Abstract
Two of the most commonly used immunosuppressants, cyclosporine A and tacrolimus (FK506), inhibit the activity of a ubiquitously expressed Ca2+/calmodulin-sensitive phosphatase, calcineurin. Because both drugs also cause profound bone loss in humans and in animal models, we explored whether calcineurin played a role in regulating skeletal remodeling. We found that osteoblasts contained mRNA and protein for all isoforms of calcineurin A and B. TAT-assisted transduction of fusion protein TAT-calcineurin Aα into osteoblasts resulted in the enhanced expression of the osteoblast differentiation markers Runx-2, alkaline phosphatase, bone sialoprotein, and osteocalcin. This expression was associated with a dramatic enhancement of bone formation in intact calvarial cultures. Calcineurin Aα-/- mice displayed severe osteoporosis, markedly reduced mineral apposition rates, and attenuated colony formation in 10-day ex vivo stromal cell cultures. The latter was associated with significant reductions in Runx2, bone sialoprotein, and osteocalcin expression, paralleled by similar decreases in response to FK506. Together, the gain- and loss-of-function experiments indicate that calcineurin regulates bone formation through an effect on osteoblast differentiation.
Keywords: FK506, osteoporosis, runx-2
Calcineurin, a Ser/Thr protein phosphatase sensitive to Ca2+ and calmodulin, plays a critical role in coupling Ca2+ signals to cellular responses (1, 2). The calcineurin heterodimer consists of catalytic and regulatory subunits, A and B (1). The three known isoforms of mammalian calcineurin A (α, β, and γ), which are products of different genes, exhibit ≈86% sequence homology, whereas the B subunits are highly conserved from yeast to man (3). Calcineurin is distributed widely in the brain and lymphocytes (4-7) and has established roles in T cell activation, vesicular trafficking, cell growth, apoptosis, neuron depotentiation, muscle development, and cardiac valve formation (reviewed in refs. 8 and 9).
Both calcineurin Aα and Aβ knockout mice show defective antigen-specific T cell responses in vivo, indicating that both isoforms are required for T cell function (10, 11). In contrast, calcineurin Aα, but not Aβ, is required specifically for kidney development (12). Additionally, mossy-fiber cytoskeletons of calcineurin Aα-/- brains appear abnormal, suggesting that calcineurin Aα regulates the phosphorylation of the microtubule-associated protein τ (13). More recently, calcineurins Aα and Aγ have been implicated in the pathogenesis of Alzheimer's disease and schizophrenia, respectively (14, 15). Thus, whereas the tissue distribution and function of the three calcineurin isoforms appears to be overlapping (16), isoform-specific functions are just beginning to be identified.
The two commonly used immunosuppressants, cyclosporin A and tacrolimus (FK506), potently inhibit calcineurin's phosphatase activity by interacting with distinct domains of the calcineurin A subunit (17). The systemic administration of either drug to rats causes a dramatic osteoporosis (18, 19). In humans, acute, rapid, severe bone loss ensues with a fracture incidence approaching 65% (20, 21). It has been speculated, but never proven in either animals or humans, that cyclosporine A or FK506 use the calcineurin pathway to exert their skeletal effects. Obviously, the likelihood of such a pathway will depend on whether osteoblasts and osteoclasts, the likely target cells for both agents, express calcineurin and whether calcineurin plays a central role in the formation and (or) function of these cells.
We and others have shown recently that calcineurin plays a critical role in regulating the genesis and function of osteoclasts (22-25). Calcineurin Aα overexpression inhibits bone resorption by mature osteoclasts (23). In contrast, there are several reports indicating that the downstream substrate of calcineurin, NFAT, is necessary for osteoclastogenesis (24, 25). Thus, stem cell precursors from NFATc1-/- mice fail to form osteoclasts (24). It is, therefore, possible that calcineurin positively regulates osteoclast formation but blocks the function of mature cells. Nonetheless, the role of calcineurin in bone formation by the osteoblast has remained unrecognized.
In this study, we demonstrate that calcineurin is expressed in osteoblasts. For gain-of-function studies, we fused the calcineurin Aα to TAT, a 12-amino-acid Arg-rich sequence that allows the transduction of full-length proteins into cells. The high-efficiency cellular uptake of TAT-calcineurin Aα resulted in a profound increase in the expression of the osteoblast differentiation markers Runx-2, alkaline phosphatase (ALP), bone sialoprotein (BSP), and osteocalcin. This increase was associated with enhanced bone formation in calvarial cultures. In contrast, the calcineurin Aα-/- mouse displayed severe osteoporosis with markedly reduced osteoblastic bone formation. Ex vivo cultures of osteoblasts derived from this mouse showed reduced colony-forming unit-fibroblastoid (CFU-F) formation and marker expression. The study, therefore, directly implicates the phosphatase calcineurin in the control of osteoblastic bone formation. Suppression of bone formation by calcineurin loss/inhibition may be the mechanism through which calcineurin inhibitors exert their clinical effects on the skeleton.
