Abstract
Extensive biochemical testing and 16S rRNA and rpoB sequence analysis revealed that clinical strain CF01Ent1, initially identified as Buttiauxella agrestis by the use of Api 32 biochemical strips, is a new organism in the Enterobacteriaceae family. It produced an inducible AmpC-type β-lactamase whose sequence shares 69 to 72% identity with those of the other AmpC-type β-lactamases of Enterobacteriaceae. This enzyme exhibits an atypical high affinity for all β-lactams tested.
In 1998, we isolated from a wound of a patient hospitalized in the intensive care ward at the teaching hospital of Clermont-Ferrand, France, an unusual strain of Enterobacteriaceae, designated CF01Ent1 and initially identified (by use of Api32E biochemical strips [BioMerieux]) with a probability of 99.7% as Buttiauxella agrestis, a species not usually recovered from human specimens (4, 11, 15). Extensive biochemical testing, which was performed with biotype-100 carbon source strips (BioMérieux), failed to identify the isolate strain as belonging to a known species, the closest being Klebsiella pneumoniae (isolate strain test results diverging from those for Klebsiella pneumoniae: negative reactions for d-melibiose, d-raffinose, palatinose, and myo-inositol).
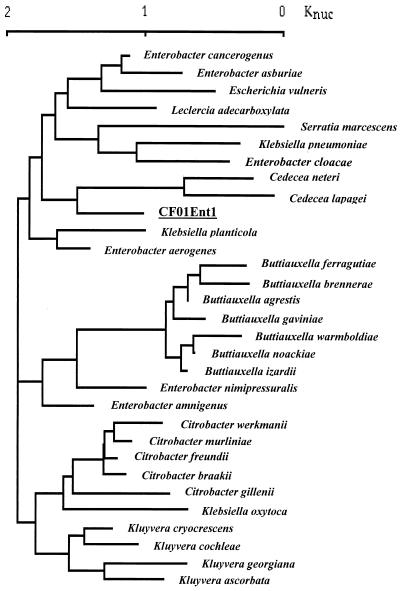
The 16S rRNA sequence of the organism was determined as previously reported (16) and compared (using Clustal software) with more than 222 16S rRNA sequences downloaded from GenBank (Fig. 1) or determined at Institut Pasteur. The tree constructed from this alignment, using neighbor-joining methods implemented by Lasergene software (DNAstar, Madison, Wis.), showed that the sequence was distinct from those of members of the branches containing species of genera Klebsiella, Buttiauxella, Kluyvera, and Citrobacter. The branching of strain CF01Ent1 differed according to the identity of the species selected for building the tree. The proximity with the Cedecea species shown in Fig. 1 was inconstant. These results were confirmed by the analysis of rpoB sequences (data not shown) (10). Using the results of the biochemical testing and 16S rRNA and rpoB sequencing, therefore, the isolated strain, which was designated CF01Ent1, was identified as the single representative of a new species of the family Enterobacteriaceae, which may be described more fully later, when other, similar isolates are collected. Although commercial microbial identification systems provide accurate identification of the common species of Enterobacteriaceae, this result shows that they are problematic with newly described organisms.
FIG. 1.
Dendrogram showing the 16S RNA sequence of strain CF01Ent1 and the 30 closest 16S RNA sequences of Enterobacteriaceae, constructed according to the neighbor-joining method and implemented using Lasergene software (DNAstar). The GenBank accession numbers for the corresponding bacteria are listed in brackets as follows: Buttiauxella gaviniae (AJ233403), Buttiauxella noackiae (AJ293689), Buttiauxella warmboldiae (AF233406), Buttiauxella izardii (AJ233404), Buttiauxella agrestis (AF233400), Buttiauxella ferragutiae (AJ233402), Buttiauxella brennerae (AJ233401), Buttiauxella gaviniae (AJ233403), Enterobacter aerogenes (AB004750), Enterobacter cloacae (AJ251469), Enterobacter amnigenus (AB004749), Enterobacter asburiae (AB004744), Kluyvera cochleae (AF047187), and Kluyvera georgiana (AF047186).
