Abstract
Long-term depression (LTD) of the parallel-fiber (PF) Purkinje synapse induced by four different experimental paradigms could be prevented in rat cerebellar slices by T-588, a neuroprotective compound. The paradigms consisted of pairing PF activation with climbing-fiber activation, direct depolarization, glutamic iontophoretic depolarization, or caffeine. In all cases, LTD was determined by patch-clamp recording of PF excitatory postsynaptic currents at the Purkinje cell somata. T-588 at 1 μM prevented the triggering of LTD reversibly and did not generate LTD on its own. Two-photon calcium-sensitive dye imaging demonstrated that T-588 reduces intracellular calcium concentration ([Ca2+]i) increase by blocking calcium release from intracellular stores. Because [Ca2+]i increase has been widely shown to trigger LTD and glutamate excitotoxicity, we propose that LTD may act as a neuroprotective mechanism. As such, LTD would serve to decrease glutamatergic-receptor sensitivity to limit deleterious [Ca2+]i increase rather than to act as a mechanism for cerebellar learning.
Keywords: caffeine, caffeine-induced cytosolic Ca2+ release, patch clamp, two-photon laser scanning microscopy
It has been known since the nineteenth century work by Ramon y Cajal (1) that the cerebellar cortex has two main afferent systems, the climbing-fiber (CF) and mossy-fiber terminals. Yet one of the debated issues in cerebellar physiology today relates to the functional role of these two main afferent systems. Indeed, their functional significance has been in hot contention since their physiological actions on Purkinje cells (PCs) were first described four decades ago (2, 3).
Thus, CFs, which are recognized as having a one-to-one relation to PCs, were found to generate very powerful all-or-none excitatory synaptic activation, in accordance with the large number of synaptic terminals they support. By contrast, the mossy fibers have a disynaptic relation to PCs via the granule cells (GrC), which, in turn, terminate on PC spines as the parallel fibers (PFs). These two afferents represent the limit of single-cell innervation in the central nervous system. That is, maximum convergence and minimum divergence for the CF-PC synapse, where a single axonal terminal establishes a one-to-one relation to a PC via hundreds of synaptic junctions, making this one of the most powerful single synapses in the brain. By contrast, the PF connectivity represents maximum divergence and minimum convergence, because each PF contacts a PC only once, and a given PC may receive input from as many as 200,000 different PFs (4). The electrical signatures of these two systems are also quite different. MF-GrC-PF inputs generate fast single spikes (20-50 Hz), whereas a CF input produces a burst of action potentials in a PC (2) that are stereotyped in waveform and are known as complex spikes (5). In the free-moving animal, complex spikes occur at low frequencies (1-2 Hz) (6) because of the intrinsic properties of their cells of origin in the inferior olive nucleus (7).
Two basic hypotheses have been proposed for the function of these two inputs: (i) The “learning hypothesis” and (ii) the “timing hypothesis.” The learning hypothesis, where the repeated coincident activation of CFs and PFs results in a longterm depression (LTD) of the PF-PC synapse, relegates the functional significance of the CF system to that of motor memory, where CF input modifies PF activity and serves no other function (8). The timing hypothesis proposes that CFs serve to determine the temporal binding required for motor execution (9, 10). CFs would act as a time-coherent group, given the electrical coupling of their cells of origin (9) and the isochronicity of their activation of PCs (11). According to this hypothesis, the MF-GrC-PF system is seen as providing the subtle inhibition of cerebellar nuclei required to execute coordinated movements within the temporal frame generated by the inferior olive.
Given the possible significance of LTD in synaptic plasticity, a large and excellent set of physiological and molecular studies relating to cerebellar LTD have been published that indicate that an intracellular calcium concentration ([Ca2+]i) increase is required for synaptic depression to be triggered (8). This article examines the possibility that LTD is a neuroprotective mechanism, evolved to prevent calcium-dependent cell damage by down-regulating glutamatergic-receptor sensitivity rather than a mechanism to implement memory storage (12).
T-588, (1R)-1-benzothiophen-5-yl-2[2-(diethylamino)ethoxy]-ethanol hydrochloride, is a neuroprotective compound that has been shown to block astrocyte apoptosis (13) and delay the progression of neuromuscular dysfunction in wobbler mouse motoneuron disease (14).
Here, we demonstrate that cerebellar LTD can be prevented by T-588. The accompanying article (15) shows that motor learning, as determined by either the rotorod method or eyeblink learning, is unimpaired under conditions where LTD is absent. This article, showing that T-588 inhibited LTD in cerebellar slices in the mouse, was presented at the Society for Neuroscience meeting in 2001.