Materials and Methods
Immune Detection and Imaging. MC3T3.E1 osteoblasts were fixed in paraformaldehyde (4% vol/vol in PBS, pH 7.4) for 20 min at 20°C and washed with Hanks' balanced salt solution (GIBCO/BRL) before being exposed to antiserum PP2BAα (goat anti-calcineurin Aα antiserum; Santa Cruz Biotechnology) or nonimmune goat IgG or antiserum PP2BAα plus a monoclonal anti-TAT antibody (Applied Biosystems/Advanced Biotechnologies, Columbia, MD) or anti-hemaglutinnin (HA) antibody (for colocalization studies) (all in DMEM, 1:100). After 6 h, the coverslips were incubated with donkey FITC-conjugated anti-goat IgG (green) or with both anti-goat IgG and tetramethylrhodamine B isothiocyanate-conjugated anti-mouse IgG (red) (for colocalization experiments) (Jackson ImmunoResearch). An epifluorescence microscope (Olympus AX-700) and, in some experiments, a Leica TCS-SP (NT) laser confocal microscope was used to visualize the labeling. To localize TAT-calcineurin Aα, between 9 and 15 1-μm-thick optical sections were obtained in the cell's coronal plane in selected experiments.
For in situ localization, mouse bones were fixed in Millonig's solution (phosphate-buffered 5% vol/vol formaldehyde in isotonic 150 mM sucrose), followed by dehydration in graded alcohols and methacrylate embedding, as described in ref. 26. The bones were then sectioned at 6 μm by using a Reichert-Jung sledge microtome (Reichert and labeled by using the antibody PP2BAα (above). Controls were performed simultaneously by omitting the primary antibody.
Osteoblast membranes were prepared from cultured cells as described in ref. 27. Protein (50 μg) was loaded onto the SDS gel and subjected to electrophoresis at 20 mA. The resolved proteins were transferred onto nitrocellulose membrane at 15°C for 1 h at 100 mV, blocked with Tween 20 (0.3%) in TBS at 20°C and incubated with PP2BAα (1:3,000). The blot was incubated for 1 h with horseradish-peroxidase-conjugated anti-goat IgG and developed by using the Pierce Super Signal Ultra Chemiluminescence kit according to the manufacturer's instruction.
Gain-of-Function Studies Using TAT-Calcineurin Aα. TAT-calcineurin Aα and TAT-HA fusion proteins were prepared as described in ref. 28. Osteoblasts were incubated with TAT-calcineurin Aα (200 nM) or TAT-HA (200 nM) just once for 10 min at 37°C and then reincubated in α-MEM for up to 5 days. The cells were then appropriately immunolabeled (see above) to check transduction efficiency, cellular retention, and subcellular localization of the fusion proteins (28). Eleven days after transduction of the cells, real-time RT-PCR was performed to examine the expression of osteoblast differentiation markers Runx-2 (early), ALP, BSP, and osteocalcin (late) with appropriately designed primers.
In separate experiments, calvaria from newborn mice were isolated, cleaned, and incubated in α-MEM with vehicle, TAT-HA (200 nM), TAT-calcineurin Aα (200 nM), or bone morphogenetic protein (BMP-2, 200 ng/ml). After incubation with calcein (5 mg/ml) for 72 h (modified from ref. 29), the calvaria were embedded in aqueous gels, frozen in blocks, and cut at -33°C in a microm cryotome equipped with a CryoJane tape-transfer system (Instrumedics, Hackensack, NJ). Calcein labels were recorded in a 14-bit charge-coupled device camera (SPOT, Diagnostic Instruments, Sterling Heights, MI). Calcein labeling relative to total bone area was calculated by threshold mapping by using fovea pro (Reindeer Graphics, Ashville, NC). Results from five fields from three or more sections were expressed as calcein-labeled/total bone area.
Skeletal Phenotyping. Calcineurin Aα-/- mice survived until ≈8 weeks. Bone mineral density (BMD) was measured by a Lunar PIXImus (Lunar, Madison, WI) small-animal densitometer (precision <1.5%). The mice were killed and their femora and tibiae dissected to remove bone marrow stromal cells, which were then cultured in α-MEM plus ascorbate-2-phosphate (1 mM) for 10 days for real-time PCR studies, as described in ref. 30. Bones were processed separately for histological examination by fixation in 5% (vol/vol) phosphate-buffered formaldehyde, decalcification with HCl, dehydration, and paraffin embedding. Sections (6 μm) were stained with hematoxylin and eosin. In separate experiments, double tetracycline labeling was carried out to examine the extent and rate of osteoblastic bone formation. Tetracycline and demeclocyline (15 mg/kg of body weight) were administered i.p. on days -10 and -2, respectively. The mice were killed on day 0, and the vertebral columns and long bones were fixed in Millonig's solution before dehydration in graded alcohols and methacrylate embedding (26). The bones were sectioned at 6 μm and examined by using a 380- to 425-nm excitation filter and a 450-nm emission filter. Morphometry was performed by using a BioQuant imaging system (Bioquant, Nashville, TN). Measurements are from unstained, trichrome-, or von Kossa-stained nondecalcified sections (31). Nonstandard units are as defined by Parfitt et al. (32). CFU-F assays were performed as described in ref. 33.