MICs for strain CF01Ent1 were determined on Mueller-Hinton agar with an inoculum of 104 CFU per spot by a dilution method (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) (1). The β-lactam resistance phenotype (Table 1) was characterized by resistance to amoxicillin (MIC of 32 μg/ml), cephalothin (64 μg/ml), and cefoxitin (32 μg/ml) and, to a lesser extent, to cefuroxime (8 μg/ml). Clavulanate (2 μg/ml) did not restore susceptibility to amoxicillin (MIC, 32 μg/ml). In contrast, the enteric bacterium CF01Ent1 was susceptible to ticarcillin, piperacillin, cefotaxime, ceftazidime, aztreonam, cefepime, cefpirome, and imipenem (MICs, 0.06 to 2 μg/ml).
TABLE 1.
Comparison of β-lactam MICs for strain CF01Ent1 and E. coli DH5α transformants containing AmpR-AmpC-encoding plasmid pCF01Ent1-8 or AmpC-encoding plasmid pCF01Ent1-3
| Substrate | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| CF01Ent1 | E. coli DH5α (pCF01BA1-8) | E. coli DH5α (pCF01BA1-3) | E. coli DH5α | |
| Amoxicillin | 32 | 256 | 1,024 | 2 |
| Amoxicillin + CLAa | 32 | 256 | 1,024 | 2 |
| Ticarcillin | 1 | 64 | 256 | 2 |
| Ticarcillin + CLA | 0.5 | 64 | 256 | 2 |
| Piperacillin | 2 | 8 | 32 | 2 |
| Piperacillin + TZB | 2 | 8 | 32 | 2 |
| Cephalothin | 64 | 256 | 512 | 4 |
| Cefuroxime | 8 | 64 | 64 | 4 |
| Cefoxitin | 32 | 128 | 128 | 4 |
| Cefotaxime | 0.25 | 2 | 8 | 0.06 |
| Cefpirome | 0.06 | 0.06 | 1 | 0.06 |
| Cefepime | 0.06 | 0.06 | 1 | 0.06 |
| Ceftazidime | 0.25 | 8 | 32 | 0.12 |
| Aztreonam | 0.12 | 2 | 2 | 0.12 |
| Imipenem | 0.5 | 0.25 | 0.25 | 0.12 |
Cla, clavulanate at a fixed concentration of 2 μg/ml; TZB, tazobactam at a fixed concentration of 4 μg/ml.
From a cell extract of strain CF01Ent1, one protein band of isoelectric point 9.2 had β-lactamase activity in analytical isoelectric focusing experiments, which were performed as previously reported (2).
The β-lactam resistance phenotype and the isoelectric point suggest the production of a class C cephalosporinase (3), but negative amplification tests were obtained with primers designed to amplify class C β-lactamase-encoding genes of Escherichia coli, Enterobacter aerogenes, Enterobacter cloacae, Citrobacter freundii, Morganella morganii, and Serratia marcescens. Cloning was undertaken in pBK-CMV vector (Stratagene, La Jolla, Calif.) with genomic DNA partially restricted with Sau3A, as previously reported (12). Two transformants harboring the recombinant plasmids pCF01Ent1-3 and pCF01Ent1-8 produced the β-lactamase of clinical strain CF01Ent1. Except for that of imipenem, the β-lactam MICs for the transformants were higher than those for the strain CF01Ent1 (Table 1).
To determine whether the β-lactamase gene was inducible, the specific β-lactamase activities of clinical strain CF01Ent1 and of the E. coli transformants were measured after induction for 2 h with cefoxitin at 16 μg/ml. Cell extracts of the induced E. coli transformant (pCF01Ent1-8) and induced strain CF01Ent1 had about 30-fold higher specific activities (148.0 ± 6 and 70.0 ± 2 U/mg of protein, respectively) than extracts from uninduced cultures (5.0 ± 0.6 and 2.5 ± 0.5 U/mg of protein, respectively). These results show that β-lactamase expression is inducible in strain CF01Ent1 and in the E. coli transformant (pCF01AB1-8). Chromosomal class C β-lactamases are generally regulated by a trans-acting protein, AmpR, which represses or induces the transcription of the ampC gene in the absence or in the presence of inducer, respectively (5, 8, 14).