Methods
Cerebellar Slice Preparation. All experiments were performed in cerebellar slices obtained from male ICR mice (3.5-5 weeks old) by using our standard methods (16, 17). Mice were anesthetized with pentobarbital before decapitation. Parasagittal cerebellar slices (200 μm thick) were superfused with an oxygenated mammalian Ringer's solution containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 20 mM glucose that was bubbled with 95% O2 and 5% CO2 (18) and maintained at room temperature. Picrotoxin (20 μM) was added to block spontaneous inhibitory postsynaptic currents. For some experiments, 10 mM caffeine was added to the Ringer's solution. All experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals at Toyama Chemical Company and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrical Recording and Stimulation. PC electrical activity was recorded by using the whole-cell-patch mode (Axopatch 200B, Axon Instruments), under direct upright observation (AX70, ×60 water-immersion lens, Olympus) by using patch pipettes with access resistances of 3-5 MΩ. Experimental parameters were controlled, electrophysiological signals were digitized, and data were analyzed on-line by using programs we developed in the labview programming environment (digitizing board PCI-MIO-16E-1, labview 6.0, National Instruments, Austin, TX).
Patch electrodes were filled with 70 mM KCl, 60 mM K d-gluconate, 4 mM MgCl2, 4 mM ATP, 0.4 mM GTP, and 10 mM Hepes, adjusted to pH 7.3 with KOH. Stimulation was achieved by using glass pipettes with fire-polished tips (access resistances <1 MΩ) filled with 0.9% NaCl. PFs were stimulated at midmolecular-layer level. Their activation was confirmed by the generation of graded excitatory postsynaptic currents (EPSCs) in PCs. CF axons were stimulated by using an electrode placed in the white matter adjacent to the PC somata. CF activation was confirmed by the generation of characteristic all-or-none complex spikes in PCs.
LTD Induction. Steps toward LTD induction were implemented in the absence and in the presence of T-588 by using four induction paradigms. Before inducing LTD, PFs were stimulated once every 5 s for 10 min, sets of five successive EPSCs were averaged, and the control EPSC amplitude was determined by plotting these average amplitudes as a function of time (see Fig. 2, -10 to 0 min). Recordings were not made during, or for one minute after, LTD induction. After LTD induction, PFs were stimulated once every 5 s for 35 min, and sets of five EPSCs were averaged (see Fig. 2, 6-40 min). The four paradigms used for LTD induction (Fig. 1 A) were (i) following Hartell (19), conjunctive paired stimuli (CF followed after 15 ms by PF stimulation) delivered at 1 Hz for 5 min (n = 16 and n = 8 in the presence of T-588); (ii) direct PC depolarization via patch-electrode clamping paired with PF stimulation (20) delivered at 1 Hz for 5 min. In this experimental arrangement, the PC was depolarized by using a somatic voltage step that maintained the membrane potential at 0 mV for the duration of the PF-stimulus trains (n = 16 and n = 8 in the presence of T-588); (iii) local Glut I paired with PF stimulation (21) delivered at 1 Hz for 5 min. Iontophoretic pulses of a solution of 10 μM glutamate chloride were applied at the dendritic level (at the same level as the PF stimulation) during PF stimulation (n = 16 and n = 8 in the presence of T-588); (iv) by using the addition of caffeine with nonconjunctive PC depolarization and PF stimulation at 1 Hz for 5 min to induce LTD in the absence (n = 3) or presence (n = 3) of T-588.
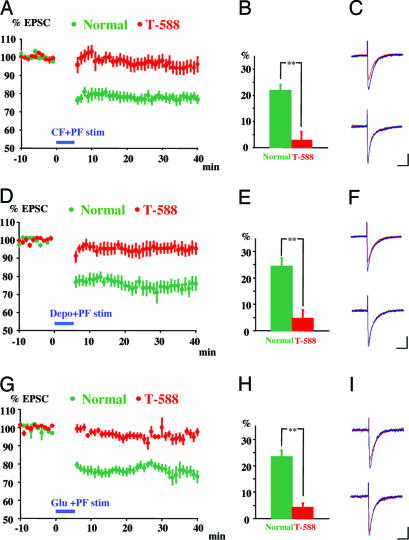
Fig. 2.
Prevention of LTD by T-588. LTD was induced by pairing CF and PF stimulation (A-C), direct depolarization and PF stimulation (D-F), or Glut I and PF stimulation (G-I) (green points and columns). T-588 (1 μM) prevented LTD evoked by all three pairing paradigms (red points and columns). (A, D, and G) Data are pooled and normalized to the mean initial PF-EPSC amplitude during the period before conjunctive stimulation (-10 to 0 min), conjunctive stimulation delivered during blue horizontal bar. (B, E, and H) Averaged EPSC decrease from 6 to 40 min after conjunctive stimulation. Data represent the mean (±SEM); **, P < 0.01, Student t test. (C, F, and I) Superimposed sample records of PF-EPSC traces are taken before (red line) and 30 min after (blue line) LTD induction in the absence (Upper) and presence (Lower) of T-588.
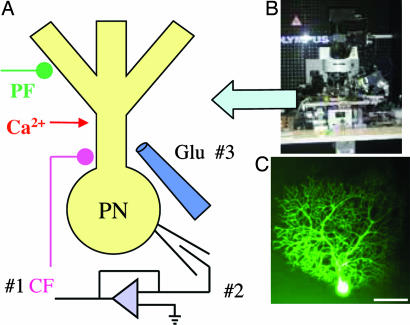
Fig. 1.