Results
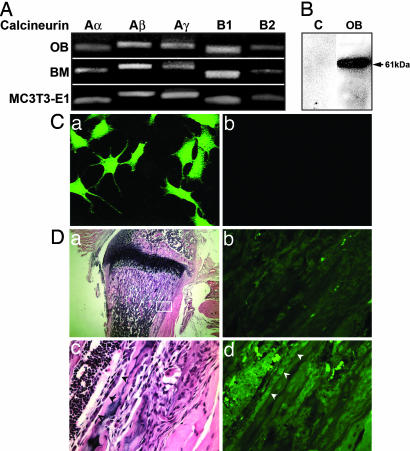
Osteoblasts Express Calcineurins. Using RT-PCR, Western blotting, and immune labeling, we explored whether bone marrow cells, bone-marrow-derived osteoblasts, and MC3T3.E1 preosteoblastic cells express the calcineurin isoforms. Fig. 1A shows a single band for each of the three isoforms of calcineurin A (α, β, and γ) and the two isoforms for calcineurin B (1 and 2). Western blotting of cell extracts revealed a single ≈61-kDa band, consistent with calcineurin Aα protein expression. Use of the same anti-calcineurin Aα antibody in immunocytochemical studies confirmed the expression of the protein in intact cells (Fig. 1Ca). Replacement of the antibody with nonimmune mouse serum did not result in any immunostaining (Fig. 1Cb). Fig. 1D shows osteoblasts in situ in bone sections (a and c), whereas d is the same section stained with an anti-calcineurin Aα antibody, demonstrating specific immune labeling of osteoblasts. Omitting the primary antibody abolished immune labeling (Fig. 1Db).
Fig. 1.
Expression of calcineurin A and B isoforms in osteoblasts. (A) RT-PCR bands showing the expression of calcineurin isoforms Aα, Aβ, Aγ, B1, and B2 in MC3T3.E1 cells, primary osteoblasts (OB), and freshly isolated bone marrow cells (BM). (B) Western blot showing a 61-kDa band in primary calvarial osteoblasts (30 μg of protein); C, control lane. (C) Immune localization of calcineurin Aα in MC3T3.E1 osteoblasts that were incubated with antibody PP2BAα (a) or nonimmune rabbit serum (b). (D) In situ labeling of osteoblasts for calcineurin Aα in the sections of wild-type mouse bones. (a)A6-μm section stained with hematoxylin and eosin at low power, with the region of interest (box) shown at high power in c, where osteoblasts are indicated by arrowheads. The same section is shown in epifluorescence after calcineurin Aα immune labeling, indicating that the osteoblasts are immunelabeled (arrowheads) (d). In an adjacent section, a comparable region in epifluorescence (b) is shown with the primary antibody omitted.
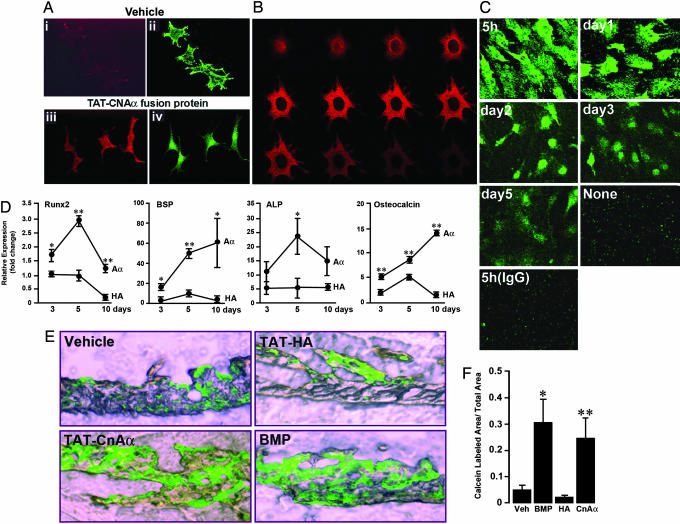
Calcineurin Overexpression Enhances Osteoblast Differentiation and Bone Formation. MC3T3.E1 osteoblasts were incubated with TAT-calcineurin Aα (10 min at 37°C) and stained, after glutaraldehyde fixation, with both anti-TAT (red) and anti-calcineurin Aα (green) antibodies. Cells transduced with vehicle alone showed only anti-calcineurin Aα (green) immune labeling, representing endogenous calcineurin Aα (Fig. 2Aii). However, transduction with TAT-calcineurin Aα resulted in a precise colocalization of the red and green immunostains within the same cells (Fig. 2 Aiii and Aiv), consistent with the influx of exogenously applied fusion protein that was detected by both antibodies. Confocal microscopy of the TAT-transduced fusion protein showed diffuse cytoplasmic staining with negligible localization to the nucleus across all coronal planes of the same cell (Fig. 2B), indicating that the TAT construct did not permeate the nucleus, an important control to rule out direct intranuclear actions of calcineurin. Finally, we showed that TAT-HA levels could be detected in the cytosol for the 5 days posttransduction, although levels diminished after day 2 (Fig. 2C).
Fig. 2.
