in targeting mRNA to mammalian P-bodies (49). It is tempting to speculate that 4E-T may be a part of both the molecular bridge between the miRNA complex in the 3′ UTR and the cap-recognition step in translation initiation, and also the machinery that targets repressed miRNA/mRNA complexes to the P-bodies.
In conclusion, the observations presented here demonstrate initiation as the step in translation that is inhibited by a miRNA, and further identify key molecular targets within initiation. These findings suggest a compelling scenario for the events that may precede miRNA-mediated sequestration of repressed mRNA into P-bodies, which is now amenable to experimental verification.
Supplementary Material
Acknowledgments
We thank Traude Beilharz and Pete Currie for critical reading of the manuscript. This work was supported by grants from the National Health and Medical Research Council and the Australian Research Council (to T.P. and D.I.K.M.) and by the Victor Chang Cardiac Research Institute. T.P. is supported by The Sylvia and Charles Viertel Charitable Foundation.
Author contributions: D.T.H. and B.J.W. performed research; D.T.H. and B.J.W. analyzed data; D.I.K.M. and T.P. designed research; and D.I.K.M. and T.P. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CrPV, cricket paralysis virus; eIF, eukaryotic initiation factor; EMCV, encephalomyocarditis virus; F-luc, firefly luciferase; IRES, internal ribosomal entry site; miRNA, microRNA; P-bodies, processing bodies; PABP, poly(A)-binding protein; R-luc, Renilla luciferase.
References
- 1.Bartel, D. P. (2004) Cell 116, 281-297. [DOI] [PubMed] [Google Scholar]
- 2.He, L. & Hannon, G. J. (2004) Nat. Rev. Genet. 5, 522-531. [DOI] [PubMed] [Google Scholar]
- 3.Meister, G. & Tuschl, T. (2004) Nature 431, 343-349. [DOI] [PubMed] [Google Scholar]
- 4.Ambros, V. (2004) Nature 431, 350-355. [DOI] [PubMed] [Google Scholar]
- 5.Cullen, B. R. (2004) Mol. Cell 16, 861-865. [DOI] [PubMed] [Google Scholar]
- 6.Giraldez, A. J., Cinalli, R. M., Glasner, M. E., Enright, A. J., Thomson, J. M., Baskerville, S., Hammond, S. M., Bartel, D. P. & Schier, A. F. (2005) Science 308, 833-838. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, S. M., Grosshans, H., Shingara, J., Byrom, M., Jarvis, R., Cheng, A., Labourier, E., Reinert, K. L., Brown, D. & Slack, F. J. (2005) Cell 120, 635-647. [DOI] [PubMed] [Google Scholar]
- 8.Poy, M. N., Eliasson, L., Krutzfeldt, J., Kuwajima, S., Ma, X., Macdonald, P. E., Pfeffer, S., Tuschl, T., Rajewsky, N., Rorsman, P. & Stoffel, M. (2004) Nature 432, 226-230. [DOI] [PubMed] [Google Scholar]
- 9.Lai, E. C. (2004) Genome Biol. 5, 115.1-115.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel, D. P. & Chen, C. Z. (2004) Nat. Rev. Genet. 5, 396-400. [DOI] [PubMed] [Google Scholar]
- 11.Lewis, B. P., Burge, C. B. & Bartel, D. P. (2005) Cell 120, 15-20. [DOI] [PubMed] [Google Scholar]
- 12.Brennecke, J., Stark, A., Russell, R. B. & Cohen, S. M. (2005) PLoS Biol. 3, e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krek, A., Grun, D., Poy, M. N., Wolf, R., Rosenberg, L., Epstein, E. J., MacMenamin, P., da Piedade, I., Gunsalus, K. C., Stoffel, M. & Rajewsky, N. (2005) Nat. Genet. 37, 495-500. [DOI] [PubMed] [Google Scholar]
- 14.Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. (2003) Cell 113, 25-36. [DOI] [PubMed] [Google Scholar]
- 15.Meister, G., Landthaler, M., Patkaniowska, A., Dorsett, Y., Teng, G. & Tuschl, T. (2004) Mol. Cell 15, 185-197. [DOI] [PubMed] [Google Scholar]
- 16.Liu, J., Carmell, M. A., Rivas, F. V., Marsden, C. G., Thomson, J. M., Song, J. J., Hammond, S. M., Joshua-Tor, L. & Hannon, G. J. (2004) Science 305, 1437-1441. [DOI] [PubMed] [Google Scholar]
- 17.Pasquinelli, A. E. & Ruvkun, G. (2002) Annu. Rev. Cell Dev. Biol. 18, 495-513. [DOI] [PubMed] [Google Scholar]
- 18.Olsen, P. H. & Ambros, V. (1999) Dev. Biol. 216, 671-680. [DOI] [PubMed] [Google Scholar]