LTD-induction paradigms and experimental setup. (A) Schematic diagram of the experimental setup. LTD was evoked in mouse parasagittal cerebellar slices by pairing PF stimulation with (i) CF activation, (ii) DC depolarization, or (iii) Glut I or by nonconjunctive stimulation and caffeine-induced cytosolic Ca2+ release (CfICR) from intracellular stores (data not shown). (B) Multiphoton laser scanning microscope (AX70). (C) PC filled with calcium green 1 and 2 through a patch pipette. (Scale bar, 50 μm.)
Two additional control experiments were carried out. In the first, T-588 was superfused to find whether it triggered LTD on its own (n = 3); the second was designed to find whether, after the determination that LTD does not occur spontaneously, paradigm ii could trigger LTD after that long an exposure to T-588 (n = 3).
Two-Photon Calcium Imaging. To determine whether T-588 had an effect on calcium release from intracellular stores, an intracellular calcium profile was recorded during caffeine superfusion and PC depolarization (n = 14 and n = 7 in the presence of T-588). PCs were patch-clamped with electrodes filled with equal amounts of calcium green 1 and 2 (50 μM each), and the dyes were allowed to diffuse into the neuronal cytosol for 30 min (see Fig. 1C) to prevent saturation of the calcium response (22). Calcium fluorescence was imaged by using two-photon laser microscopy (22). Our microscope system (23, 24) has an Olympus AX70 frame (Fig. 1B). The laser, a Tsunami Ti:sapphire system, was activated with a Millenia X diode-pump laser (Spectra-Physics) that enabled a pulse frequency of 80 MHz and pulse duration of 70 fsec with a pixel dwell time of 10 μsec. The output-beam wavelength was tuned to 860 nm, and the laser intensity was regulated to 3 mW at tissue level through an Olympus ×60 (NA 0.90) lens. A labview-based (version 6.0, National Instruments) program drove the scan system [x-y scanner model 6800HP(L), mirrors 6M8003S-S, (Cambridge Technology, Cambridge, MA) via an electronics circuit designed and built in-house. Laser-beam drift and the intensity fluctuations were compensated by using an in-house servo system. The coupling pupil lens between the scanning mirror and microscope was an Olympus eyepiece (model GSWH10X), and the dichroic mirror was manufactured by Chroma Technology, Rockingham, VT (model 650DSPXR). Fluorescence light was detected with a photomultiplier tube (Hamamatsu Photonics model R3896). The microscope z axis was moved by using a high-resolution stepper motor that was computer-controlled (ZETA6104, Parker Hannifin, Irvine, CA). Frames comprised left-to-right scan steps that were sequentially repeated to cover a top-to-bottom viewing area. Computer control defined the number of control-frame averages and the real-time image subtraction and/or ratio between data frames.
Data Analysis. The mean and SEM of sets of five sequential EPSCs before and after LTD induction was determined and plotted as a function of time for experiments using LTD induction (Figs. 2, 3, 4). These means were normalized to compare across experiments. The mean reduction in EPSC amplitude was determined for each experiment, and the grand mean for all animals was determined for each induction paradigm and plotted as bar graphs. For two-photon experiments, the retention times were expressed as the mean (±SEM). The program sas 6.12 (SAS Institute, Cary, NC) was used for statistical analysis. A level of P < 0.05 was considered to be statistically significant. Statistics are given in Results.
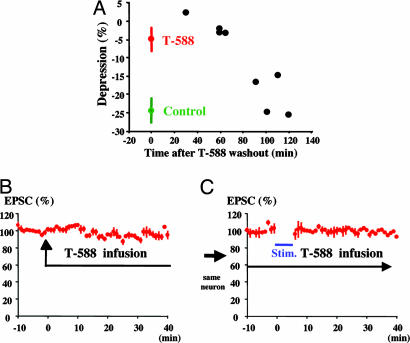
Fig. 3.
Washout of T-588 and control experiments. (A) LTD induction during T-588 (1 μM) washout. LTD recovery was determined at eight times after washout onset in eight experiments. LTD recovery time was ≈90 min. LTD was produced by pairing PC depolarization and PF stimulation at 1 Hz for 5 min. (Means are from the experiment shown in Fig. 2E.) (B) T-588 does not itself produce LTD. No reduction in PF-EPSCs was observed after a T-588 exposure time of 60 min. (C) LTD was not induced in the presence of T-588.
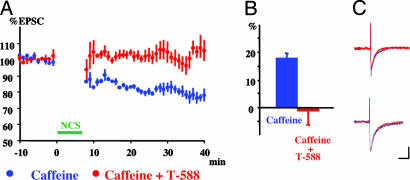
Fig. 4.
LTD produced by caffeine (10 mM) and nonconjunctive stimulation. (A) LTD was evoked by nonconjunctive stimulation (PC depolarization followed by PF stimulation) in the presence of caffeine (green symbols). LTD was prevented by 1 μM T-588 (red symbols). Data were pooled and normalized to the mean initial PF-EPSC amplitude during the control period before stimulation. (B) Average change in PF EPSC amplitude before (green) and after (red) T-588. Data represent the mean (±SEM). (C) Superimposed sample records of PF-EPSC traces are taken before (red traces) and 30 min after (blue traces) LTD induction in the absence (Upper) and presence (Lower) of T-588.