Effect of overexpressing calcineurin Aα as a TAT fusion protein on osteoblast differentiation and bone formation. (A) Immunohistochemical detection of calcineurin Aα (CNAα) and TAT, respectively, with anti-CNAα antiserum (PPB2Aα, green) and anti-TAT antibody (red) in MC3T3.E1 osteoblasts that were transduced with TAT-CNAα fusion protein (200 nM) (Aiii and Aiv) or vehicle (Ai and Aii) for 10 min at 37°C. Note the similar distribution of red and green staining after transduction. (B) Confocal micrographs (1-μm-thick coronal sections of the same cell) demonstrating the mainly cytosolic localization of TAT-CNAα fusion protein in intact cells by using an anti-TAT antibody (red). Note that the nucleus is spared, indicating that the TAT construct does not permeate into the nuclear compartment. (C) Immunolabeling for HA (green) in primary osteoblasts treated with TAT-HA (200 nM for 10 min), demonstrating the persistence of TAT-HA for at least 5 days. No green staining was seen without transduction or with the use of an irrelevant IgG. (D) Enhanced expression of osteoblast differentiation markers, Runx-2 (early), BSP, ALP, and osteocalcin in MC3T3.E1 cells at 3, 5, and 10 days after transduction with TAT-CNAα (Aα) or TAT-HA, (control) (200 nM each for 10 min). The real-time PCR results are expressed as fold-change normalized to GAPDH and cell control (+SEM). Statistics by Student's t test; **, P < 0.01; *, P < 0.05, comparisons with HA at every time point. (E and F) Sections of calvaria from newborn mice incubated in the presence of calcein with vehicle, TAT-HA (200 nM), TAT-CNAα (200 nM), or BMP-2, 200 ng/ml. Quantitative estimates of calcein-labeled surfaces expressed relative to total surface area (F), measured morphometrically by using fovea pro (Reindeer Graphics). Mean ± SEM from five fields from three or more sections were analyzed by Student's t test, comparing each treatment with vehicle/TAT-HA; *, P < 0.05; **, P < 0.01.
Transduced MC3T3.E1 cells were grown under differentiating conditions in medium containing 10 mM ascorbic-2-phosphate for up to 10 days. We performed real-time RT-PCR to quantitate markers of osteoblast differentiation Runx-2, ALP, BSP, and osteocalcin. Compared with TAT-HA, TAT-calcineurin Aα produced a dramatic increase in the expression of Runx-2, ALP, BSP, and osteocalcin (normalized to GAPDH, a housekeeping gene) (Fig. 2D). That these effects were not reproduced by a control peptide, TAT-HA, indicated that the effects were not due to TAT introduction per se.
In separate experiments, we found that TAT-calcineurin Aα substantially enhanced calcein labeling of calvarial bones similarly to a known bone-forming molecule, BMP-2. Again, TAT-HA was without significant effect compared with vehicle treatment (Fig. 2 E and F). The results show directly that calcineurin Aα promotes bone formation and are consistent with the diminished bone formation seen in the calcineurin Aα knockout mouse (see below).
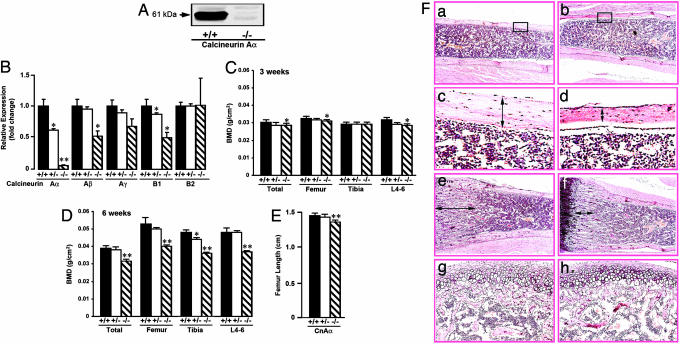
Calcineurin Aα-/- Mice Show Diminished Bone Formation and Severe Osteoporosis. Calcineurin Aα-/- mice were kindly provided by D. O'Conner and J. Seidman (Harvard Medical School, Boston) (10). We confirmed by Western blotting that whole-bone extracts from the calcineurin Aα-/- mouse did not express the ≈61-kDa band, consistent with deletion of the calcineurin Aα gene (Fig. 3A). This finding was also confirmed in real-time quantitative PCR studies on RNA derived from bone marrow osteoblast precursors. The Aα heterozygotes were haploinsufficient, whereas the homozygotes had no detectable protein (Fig. 3B). Calcineurin Aβ and B1 showed modest decrements in expression, whereas calcineurin Aγ and B2 showed no significant change (Fig. 3B), indicating that the phenotype resulted primarily from Aα deficiency, with minor contributions, if any, from the other catalytic and regulatory isoforms.
Fig. 3.
Phenotypic characterization of the calcineurin Aα-/- mice. (A) Western blot of a whole-bone extract showing the absence of the 61-kDa calcineurin Aα band in null mice. (B) Real-time PCR on RNA isolated from bone-marrow-derived osteoblasts to examine the expression of the calcineurin A and B isoforms (shown) in calcineurin Aα+/+,Aα+/-, and Aα-/- mice. (C and D) Severe osteoporosis in the calcineurin Aα-/- mouse at 6 weeks. Comparisons of BMD (g/cm2) at femur, tibia, lumbar spine (L4-L6), and total body in 3- (C) and 6-week-old (D) calcineurin Aα+/+,Aα+/-, and Aα-/- mice by using a Lunar PIXImus small animal densitometer (Lunar, precision <1.5%). Student's t test was used to compare the BMDs of homozygotes and heterozygotes with wild-type littermates at each site. **, P < 0.01; *, P < 0.05. (E) Estimates of femur length (cm) in calcineurin Aα+/+, Aα+/-, and Aα-/- mice. Statistics by Student's t test; **, P < 0.01. (F) Histological comparisons of cortical thickness (a and b at magnification ×1 and c and d at magnification ×2), overall trabecular volume (e and f), and growth plate (g and h) between 6-week-old calcineurin Aα-/- and Aα+/+ mice. There was ≈40% reduction in diaphyseal cortical thickness (Table 1) and a mild reduction in trabecular bone (not quantifiable precisely at this age) in calcineurin Aα-/- mice. The respective articular surfaces showed no apparent difference (data not shown).