In contrast, cell extracts of induced and uninduced E. coli harboring pCF01Ent1-3 had higher and similar specific activities (1,245.0 ± 10 and 1,516.5 ± 15 U/mg of protein, respectively). As a result of the alteration of the β-lactamase regulon in the recombinant plasmid pCF01Ent1-3, the β-lactamase expression is not repressed in this E. coli transformant, a finding similar to that for natural plasmid-encoded class C β-lactamases (8, 9).
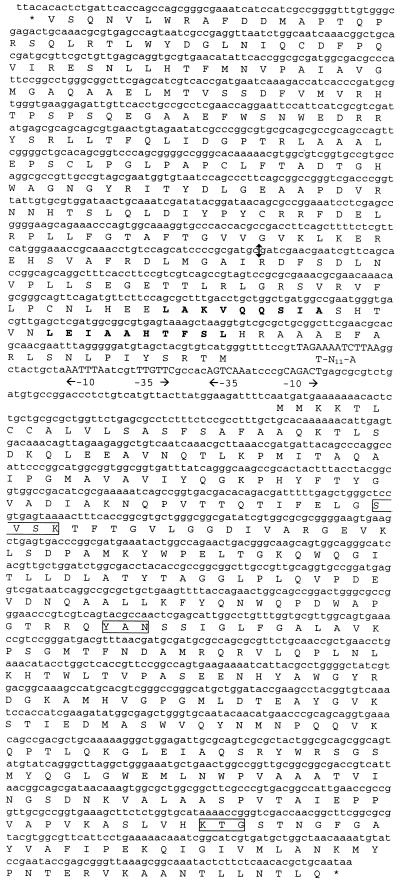
Recombinant plasmids pCF01Ent1-3 and pCF01Ent1-8, which harbor inserts of 3.5 and 8 kb, respectively, were used to sequence the β-lactamase gene of the clinical isolate by the dideoxy chain termination procedure of Sanger et al. (13). The sequence revealed an ampC-type β-lactamase gene downstream of, and oppositely oriented to, the sequence of a typical ampR regulator gene (Fig. 2), as previously reported for the chromosome-mediated class C β-lactamases genes (5, 8,14). The 86-bp ampR-ampC intercistronic region contained two overlapping and divergently oriented promoters as well as a Lys-R-type sequence, which was probably the fixation site of AmpR protein. As plasmid pCF01BA1-3 contained a partial 234-bp AmpR-encoding gene, the complete sequence of the ampR gene was obtained by sequencing plasmid pCF01Ent1-8. The deduced amino acid sequence of the 873-bp AmpR-type open reading frame was 81 to 85% identical to the known AmpR-type protein and harbored a helix-turn-helix motif, which is typical of transcriptional activators of the Lys-R family, as observed in other AmpR proteins (5, 14).
FIG. 2.
The nucleotide sequences of ampR-ampC genes and their deduced amino acid sequences. The putative promoter regions are indicated by −10 and −35, and the nucleic Lys-R motif is indicated by T-N11-A. The consensus sites of class C β-lactamases are boxed, and the predicted helixes of AmpR helix-turn-helix motif are in bold. The double arrow in the ampR gene sequence indicates the site of cloning in recombinant plasmid pCF01BA1-3.
The 1,194-bp-long AmpC-type type open reading frame encoded a 390-amino acid sequence, which contained the consensus sites SXXK, YAN, and KTG of class C β-lactamases (6). Analysis of this precursor protein suggested that the leader peptide comprised 20 residues. The molecular mass and pI of the deduced mature protein were 39,822 kDa and 9.26, respectively, which is in agreement with the experimental pI value.