Results
A total of 74 experiments were implemented in this study. In the initial LTD experiments (Fig. 2), three sets of recordings were implemented in 48 animals. In each set, eight experiments were carried out in the presence of T-588 and eight in the absence of this agent. These results are organized according to the LTD-induction paradigm.
LTD Induction by Pairing CF and PF Stimulation Is Prevented by T-588. Sixteen LTD experiments were performed pairing CF stimulation with PF stimulation (19). Eight of these experiments were in the presence of T-588. Each experiment began with a 10-min recording of PC EPSCs generated by PF stimulation at 0.2 Hz, as shown in Fig. 2 A for one experiment (-10 to 0 min, green). Each point represents the mean of five responses normalized to 100% of the PF response. This PF stimulation was followed by 5 min of PF and CF conjunctive stimulation at 1 Hz (Fig. 2 A, blue horizontal bar). The amplitudes of the PF EPSCs were reduced by ≈20% from the control level, as shown for one experiment by the green symbols in Fig. 2 A. The mean LTD was 22 ± 2.2% (n = 8), as shown by the green column in Fig. 2B. The EPSC amplitude before CF-PF pairing (Fig. 2C Upper, blue trace) was larger than the amplitude after LTD induction, as shown in these representative traces (Fig. 2C Upper, red trace). Each trace is the mean of five responses.
The same paradigm was used in eight experiments, after incubation of the cerebellar slices for 1 h in the presence of 1 μM T-588. Under these conditions, there was an insignificant reduction of the PF EPSC, as shown for one case in Fig. 2 A (red). The mean reduction in the PF EPSC in the presence of T-588 (2.8 ± 3.2%, n = 8) was significantly less (P < 0.01) than under control conditions (Fig. 2B). The EPSC amplitude before CF-PF pairing (Fig. 2C, lower blue trace) was indistinguishable from that after LTD induction in the presence of T-588, as shown in these representative traces (Fig. 2C Lower, red trace). Each trace is the mean of five responses. (The Ringer's solution contained 1 μM T-588 superfused throughout the recording period to avoid T-588 washout.)
LTD Induced by Pairing Direct PC Depolarization with PF Stimulation Is Prevented by T-588. In the next set of experiments, PC depolarization was paired with PF stimulation (20) (Fig. 2 D-F). The depolarization consisted of a 100-msec voltage step to 0 mV from a -70-mV holding potential. The mean LTD induced with this method was a 24 ± 3.2% (n = 8) reduction in PF EPSC amplitude (Fig. 2 D and E, green). The EPSC after LTD induction was smaller than that before LTD induction, as shown in the upper traces of Fig. 2F (after LTD, red trace; before LTD, blue trace). Each trace is the mean of five responses. In the presence of T-588, this LTD-induction paradigm reduced the mean EPSC amplitude by only 4.8 ± 3.1% (n = 8) (Fig. 2 D and E, red), significantly less than in the absence of the drug (P < 0.01). In the presence of T-588, an EPSC before LTD induction (Fig. 2F Lower, blue trace) and one recorded after LTD induction (Fig. 2F Lower, red trace) were indistinguishable. Each trace is the mean of five responses.
LTD Induced by Pairing Glut I with PF Stimulation Is Prevented by T-588. The activation of glutamate receptors (GluRs) in PCs has been shown to trigger LTD by the entry of calcium via voltage- and ligand-gated calcium channels and by the metabotropic activation of the release of calcium from intracellular stores (25-27). We found that pairing Glut I and PF stimulation induced LTD, as shown for one experiment in Fig. 2G (green symbols). The mean reduction in the PF EPSC was 23.5 ± 2.3% (n = 8) (Fig. 2H, green). A representative PF EPSC recorded before LTD induction (Fig. 2I Upper, blue trace) was larger than a representative EPSC recorded after LTD induction (Fig. 2I Upper, red trace).
LTD generated by Glut I-PF pairing was prevented by the addition of T-588. The PF EPSC was reduced by only 3.9 ±1.5% (n = 8) (Fig. 2 G and H, red). As in the two previous sets of findings, there was a significant difference between the LTD induced in the presence and in the absence of this drug (P < 0.01). In the presence of T-588, a PF EPSC before LTD induction (Fig. 2I Lower, blue trace) and one recorded after LTD induction (Fig. 2I Lower, red traces) were very similar. (The Ringer's solution contained 1 μM T-588 superfused throughout the recording period to avoid T-588 washout.)
T-588 Has a Reversible Effect on LTD and Does Not Itself Produce LTD. As a continuation of the experiment shown in Fig. 2 D-F, we measured the effect of the washout of T-588 to determine whether the effect of this drug on LTD was reversible. The mean LTD induced by pairing PC depolarization with PF stimulation (Fig. 2E), is shown at time 0 in the presence of T-588 (Fig. 3A, red symbol) and in its absence (Fig. 3A, green symbol). LTD was determined at a different time after the beginning of drug washout in each of eight experiments (Fig. 3A, black symbols). The LTD showed a return to control levels 90 min after the initiation of the washout procedure.