Lunar PIXImus small-animal densitometry showed significant reductions in BMD at the lumbar spine (L4-L6), femur, and tibia, and total body in Aα-/- mice compared with both Aα+/+ and Aα+/- mice at 6 weeks and reduced BMD at the lumbar spine as early as 3 weeks (Fig. 3 C and D). No significant BMD differences were noted at any site between the wild type and heterozygotes, although there was a general trend toward lower BMDs in the Aα+/- mice compared with wild-type littermates. The mice were slightly runted, there was a <10% difference in femur lengths between Aα+/+ and Aα-/- mice, and the heterozygotes showed no difference compared with wild-type mice (Fig. 3E).
The low bone density in the calcineurin Aα-/- mouse was consistent with a dramatic ≈40% reduction in cortical thickness compared with wild-type littermates (Table 1). Fig. 3F confirms tibial cortical thinning (a-d) together with mild reductions in trabecular bone (e and f). No differences in the growth plate were noted (g and h). Tetracycline-labeling studies showed that the interlabel distance and mineral apposition rates were reduced significantly in the calcineurin Aα-/- mouse, indicating a lower rate of osteoblastic bone formation (Table 1). In contrast, tartrate-resistant acid phosphatase (TRAP)-positive resorption surfaces, osteoid area, and bone area were not reduced significantly in calcineurin Aα-/- mice compared with wild-type littermates (Table 1). Thus, there does not appear to be a significant osteoclast defect, at least in vivo.
Table 1. Histomorphometric measurements.
| Bone and parameter | Units | Aα+/+ | Aα–/– |
|---|---|---|---|
| Tibia, diaphysis | |||
| Cortex | μm | 105 ± 8 | 62 ± 6* |
| Bone diameter | μm | 610 ± 15 | 550 ± 50 |
| Vertebra, L3 | |||
| Mineralizing surface | % of surface | 39 ± 2.0 | 42.6 ± 1.5 |
| Resorbed surface | % of surface | 25 ± 7 | 21 ± 6 |
| Interlabel distance | μm | 20 ± 7 | 7 ± 3† |
| Mineral apposition rate | μm/day | 2.2 ± 0.8 | 0.8 ± 0.3† |
| Osteoid area | mm2 | 0.40 ± 0.08 | 0.38 ± 0.08 |
| Bone area | mm2 | 3.62 ± 0.14 | 3.22 ± 0.50 |
Selected histological measurements and calculated parameters in tibial cortex and L3 vertebral trabecular bone of 6-week-old tetracycline-labeled wild-type and calcineurin Aα–/– mice. Measurements are from unstained, trichrome, or von Kossa-stained undecalcified sections and hematoxylin and eosin-stained decalcified sections by digital histomorphometry as described in ref. 26. Nonstandard units are as defined by Parfitt et al. (32). Mean ± SD for cross sections from each of four animals per group. *, P < 0.01; †, P < 0.05; relative to wild type by Student's t test.
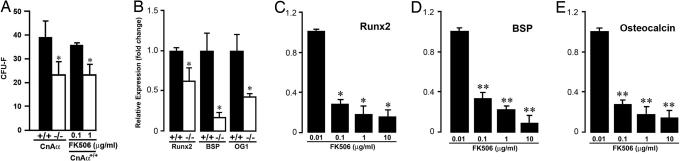
Calcineurin Aα Deletion or Inhibition Reduces Osteoblast Differentiation. To study the cellular basis of the reduced osteoblastic bone formation in vivo, we cultured calvarial osteoblasts ex vivo for 10 days, measured CFU-F formation, and used real-time PCR to compare the expression of the differentiation markers Runx2, BSP, and osteocalcin. Fig. 4A shows an ≈40% reduction in ALP-positive CFU-F formation in Aα-/- mice and in wild-type mice treated with FK506 compared with respective controls (Fig. 4A). Fig. 4B shows a significant decrease in Runx 2 expression and a profound inhibition of BSP and osteocalcin expression in Aα-/- CFU-Fs, indicating that calcineurin Aα deletion results in reduced osteoblast differentiation. Calvarial cells from wild-type mice were also treated with FK506 (0.01, 0.1, and 10 μg/ml), with the expectation that calcineurin Aα gene deletion and calcineurin enzyme inhibition would have similar effects. There was a concentration-dependent decrease in Runx2, BSP, and osteocalcin expression in response to FK506 (Fig. 4 C-E).
Fig. 4.
Effect of either calcineurin Aα gene deletion or the inhibition of calcineurin enzyme activity by FK506 on the number of CFU-F (A) and the expression of osteoblast genes (B-E), namely Runx2, BSP, and osteocalcin in mouse primary osteoblasts after 10 days of culture in the presence of ascorbate-5-phosphate (1 mM). Student's t test was used to compare with wild-type littermates or zero-dose. *, P < 0.06; **, P < 0.01.
Discussion
Together, the gain- and loss-of-function experiments indicate that calcineurin Aα enhances osteoblast differentiation to positively regulate bone formation. All calcineurin isoforms are expressed in the osteoblast. Calcineurin Aα promotes cell differentiation and bone formation when introduced into osteoblasts by using a high-efficiency TAT transduction system. The deletion or inactivation of calcineurin inhibits osteoblast differentiation. This attenuated osteoblast differentiation translates into markedly reduced bone-formation rates and profound osteoporosis in Aα-/- mice. In these mice, there is no major decrement in the expression of other calcineurin A isoforms, suggesting that the phenotype resulted mainly from a loss of the Aα isoform.