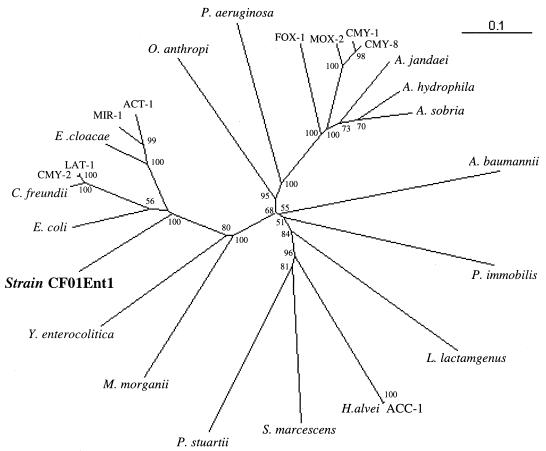
The AmpC-type β-lactamase was compared with 25 other chromosomally encoded and plasmid-encoded class C cephalosporinases (data not shown). Plasmid-mediated class C cephalosporinases have about 87 to 99% identity with the chromosomally encoded class C β-lactamases of C. freundii (LAT group and CMY-2 to CMY-7), E. cloacae, Enterobacter asburiae (MIR-1 and ACT-1), and Hafnia alvei (ACC-1) and therefore probably originate from these species by transfer of their β-lactamase genes into plasmids. The plasmid-encoded enzymes MOX-2, FOX-1, CMY-1, and CMY-8 to CMY-11 are only distantly related (<75% identity) to the known chromosomally encoded class C β-lactamases and are therefore “orphans.” AmpC β-lactamase of strain CF01Ent1, designated Ent-1, was only about 40% identical to these orphan plasmid-mediated class C cephalosporinases and therefore cannot be the origin of the plasmidic class C β-lactamases. The most closely related class C β-lactamases were from the genus Enterobacter, with about 70% identity (and from that corresponding to the unpublished GenBank sequence no. AJ415568, with 98% identity). The phylogenic tree (Fig. 3), which was constructed as previously reported (2), showed that the class C cephalosporinase of strain CF01Ent1 clustered with chromosomally mediated class C β-lactamases of Enterobacteriaceae, between those of a species group comprising E. coli, C. freundii, and E cloacae and that of Yersinia enterocolitica (69 to 70% and 51% identity, respectively).
FIG. 3.
Dendrogram of representative plasmid-encoded and chromosomally encoded AmpC β-lactamases. Branch lengths are to scale according to the amino acid changes determined using the Blosum matrix. The percentages at the branch points refer to the number of times a particular node was found in 100 bootstrap replications. The GenBank accession numbers for AmpC are listed in brackets as follows: Enterobacter aerogenes (AF211348), E. cloacae (X07274), E. coli (U14003), Citrobacter freundii (AF349569), Morganella morganii (AF055067), Yersinia enterocolitica (X63149), Providencia stuartii (Y17315), Serratia marcescens (AF327324), Hafnia alvei (AF180960), Aeromonas hydrophila (AF2766030), Aeromonas sobria (X80276), Aeromonas jandaei (S13408), Pseudomonas aeruginosa (X54719), Ochrobactrum anthropi (AJ401618), Acinetobacter baumannii (AJ009979), Lysobacter lactamgenus (X56660), and Psychrobacter immobilis (X83586). The GenBank accession numbers for the representative plasmid-mediated AmpC β-lactamases are listed in brackets as follows: FOX-1 (X77455), CMY-1 (X92508), CMY-2 (Y16784), CMY-8 (AF167990), LAT-1 (X78117), MOX-2 (AJ276453), and ACC-1 (AJ133121).
The kinetic constants of the β-lactamase were obtained with partially purified extracts as reported previously (7) (Table 2). The best substrates of this β-lactamase were benzylpenicillin (relative maximum rate of hydrolysis [Vmax], 100%), cephalothin (relative Vmax, 130%) and cefazolin (relative Vmax, 770%), and no hydrolysis was detected for ticarcillin and piperacillin, which are expanded- and broad-spectrum cephalosporins, respectively. Except for cephalothin (Km, 80 μM), the affinity of the β-lactamase for all substrates (Ki and Km, 0.05 to 20 μM) was higher than those previously reported for AmpC-type β-lactamases (3). This difference could be explained by a very low deacylation step during the hydrolysis of the substrates.
TABLE 2.