The possibility that the effects shown in Fig. 2 could occur because T-588 itself generates LTD (perhaps explaining why PF EPSCs showed no LTD) was tested in two sets of experiments. In the first set, the drug was added after the beginning of the PF stimulation sequence. If T-588 were to induce LTD, a reduction in the amplitude of the EPCS would be observed. This result was not the case, as shown in Fig. 3B (n = 3). In the second set of experiments, direct PC depolarization paired with PF stimulation (Fig. 3C, blue bar) failed to induce LTD (Fig. 3C), demonstrating that the drug effect was, indeed, present.
LTD Triggering by Caffeine Is Blocked by T-588. All manipulations capable of triggering LTD have in common an increase in [Ca2+]i from intracellular stores (28-30). The two main mechanisms responsible are IP3- (31) or ryanodine-receptor- (32) triggered release (see Fig. 5A). However, these two mechanisms are rather entangled with each other, and their separation is difficult (33, 34). In general terms, IP3 seems to be associated with apoptosis, whereas ryanodine receptors may be more closely related to the necrosis pathway. We designed an experiment to test the hypothesis that T-588 acted to block the release of calcium from intracellular stores, preventing or reducing the [Ca2+]i increase that was necessary to trigger LTD. We concluded that the best paradigm would be CfICR, because it is known that caffeine triggers nonconjunctive LTD (35). We found that, in the presence of 10 mM caffeine, LTD was induced by nonconjunctive stimulation (PC depolarization followed by PF stimulation), as shown for one experiment in Fig. 4A (blue symbols). The mean reduction in the PF EPSC was 19.6 ± 0.3% (n = 3) (Fig. 4B, blue column). Consistent with this finding, a representative PF EPSC recorded before LTD induction (Fig. 4C Upper, blue trace) was larger than a representative EPSC recorded after induction (Fig. 4C Upper, red trace). Each trace is the mean of five responses.
Fig. 5.
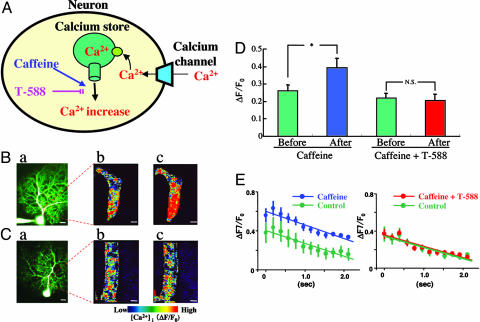
Two-photon imaging of the effect of CfICR and of T-588 on [Ca2+]i dynamics in PCs. (A) Diagram of caffeine increase and T-588 block of CfICR. (B) Effect of caffeine on response to PC depolarization. (a) Low-power image of PC filled with calcium-sensitive dyes (calcium green 1 and 2). (b and c) Magnified views of the region outlined by the box in a during PC depolarization before (b) and after (c) addition of 10 mM caffeine to the bathing solution. (C) Effect of T-588. (a) Low-power image of dye-filled PC. (b and c) Magnified views of the region outlined by the box in a during PC depolarization in the presence of T-588 before (b) and after (c) addition of caffeine (10 mM). (D) The mean ratio of stimuli-induced calcium changes in a PC dendrite before (first green column) and after (blue column) caffeine infusion (10 mM). Mean ratio in the presence of T-588 before (second green column), and after (red column) addition of 10 mM caffeine. Note that T-588 reduced the increase of [Ca+]i by caffeine (mean ± SEM; *, P < 0.05, paired t test). (E) Time course of calcium changes immediately after depolarizing pulse in a PC dendrite under control conditions before (Left green symbols) and after (blue symbols) caffeine infusion (10 mM). Same protocol but in the presence of T-588 (10 μM) before (Right green symbols) and after (red symbols) addition of caffeine (10 mM) (mean ± SEM). A significant difference was seen between control before and after caffeine infusion (P < 0.05, Scheffé's post hoc test). (Scale bars, 20 μm in Ba and Ca; 2 μm in Bb, Bc, Cb, and Cc.)
After superfusion with 1 μm of T-588, there was a mean increase (rather than a decrease) in the PF EPSC (-0.9 ± 5.3%, n = 3) (Fig. 4 A and B, red). An EPSC recorded before nonconjunctive stimulation (Fig. 4C Lower, blue trace) was similar to one recorded after stimulation (Fig. 4C Lower, red trace). Thus, nonconjunctive stimulation is able to elicit LTD in the presence of increased [Ca2+]i (due to caffeine, in this case) blocked by T-588. Increased [Ca2+]i is a necessary condition for LTD induction, but the conjunctive paring of PF stimulation with membrane depolarization is not needed.