It is well known that FK506 complexes with the ubiquitously expressed FK-binding protein. This complex competitively binds to and inhibits calcineurin activation by Ca2+ and calmodulin (34, 35). With such specificity, one would expect that the skeletal effects of calcineurin inhibition by FK506 and calcineurin gene deletion are identical. In other words, reduced osteoblast differentiation and bone formation should be the mechanism through which calcineurin inhibitors cause bone loss (18-21). Although this may be the case, the osteoporosis resulting from calcineurin inhibitors is characterized by a high-turnover state in which both bone resorption and through-coupling bone formation are elevated (18, 19). Several mechanisms could underlie the osteoclastic activation seen with calcineurin inhibitors. Because calcineurin overexpression directly attenuates osteoclast activity (23), calcineurin inhibitors would be expected to stimulate bone resorption. Alternatively, FK506 could enhance resorption independently of calcineurin or via T cells (36). It is becoming clear that T cells can mediate the bone loss after estrogen withdrawal (37). Thus, T cell deficiency abrogates the bone loss due to both estrogen withdrawal and immunosuppressant therapy (36, 37).
In contrast to acute calcineurin inhibition, relatively chronic calcineurin deficiency in Aα-/- mice does not result in an in vivo osteoclastic phenotype. This compensation may arise from the dual actions of calcineurin: its facilitation of osteoclastogenesis (24, 25) versus its propensity to reduce the resorptive activity of mature cells (23). Thus, the deficiency of calcineurin should stimulate resorption but, paradoxically, reduce osteoclast formation, with no net change in histomorphometrically determined tartrate-resistant acid phosphatase (TRAP)-labeled surfaces (overall resorption). In the absence of elevated resorption and consistent with the action of calcineurin on osteoblast differentiation and function, the osteoporosis in Aα-/- mice arises from a defect in bone formation. Nonetheless, it is difficult, without the development of cell-specific knockout mice, to speculate on which action, osteoclastic or osteoblastic, is most relevant to calcineurin's role in regulating skeletal homeostasis.
Expression of the master gene for osteoblast differentiation Runx-2 is modestly decreased in the Aα-/--null mouse but is more dramatically attenuated by FK506. Runx-2's relatively modest suppression in the Aα-/- mice likely results from compensation by the other isoforms Aβ and Aγ; FK506, on the other hand, suppresses the activity of all calcineurin isoforms and, thus, inhibits Runx2 profoundly. Consistent with this finding, TAT-calcineurin Aα stimulates Runx-2 expression. It is unclear, however, whether Runx-2 mediates the enhanced osteoblastic differentiation or whether its up-regulation is a secondary effect.
Indeed, it is possible that effects of calcineurin on osteoblast differentiation are exerted through the traditional NFAT signaling pathway. In lymphocytes, for example, activation of calcineurin results in the NFAT-dependent activation of several genes, including the GM-CSF, interleukins 2, 3, 4, and 5, CD40, and the Fas genes (38). From our studies on myoblastic cells, we can infer additional dephosphorylation targets for calcineurin in the osteoblast, notably IκBβ (39). It is also possible that critical genes for Ca2+-release channels, including IP3 receptors and ryanodine receptors (RyRs) that are abundantly expressed in the osteoblast, are potential targets for osteoblastic calcineurin. The overexpression of calcineurin Aα in pleuripotent C2C12 cells results in dramatic increases in the expression of RyR-1 (40). It is notable that C2C12 cells can form myocytes, osteoblasts, or adipocytes under appropriate differentiating conditions (41). Oxidative stress resulting from mitochondrial DNA deletion is also associated with the enhanced expression of both RyR-1 and calcineurin Aα (39). Thus, there is a direct relationship between the expression of calcineurin Aα and target RyR genes. In addition, inhibitors such as FK506 decouple the molecular interaction between RyRs and calcineurin (42, 43).
An interesting clinical paradigm has emerged that significantly enhances the importance of our discovery of a key role for calcineurin in osteoblastic bone formation. The DSCR1 gene, a potent inhibitor of calcineurin activity located on human chromosome 21 is overexpressed in Down's syndrome as a result of trisomy (44). It is has been proposed that such overexpression results in defects in the development of the brain, immune system, heart, and skeleton in children with Down's syndrome (44). A peptide fragment of the DSCR1 gene has been identified as a calcineurin competitor (45). Nonetheless, restoration of the DSCR1 gene to disomy in the trisomy-16 mouse model of Down's syndrome has been found not to rescue the cardiac or craniofacial abnormalities (46), calling a role for calcineurin into question.
Finally, even though the phenotypes of calcineurin-inhibitor-treated and calcineurin Aα knockout animals differ somewhat, both cyclosporine and FK506 do reduce osteoblastic differentiation. Indeed, the drugs' ultimate effects in causing skeletal fractures may arise from poorly healing microcracks due to reduced bone formation. When the drugs are administered with glucocorticoids, these suppressive effects on bone formation may be even more pronounced. We anticipate that studies examining glucocorticoid effects in calcineurin-null mice will provide more definitive answers.