- 19.Seggerson, K., Tang, L. & Moss, E. G. (2002) Dev. Biol. 243, 215-225. [DOI] [PubMed] [Google Scholar]
- 20.Doench, J. G., Petersen, C. P. & Sharp, P. A. (2003) Genes Dev. 17, 438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doench, J. G. & Sharp, P. A. (2004) Genes Dev. 18, 504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng, Y., Yi, R. & Cullen, B. R. (2003) Proc. Natl. Acad. Sci. USA 100, 9779-9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng, Y., Wagner, E. J. & Cullen, B. R. (2002) Mol. Cell 9, 1327-1333. [DOI] [PubMed] [Google Scholar]
- 24.Pillai, R. S., Artus, C. G. & Filipowicz, W. (2004) RNA 10, 1518-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillai, R. S., Bhattacharyya, S. N., Artus, C. G., Zoller, T., Cougot, N., Basyuk, E., Bertrand, E. & Filipowicz, W. (2005) Science 309, 1573-1576. [DOI] [PubMed] [Google Scholar]
- 26.Jing, Q., Huang, S., Guth, S., Zarubin, T., Motoyama, A., Chen, J., Di Padova, F., Lin, S. C., Gram, H. & Han, J. (2005) Cell 120, 623-634. [DOI] [PubMed] [Google Scholar]
- 27.Jopling, C. L., Yi, M., Lancaster, A. M., Lemon, S. M. & Sarnow, P. (2005) Science 309, 1577-1581. [DOI] [PubMed] [Google Scholar]
- 28.Sonenberg, N. & Dever, T. E. (2003) Curr. Opin. Struct. Biol. 13, 56-63. [DOI] [PubMed] [Google Scholar]
- 29.Preiss, T. & Hentze, M. W. (2003) Bioessays 25, 1201-1211. [DOI] [PubMed] [Google Scholar]
- 30.Gebauer, F. & Hentze, M. W. (2004) Nat. Rev. Mol. Cell Biol. 5, 827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, J., Valencia-Sanchez, M. A., Hannon, G. J. & Parker, R. (2005) Nat. Cell Biol. 7, 719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sen, G. L. & Blau, H. M. (2005) Nat. Cell Biol. 7, 633-636. [DOI] [PubMed] [Google Scholar]
- 33.Cougot, N., Babajko, S. & Seraphin, B. (2004) J. Cell Biol. 165, 31-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teixeira, D., Sheth, U., Valencia-Sanchez, M. A., Brengues, M. & Parker, R. (2005) RNA 11, 371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagga, S., Bracht, J., Hunter, S., Massirer, K., Holtz, J., Eachus, R. & Pasquinelli, A. E. (2005) Cell 122, 553-563. [DOI] [PubMed] [Google Scholar]
- 36.Iizuka, N., Najita, L., Franzusoff, A. & Sarnow, P. (1994) Mol. Cell. Biol. 14, 7322-7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson, J. E., Powell, M. J., Hoover, S. E. & Sarnow, P. (2000) Mol. Cell. Biol. 20, 4990-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostareck, D. H., Ostareck-Lederer, A., Shatsky, I. N. & Hentze, M. W. (2001) Cell 104, 281-290. [DOI] [PubMed] [Google Scholar]
- 39.Poyry, T. A., Kaminski, A. & Jackson, R. J. (2004) Genes Dev. 18, 62-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellen, C. U. & Sarnow, P. (2001) Genes Dev. 15, 1593-1612. [DOI] [PubMed] [Google Scholar]
- 41.Pestova, T. V. & Hellen, C. U. (2003) Genes Dev. 17, 181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergamini, G., Preiss, T. & Hentze, M. W. (2000) RNA 6, 1781-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallie, D. R. (1991) Genes Dev. 5, 2108-2116. [DOI] [PubMed] [Google Scholar]
- 44.Preiss, T. & Hentze, M. W. (1998) Nature 392, 516-520. [DOI] [PubMed] [Google Scholar]
- 45.Svitkin, Y. V., Imataka, H., Khaleghpour, K., Kahvejian, A., Liebig, H. D. & Sonenberg, N. (2001) RNA 7, 1743-1752. [PMC free article] [PubMed] [Google Scholar]
- 46.Richter, J. D. & Sonenberg, N. (2005) Nature 433, 477-480. [DOI] [PubMed] [Google Scholar]
- 47.Cho, P. F., Poulin, F., Cho-Park, Y. A., Cho-Park, I. B., Chicoine, J. D., Lasko, P. & Sonenberg, N. (2005) Cell 121, 411-423. [DOI] [PubMed] [Google Scholar]
- 48.Kahvejian, A., Svitkin, Y. V., Sukarieh, R., M'Boutchou, M. N. & Sonenberg, N. (2005) Genes Dev. 19, 104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrei, M. A., Ingelfinger, D., Heintzmann, R., Achsel, T., Rivera-Pomar, R. & Luhrmann, R. (2005) RNA 11, 717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.