Two-Photon Imaging of [Ca2+]i Modulation by T-588. The above set of experiments supports the hypothesis that LTD has a neuroprotective function that is triggered once the [Ca2+]i has reached a certain critical level. In this way, LTD limits release of calcium from intracellular stores and further increases in [Ca2+]i. These experiments further indicate that T-588 prevents LTD induction by reducing calcium release from intracellular stores. To examine directly whether this was the case, we imaged changes in PCs [Ca2+]i by using direct PC depolarization in the presence of caffeine. Two-photon calcium images were collected after diffusion of calcium sensitive dyes (calcium green 1 and 2) (22) through the patch perforation (see Fig. 5 Ba and Ca). PC proximal dendrites were chosen for the frame scanning of the change in intracellular calcium. Imaging was implemented continuously for 2.112 sec, divided into 192-msec frames. An image of the intradendritic-calcium-concentration profile obtained during a depolarization pulse is shown in Fig. 5Bb. After the addition of 10 mM caffeine to the bath, the amplitude and duration of the calcium response to the depolarizing pulse was markedly increased (Fig. 5Bc). The mean values for seven experiments are shown in the first two columns in Fig. 5D (before caffeine, 0.261 ± 0.034, n = 7; after caffeine, 0.393 ± 0.054, n = 7, P < 0.05).
When the cerebellar slice was preincubated with 1 μM T-588 (Fig. 5C), the response to PC depolarization was the same as before the drug was added to the superfusate (compare Fig. 5 Bb and Cb). After addition of 10 mM caffeine to the superfusate (Fig. 5Cc), the response-amplitude increase to the depolarizing pulse was absent (compare Fig. 5 Cb and Cc). Mean values for seven experiments are shown in the last two columns in Fig. 5D (T-588, no caffeine, 0.218 ± 0.029; T-588 and caffeine, 0.205 ± 0.038).
These results indicate that T-588 has a clearly regulative effect on the CfICR, which has been shown to exist in PCs (32, 36, 37). Indeed, these experiments demonstrated that T-588 had a suppressing effect on CfICR increase, correlated with a reduction in LTD (Fig. 4). Although the absolute level of calcium was greater in the presence of caffeine, the mean time course of the change in intradendritic calcium concentration immediately after the depolarizing pulse was the same before (Fig. 5E Left, green, n = 7) and after (Fig. 5E, blue, n = 7) caffeine infusion. In the presence of T-588, both the mean amplitude and rate of change of [Ca2+]i were identical before (Fig. 5E Right, green, n = 7) and after (Fig. 5E, red, n = 7) infusion of caffeine.
We conclude that membrane depolarization of sufficient amplitude and duration, regardless of mechanism, can result in a reduction of the sensitivity of GluRs in the spines of PC dendrites. This conclusion confirms similar results found by a substantial number of investigators (21, 31, 38, 39). Our results further demonstrate that a drug that is known to have neuroprotective properties also prevents LTD, suggesting that LTD may be considered to be a neuroprotective mechanism, because it is known that increased [Ca2+]i can lead to dendritic damage in PCs (40).
Discussion
The results illustrated here indicate three salient experimental findings: (i) that T-588, a drug that reduces calcium release from intracellular stores, prevents LTD independently from the experimental methodology used to trigger it; (ii) that LTD occurs when [Ca2+]i increases, an event that is known to activate dendritic death in PCs, and, when sufficiently high, whole-cell damage ensues (16, 17); and (iii) that LTD, rather than being a mechanism for cerebellar learning, is a neuroprotective mechanism by which overactivated PCs reduce dendritic excitability by lowering the gain of glutamatergic synaptic transmission. Indeed, it is well documented that alkaloids, such as harmaline and ibotenic acid, will produce PC death when the CF system is overactivated (41, 42).
These findings question the view that the CF input regulates the PF input and, more fundamentally, the functional role of these afferent systems. It is well known that, in brain slices, LTD can occur by synaptic interaction of CFs on each other and of PFs on each other (43) and that LTD is enhanced by inhibitory block. This type of finding is contrary to the original hypothesis by which cerebellar learning was supposed to occur, i.e., the exclusive interaction between CF and PF synaptic systems on the PC. Interestingly, it has been known that LTD is observed in vivo only in the presence of GABA blockers (44), a condition in which calcium conductance is increased to the level of triggering secondary calcium release.
We suggest that LTD functions to maintain the level of intracellular calcium within a narrow range that is below the threshold for triggering cell damage and is, therefore, neuroprotective. It must be understood that T-588 does not block the LTD response directly, but rather prevents the triggering of LTD by reducing calcium release from intracellular stores. It is well known that, as mentioned above, high [Ca2+]i induces cell death and that when the [Ca2+]i increases beyond a certain level, LTD is triggered (40). Such a calcium increase triggers a reduction in ionotropic GluR sensitivity and a reduction in calcium influx via voltage-dependent channels (8). We propose that the desensitization of GluRs occurs to reduce further increases in [Ca2+]i. We reasoned that a drug that reduces [Ca2+]i would act as a neuroprotective compound, because it prevents increases in [Ca2+]i. In the case where T-588 is used, there is a reduction of [Ca2+]i due to its action on intracellular release, and there is no need to reduce GluR sensitivity through LTD. Our results with T-588 are consistent with this view. Although the proposed neuroprotective role of LTD has a different mechanism than that of T-588, the drug and LTD both lead to a decrease in [Ca2+]i and protect PCs from calcium-induced cell death.