Acknowledgments
We thank Drs. O'Conner and Seidman for providing us with the knockout mouse. This work was supported by National Institutes of Health Grants R01 AG14197, DK70526, and AG23176 (to M.Z.) and AG12951 and AR47700 (to H.C.B.). M.Z., H.C.B., and B.S.M. also acknowledge the support of the Department of Veterans Affairs (Merit Review).
Author contributions: L.S., H.C.B., Y.P., and B.S.M. designed research; L.S., H.C.B., Y.P., N.Z., O.A.A., X.B.W., X.Y.W., and M.Z. performed research; L.S., Y.P., J.I., S.E., and E.A. contributed new reagents/analytic tools; L.S., H.C.B., and J.I. analyzed data; and J.I. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: ALP, alkaline phosphatase; BMD, bone mineral density; BSP, bone sialoprotein; CFU-F, colony-forming unit-fibroblastoid; HA, hemagglutinin; RyRs, ryanodine receptors.
References
- 1.Klee, C. B., Crouch, T. H. & Krinks, M. H. (1980) Proc. Natl. Acad. Sci. USA 76, 6270-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klee, C. B., Draetta, G. F. & Hubbard, M. J. (1988) Adv. Enzymol. Relat. Areas Mol. Biol. 61, 149-200. [DOI] [PubMed] [Google Scholar]
- 3.Klee, C. B., Ren, H. & Wang, X. (1998) J. Biol. Chem. 273, 13367-13370. [DOI] [PubMed] [Google Scholar]
- 4.Buttini, M., Limonta, S., Luyten, M. & Boddeke, H. (1995) Histochem. J. 27, 291-299. [DOI] [PubMed] [Google Scholar]
- 5.Guerini, D. (1997) Biochem. Biophys. Res. Commun. 235, 271-275. [DOI] [PubMed] [Google Scholar]
- 6.Kincaid, R. L., Takayama, H., Billingsley, M. L. & Sitkovsky M. V. (1987) Nature 330, 176-178. [DOI] [PubMed] [Google Scholar]
- 7.Liu, J., Albers, M. W., Wandless, T. J., Luan, S., Alberg, D. G., Belshaw, P. J., Cohen, P., MacKintosh, C., Klee, C. B. & Schreiber, S. L. (1992) Biochemistry 31, 3896-3901. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura, R., Sio, S. O., Shuntoh, H. & Kuno, T. (2001) Cell. Mol. Life Sci. 55, 278-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibasaki, F., Hallin, U. & Hiroyuki, U. (2002) J. Biochem. 131, 1-15. [DOI] [PubMed] [Google Scholar]
- 10.Zhang, W., Zimmer, G., Chen, J., Ladd, D., Li, E., Alt, F. W., Wiederrecht, G., Cryan, J., O'Neill, E. A., Seidman, C. E., et al. (1996) J. Exp. Med. 183, 413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno, O. F., Brandt, E. B., Rothenberg, M. E. & Molkentin, J. D. (2002) Proc. Natl. Acad. Sci. USA 99, 9389-9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooch, J. L., Toro, J. J., Guler, R. L. & Barnes, J. L. (2004) Am. J. Pathol. 165, 1755-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayyali, U. S., Zhang, W., Yee, A. G., Seidman, J. G. & Potter, H. (1997) J. Neurochem. 68, 1668-1678. [DOI] [PubMed] [Google Scholar]
- 14.Norris, C. M., Kadish, I., Blalock, E. M., Chen, K. C., Thibault, V., Porter, N. M., Landfield, P. W. & Kraner, S. D. (2005) J. Neurosci. 25, 4649-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastwood, S. L., Burnet, P. W. & Harrison, P. J. (2005) Biol. Psychiatry 57, 702-710. [DOI] [PubMed] [Google Scholar]
- 16.Usuda, N., Arai, H., Sasaki, H., Hanai, T., Nagata, T., Muramatsu, T., Kincaid, R. L. & Higuchi S. (1996) J. Histochem. Cytochem. 44, 13-18. [DOI] [PubMed] [Google Scholar]
- 17.Aramburu, J., Rao, O. & Klee, C. B. (2000) Curr. Top. Cell. Regul. 36, 237-295. [DOI] [PubMed] [Google Scholar]
- 18.Cvetkovic, M., Mann, G. N., Romero, D. F., Liang, X. G., Ma, Y., Jee, W. S. & Epstein, S. (1994) Transplantation 57, 1231-1237. [DOI] [PubMed] [Google Scholar]
- 19.Sass, D. A., Bowman, A. R., Yuan, Z., Ma, Y., Jee, W. S. & Epstein, S. (1997) Bone 21, 65-70. [DOI] [PubMed] [Google Scholar]
- 20.Epstein, S., Shane, E. & Bilezikian, J. P. (1995) Curr. Opin. Rheumatol. 7, 255-261. [DOI] [PubMed] [Google Scholar]
- 21.Epstein, S., Inzerillo, A. M., Caminis, J. & Zaidi, M. (2003) J. Bone Miner. Res. 18, 2083-2094. [DOI] [PubMed] [Google Scholar]
- 22.Awumey, E. M., Moonga, B. S., Sodam, B. R., Koval, A. P., Adebanjo, O. A., Kumegawa, M., Zaidi, M. & Epstein, S. (1999) Biochem. Biophys. Res. Commun. 254, 248-252. [DOI] [PubMed] [Google Scholar]