Substrate profile of AmpC-type β-lactamase of strain CF01Ent1
| Substrate | Relative Vmaxa (%) | Km or Kib (μM) | Relative Vmax/Kmc (%) |
|---|---|---|---|
| Benzylpenicillin | 100 | 4.5 | 100 |
| Amoxicillin | 5 | 12 | 2 |
| Ticarcillin | <1 | 0.5 | |
| Cephalothin | 130 | 6 | 100 |
| Cefazolin | 770 | 80 | 45 |
| Cefuroxime | <1 | 0.05 | |
| Cefoxitin | <1 | 0.7 | |
| Cefotaxime | <1 | 0.2 | |
| Ceftazidime | <1 | 20 |
Vmax values are given as percentages of benzylpenicillin taken as 100%.
Ki values were determined by substrate competition with cephalothin.
Vmax/Km values are given as percentages of the relative Vmax/Km ratio for benzylpenicillin, which was defined as 100%.
We characterized a new inducible AmpC-type β-lactamase from a clinical isolate of a new member of the Enterobacteriaceae family. Its enzymatic properties were characterized by a high affinity for all substrates and weak hydrolytic activity.
Nucleotide sequence accession number.
The ampR-ampC, rpoB, and 16S rRNA sequences are listed under GenBank accession no. AF440406, AJ489827, and AJ489826, respectively.
Acknowledgments
We thank Rolande Perroux, Marlène Jan, and Dominique Rubio for technical assistance and Jeffrey Watts for reading the English manuscript.
This work was supported in part by a grant from Ministère de l'Education Nationale, de la Recherche et de la Technologie.
REFERENCES
- 1.Bonnet, R., C. De Champs, D. Sirot, C. Chanal, R. Labia, and J. Sirot. 1999. Diversity of TEM mutants in Proteus mirabilis. Antimicrob. Agents Chemother. 43:2671-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet, R., C. Dutour, J. L. M. Sampaio, C. Chanal, D. Sirot, R. Labia, C. De Champs, and J. Sirot. 2001. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240→Gly. Antimicrob. Agents Chemother. 45:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freney, J., M. O. Husson, F. Gavini, S. Madier, A. Martra, D. Izard, H. Leclerc, and J. Fleurette. 1988. Susceptibilities to antibiotics and antiseptics of new species of the family Enterobacteriaceae. Antimicrob. Agents Chemother. 32:873-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henikoff, S., G. W. Haughn, D. L. Wulff, and K. Kersters. 1988. A large family of bacterial activator proteins. Proc. Natl. Acad. Sci. USA 85:6602-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joris, B., J. M. Ghuysen, G. Dive, A. Renard, O. Dideberg, P. Charlier, J. M. Frere, J. A. Kelly, J. C. Boyington, P. C. Moews, and J. R. Knox. 1988. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Eur. J. Biochem. 250:313-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labia, R., J. Andrillon, and F. Le Goffic. 1973. Computerized microacidimetric determination of β-lactamase Michaelis-Menten constants. FEBS Lett. 33:42-44. [DOI] [PubMed] [Google Scholar]
- 8.Lindberg, F., L. Westman, and S. Normark. 1987. Regulatory components in Citrobacter freundii ampC β-lactamase induction. Proc. Natl. Acad. Sci. USA 82:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumura, N., S. Minami, and S. Mitsuhashi. 1998. Sequences of homologous β-lactamases from clinical isolates of Serratia marcescens with different substrate specificities. Antimicrob. Agents Chemother. 42:176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollet, C., M. Drancourt, and D. Raoult. 1997. rpoB sequence analysis as a novel basis for bacterial identification. Mol. Microbiol. 26:1005-1011. [DOI] [PubMed] [Google Scholar]
- 11.Müller, H. E., D. J. Brenner, G. R. Fanning, P. A. D. Grimont, and P. Kämfper. 1996. Emended description of Buttiauxella agrestis with recognition of six new species of Buttiauxella and two new species of Kluyvera. Int. J. Syst. Bacteriol. 46:50-63. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schell, M. A. 1993. Molecular biology of the Lys-R family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 15.Spröer, C., U. Mendrock, J. Swiderski, E. Lang, and E. Stackebrandt. 1999. The phylogenetic position of Serratia, Buttiauxella and some other genera of the family Enterobacteriaceae. Int. J. Syst. Bacteriol. 49:1433-1438. [DOI] [PubMed] [Google Scholar]
- 16.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]