An important issue here is to differentiate the motor-learning problems that arise in mGluR1 mutant mice (where the ability to learn and LTD are impaired) (18, 35, 45, 46) from problems related to altered motor coordination. Indeed, it must be noted that, in addition to learning difficulties, these mutant animals show severe ataxia, intention tremor, and poor motor coordination, so it is difficult to separate motor abnormalities from motor-learning disability. An important issue to consider is the introduction of transgene (L7-mGluR1) that expressed mGluR1a under the control of PC-specific L7 promoter into mGluR1 (-/-) mice (mGluR1-rescued only on PCs). In this case, there was no fundamental difference in LTD expression or motor function between mGluR1(+/+) and the mGluR1-rescued mice. We have observed that brain levels of T-588 that prevent LTD triggering in vivo do not produce obvious changes in motor function in both rats and mice but do prevent LTD triggering (15). Thus, a clear difference exists between the mGluR1(-/-) and the T-588-treated mouse. In the former, suppression of LTD is accompanied by motor behavior changes (18), whereas, in the latter, no motor problems are present. We can conclude, therefore, that mGluR1 is essential for proper motor execution and, thus, for motor learning and that its modification leads to abnormal learning and LTD block. By contrast, T-588 prevents LTD but does not affect motor execution or motor learning.
As will be seen in the companion paper, T-588 can also prevent the LTD that can be evoked after GABA blockers in vivo, at concentrations similar to those used in these experiments. The present set of results, indeed, suggests that the olivocerebellar system, rather than being a “teaching circuit” (47), is more likely involved in the timing organization of movement, by implementing temporal motor binding (9, 10). Indeed, increasing evidence supports a role for the inferior olive in the actual organization of movement, in agreement with the very special properties of this system from a temporal perspective. In particular, the oscillatory properties of olivary cells, the fact that they are electrotonically coupled, and that their conduction velocity is matched to produce isochronous activation of PCs (11) all point to a well defined function for the CF system in its own right.
These results leave untouched the role of the cerebellum in motor learning. It is quite clear that the cerebellum is necessary for motor learning to occur. However, the fact that it is thus involved never implied that the actual learning, the proposed cerebellar “learning engram,” was to be found in the interaction between the CF and PF systems. Perhaps as we reexamine the functional properties of the parallel and climbing afferent system in the organization of PC activity, a more complete understanding of cerebellar dynamics in motor coordination and in the acquisition of new motor tasks will emerge from such perspective.
Author contributions: R.R.L. designed research; T.K. and M.S. performed research; T.K., M.S., and R.R.L. analyzed data; and T.K., M.S., and R.R.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: [Ca2+]i, intracellular calcium concentration; CF, climbing fiber; CfICR, caffeine-induced cytosolic Ca2+ release; EPSCs, excitatory postsynaptic currents; GluR, glutamate receptor; Glut I, glutamate iontophoresis; LTD, long-term depression; PC, Purkinje cell; PF, parallel fiber; T-588, (1R)-1-benzothiophen-5-yl-2[2-(diethylamino)ethoxy]ethanol hydrochloride.
References
- 1.Cajal, R. (1888) Trimest. Histol. 1, 1-102. [Google Scholar]
- 2.Eccles, J. C., Llinás, R. & Sasaki, K. (1965) Proc. Aust. Assoc. Neurol. 3, 7-14. [PubMed] [Google Scholar]
- 3.Eccles, J. C., Llinás, R. & Sasaki, K. (1966) J. Physiol. 182, 268-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palay, S. & Chan-Palay, V. (1974) Cerebral Cortex Cytology and Organization (Springer, New York).
- 5.Thach, W. T. (1967) J. Neurophysiol. 30, 675-696. [DOI] [PubMed] [Google Scholar]
- 6.Lang, E. J., Sugihara, I., Welsh, J. P. & Llinás, R. (1999) J. Neurosci. 19, 2728-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llinás, R. & Yarom, Y. (1981) J. Physiol. (London) 315, 549-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito, M. (2001) Physiol. Rev. 81, 1143-1195. [DOI] [PubMed] [Google Scholar]
- 9.Llinás, R. (1991) in Motor Control: Concepts and Issues, eds. Humphrey, D. & Freund, H. (Wiley, New York), pp. 223-242.