- 23.Sun, L., Moonga, B. S., Lu, M., Zaidi, N., Iqbal, J., Blair, H. C., Epstein, S., Abe, E., Troen, B. R., Huang, C. L.-H. & Zaidi, M. (2003) Am. J. Physiol. 284, F575-F583. [DOI] [PubMed] [Google Scholar]
- 24.Takayanagi, H. (2005) J. Mol. Med. 83, 170-179. [DOI] [PubMed] [Google Scholar]
- 25.Hirotani, H., Tuohy, N. A., Woo, J. T., Stern, P. H. & Clipstone, N. A. (2004) J. Biol. Chem. 279, 13984-13992. [DOI] [PubMed] [Google Scholar]
- 26.Abe, E., Marians, R. C., Yu, W., Wu, X. B., Ando, T., Li, Y., Iqbal., J., Eldiery, L., Rajendren, G., Blair, H. C., Davies, T. F. & Zaidi, M. (2003) Cell 115, 151-162. [DOI] [PubMed] [Google Scholar]
- 27.Sun, L., Adebanjo, O. A., Moonga, B. S., Corisdeo, S., Anandatheerthavarada, H. K., Biswas, G., Arakawa, T., Hakeda, Y., Koval, A., Sodam, B., et al. (1999) J. Cell Biol. 146, 1161-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolgilevich, S., Zaidi, N., Song, J., Abe, E., Moonga, B. S. & Sun, L. (2002) Biochem. Biophys. Res. Commun. 299, 505-509. [DOI] [PubMed] [Google Scholar]
- 29.Mundy, G., Garrett, R., Harris, S., Chan, J., Chen, D., Rossini, G., Boyce, B., Zhao, M. & Gutierrez, G. (1999) Science 286, 1946-1949. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, L. L., Zaidi, S., Moonga, B. S., Troen, B. R. & Sun, L. (2004) Biochem. Biophys. Res. Commun. 326, 131-135. [DOI] [PubMed] [Google Scholar]
- 31.Eberhardt, A. W., Yaeger-Jones, A. & Blair, H. C. (2001) Endocrinol. 142, 1333-1340. [DOI] [PubMed] [Google Scholar]
- 32.Parfitt, A. M., Drezner, M. K., Glorieux, F. H., Kanis, J. A., Malluche, H., Meunier, P. J., Ott, S. M. & Recker, R. (1987) J. Bone Miner. Res. 2, 595-610. [DOI] [PubMed] [Google Scholar]
- 33.Wu, X. B., Li, Y., Schneider, A., Yu, W., Rajendren, G., Iqbal, J., Yamamoto, M., Alam, M., Brunet, L. J., Blair, H. C., et al. (2003) J. Clin. Invest. 112, 924-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomson, A. W., Bonham, C. A. & Zeevi, A. (1995) Ther. Drug Monit. 2, 713-718. [DOI] [PubMed] [Google Scholar]
- 35.Luo, Z. D., Wang, Y., Werlin, G., Camp, S., Chien, K. R. & Taylor, P. (1999) Mol. Pharmacol. 56, 886-894. [PubMed] [Google Scholar]
- 36.Buchinsky, F. J., Ma, Y., Mann, G. N., Rucinski, B., Bryer, H. P., Romero, D. F., Jee, W. S. & Epstein, S. (1996) Endocrinology 137, 2278-2285. [DOI] [PubMed] [Google Scholar]
- 37.Roggia, C., Gao, Y., Cenci, S., Weitzmann, M. N., Toraldo, G., Isaia, G. & Pacifici, R. (2001) Proc. Natl. Acad. Sci. USA 98, 13960-13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crabtree, G. R. (2001) J. Biol. Chem. 276, 2313-2316. [DOI] [PubMed] [Google Scholar]
- 39.Biswas, G., Adebanjo, O. A., Freedman, B. D., Anandatheerthavarada, H. K., Vijayasarathy, C., Zaidi, M., Kotlikoff, M. & Avadhani, N. G. (1999) EMBO J. 18, 522-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas, G., Anandathreethavarada, H. K., Zaidi, M. & Avadhani, N. G. (2003) J. Cell Biol. 161, 507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katagiri, T., Yamaguchi, A., Komaki, M., Abe, E., Takahashi, N., Ikeda, T., Rosen, V., Wozney, J. M., Fujisawa-Sehara, A. & Suda, T. (1994) J. Cell Biol. 127, 1755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayaraman, T., Brillantes, A. M., Timerman, A. P., Fleischer, S., Erdjiment-Bromaga, H., Tempst, P. & Marks, A. R. (1992) J. Biol. Chem. 267, 9474-9477. [PubMed] [Google Scholar]
- 43.Cameron, A. M., Steiner, J. P., Roskams, A. J., Ali, S. M., Ronnett, G. V. & Snyder, S. H. (1995) Cell 83, 463-472. [DOI] [PubMed] [Google Scholar]
- 44.Fuentes, J. J., Genesca, L., Kingsbury, T. J., Cunningham, K. W., Perez-Riba, M., Estivill, X. & de la Luna, S. (2000) Hum. Mol. Genet. 9, 1681-1690. [DOI] [PubMed] [Google Scholar]
- 45.Chan, B., Greenan, G., McKeon, F. & Ellenberger, T. (2005) Proc. Natl. Acad. Sci. USA 102, 13075-13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lange, A. W., Rothermel, B. A. & Yutzey, K. E. (2005) Dev. Dyn. 233, 954-963. [DOI] [PubMed] [Google Scholar]