- 10.Welsh, J. P., Lang, E. J., Suglhara, I. & Llinás, R. (1995) Nature 374, 453-457. [DOI] [PubMed] [Google Scholar]
- 11.Sugihara, I., Lang, E. J. & Llinás, R. (1993) J. Physiol. (London) 470, 243-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llinás, R., Lang, E. J. & Welsh, J. P. (1997) Learn. Mem. 3, 445-455. [DOI] [PubMed] [Google Scholar]
- 13.Takuma, K., Fujita, T., Kimura, Y., Tanabe, M., Yamamuro, A., Lee, E., Mori, K., Koyama, Y., Baba, A. & Matsuda, T. (2000) Eur. J. Pharmacol. 399, 1-8. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda, K., Iwasaki, Y., Kinoshita, M., Marubuchi, S. & Ono, S. (2000) Brain Res. 858, 84-91. [DOI] [PubMed] [Google Scholar]
- 15.Welsh, J., Yamaguchi, H., Zeng, X.-H., Kojo, M., Nakada, Y., Takagi, A., Sugimori, M. & Llinás, R. R. (2005) Proc. Natl. Acad. Sci. USA 102, 17166-17171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llinás, R. & Sugimori, M. (1980) J. Physiol. (London) 305, 197-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llinás, R. & Sugimori, M. (1980) J. Physiol. (London) 305, 171-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichise, T., Kano, M., Hashimoto, K., Yanagihara, D., Nakao, K., Shigemoto, R., Katsuki, M. & Aiba, A. (2000) Science 288, 1832-1835. [DOI] [PubMed] [Google Scholar]
- 19.Hartell, N. A. (2001) Neuropharmacology 40, 148-161. [DOI] [PubMed] [Google Scholar]
- 20.Blond, O., Daniel, H., Otani, S., Jaillard, D. & Crepel, F. (1997) Neuroscience 77, 945-954. [DOI] [PubMed] [Google Scholar]
- 21.Linden, D. J. & Connor, J. A. (1991) Science 254, 1656-1659. [DOI] [PubMed] [Google Scholar]
- 22.Denk, W., Sugimori, M. & Llinás, R. (1995) Proc. Natl. Acad. Sci. USA 92, 8279-8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata, K., Nakagawa, M., Urbano, F. J., Rosato-Siri, M. D., Moreira, J. E., Uchitel, O. D., Sugimori, M. & Llinás, R. (1999) Proc. Natl. Acad. Sci. USA 96, 14588-14593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimori, M., Tong, C. K., Fukuda, M., Moreira, J. E., Kojima, T., Mikoshiba, K. & Llinás, R. (1998) Neuroscience 86, 39-51. [DOI] [PubMed] [Google Scholar]
- 25.Berridge, M. J. (1998) Neuron 21, 13-26. [DOI] [PubMed] [Google Scholar]
- 26.Kumar, A. & Foster, T. C. (2005) Brain Res. 1031, 125-128. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama, M., Hong, K., Mikoshiba, K., Poo, M. M. & Kato, K. (2000) Nature 408, 584-588. [DOI] [PubMed] [Google Scholar]
- 28.Kasono, K. & Hirano, T. (1994) NeuroReport 6, 17-20. [DOI] [PubMed] [Google Scholar]
- 29.Khodakhah, K. & Armstrong, C. M. (1997) Proc. Natl. Acad. Sci. USA 94, 14009-14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konnerth, A., Dreessen, J. & Augustine, G. J. (1992) Proc. Natl. Acad. Sci. USA 89, 7051-7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y. T. & Linden, D. J. (2000) Neuron 25, 635-647. [DOI] [PubMed] [Google Scholar]
- 32.Kohda, K., Inoue, T. & Mikoshiba, K. (1995) J. Neurophysiol. 74, 2184-2188. [DOI] [PubMed] [Google Scholar]
- 33.Gruol, D. L., Netzeband, J. G. & Parsons, K. L. (1996) J. Neurophysiol. 76, 3325-3340. [DOI] [PubMed] [Google Scholar]
- 34.Sharp, A. H., McPherson, P. S., Dawson, T. M., Aoki, C., Campbell, K. P. & Snyder, S. H. (1993) J. Neurosci. 13, 3051-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Womack, M. D., Walker, J. W. & Khodakhah, K. (2000) J. Gen. Physiol. 115, 339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kano, M., Garaschuk, O., Verkhratsky, A. & Konnerth, A. (1995) J. Physiol. (London) 487, 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llano, I., DiPolo, R. & Marty, A. (1994) Neuron 12, 663-673. [DOI] [PubMed] [Google Scholar]
- 38.Daniel, H., Hemart, N., Jaillard, D. & Crepel, F. (1992) Exp. Brain. Res. 90, 327-331. [DOI] [PubMed] [Google Scholar]
- 39.Narasimhan, K., Pessah, I. N. & Linden, D. J. (1998) J. Neurophysiol. 80, 2963-2974. [DOI] [PubMed] [Google Scholar]
- 40.Llinás, R. & Sugimori, M. (1990) in Neurotoxicity of Excitatory Amino Acids, ed. Guidotti, A. (Raven, New York), pp. 1-10.
- 41.O'Herne, E. & Molliver, M. (1993) Neuroscience 55, 303-310. [DOI] [PubMed] [Google Scholar]
- 42.O'Herne, E. & Molliver, M. (1997) J. Neurosci. 17, 8828-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartell, N. A. (2002) Cerebellum 1, 3-18. [DOI] [PubMed] [Google Scholar]
- 44.Otani, S. & Connor, J. A. (1998) J. Physiol. (London) 511, 761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiba, A., Kano, M., Chen, C., Stanton, M. E., Fox, G. D., Herrup, K., Zwingman, T. A. & Tonegawa, S. (1994) Cell 79, 377-388. [PubMed] [Google Scholar]
- 46.Conquet, F., Bashir, Z. I., Davies, C. H., Daniel, H., Ferraguti, F., Bordi, F., Franz-Bacon, K., Reggiani, A., Matarese, V. & Conde, F. (1994) Nature 372, 237-243. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy, P. R. (1975) Med. Hypotheses 5, 799-807. [DOI] [PubMed] [Google Scholar]