Abstract
Lower-limb ischemia is a major health problem. Because of the absence of effective treatment in the advanced stages of the disease, amputation is undertaken to alleviate unbearable symptoms. Novel therapeutic approaches include the intramuscular use of autologous bone marrow cells (BMCs). Because tissue ischemia is associated with an overwhelming generation of oxygen radicals and negative effects due to perturbed shear-stress, metabolic intervention with antioxidants and l-arginine could potentially induce beneficial effects beyond those achieved by BMCs. The protective effect of autologous BMCs and vascular protection by metabolic cotreatment (1.0% vitamin E added to the chow and 0.05% vitamin C and 6% l-arginine added to the drinking water) were examined in ischemia-induced angiogenesis in the mouse hindlimb, a model of extensive acute peripheral arterial occlusion. i.v. BMC therapy improved blood flow and increased capillary densities and expression of Ki-67, a proliferation-associated protein. This beneficial effect was amplified by metabolic cotreatment, an intervention inducing vascular protection, at least in part, through the nitric oxide pathway, reduction of systemic oxidative stress, and macrophage activation. Therefore, although a cautious approach is mandatory when experimental findings are extended to human diseases, autologous BMCs together with metabolic intervention could be an effective clinical treatment for peripheral arterial disease.
Keywords: antioxidants, ischemic hindlimb, l-arginine, peripheral arterial disease, nitric oxide
The concept of “therapeutic angiogenesis” has become widely accepted during the past few years (1, 2). Vasculogenesis and angiogenesis are the major pathophysiological mechanisms contributing to neovascularization. Briefly, vasculogenesis requires the novel recruitment of “exogenous” stem cell progenitors, whereas angiogenesis involves the quiescent endothelial cell progenitors of the preexisting vessels (2). Bone marrow consists of multiple cell populations, including endothelial progenitor cells, which can differentiate into endothelial cells and release several angiogenic factors (3, 4). Local intramuscular autologous bone marrow cell (BMC) therapy (3, 5–7) or adipose tissue-derived stem cells injected i.v. (8) have been demonstrated to induce therapeutic angiogenesis in experimental ischemic limb models. This research goal is important, because lower-limb ischemia induced by peripheral arterial disease (PAD) is a major health problem. Because of the absence of effective pharmacological treatment, amputation is undertaken at the end stage as a unique solution to unbearable symptoms (9). Therefore, the efficacy and safety of autologous implantation of BMCs were studied in patients with PAD (10, 11). Specifically, by using the same approach used in the preclinical studies (3, 5–7), the gastrocnemius muscle of the ischemic limb was injected with BMCs, whereas the less ischemic limb was injected with saline. At 24 weeks after treatment, the ankle–brachial index in legs injected with BMCs was significantly improved, as were transcutaneous oxygen pressure, resting pain, and pain-free walking time. Thus, although not conclusive, these data suggest that autologous implantation of BMCs could be safe and effective for achievement of therapeutic angiogenesis in PAD, because of the natural ability of BMCs to supply endothelial progenitor cells and to secrete various angiogenic factors. Interestingly, a recent study showed a method for inducing skeletal muscle lineage cells from human stromal BMCs (12). This finding could be important, because advanced stages of PAD are associated with muscular problems (9). Indeed, the induction of BMCs into specific cell types damaged during tissue ischemia could be another promising therapeutic approach.
Because tissue ischemia is associated with the generation of oxygen radicals (13, 14), negative effects due to perturbed shear-stress (15, 16), and microcirculatory damage (17, 18), metabolic intervention with antioxidants and l-arginine, the precursor of nitric oxide (NO), might promote beneficial effects in ischemia-induced angiogenesis in the mouse hindlimb over that provided by BMC therapy alone. Overall, a plethora of studies show beneficial effects of antioxidants and l-arginine in vascular damage (14, 16, 19–22). Accordingly, in the present study, we investigated the safety and effectiveness of i.v. therapy with autologous BMCs alone, or in combination with metabolic cotreatment afforded by antioxidants and l-arginine, in ischemia-induced angiogenesis in the mouse hindlimb model.
Methods
Experimental Protocol and Hindlimb Ischemia Model. This study was conducted according to the Guidelines for Animal Experiments of the American Heart Association and in accordance with guidelines published by the National Institutes of Health (23). Quality standards of Laboratories at the University of Naples are in accordance with rules establish from the Italian Ministry of Health and the European College of Laboratory Animal Medicine, while Laboratories of the University of California at Los Angeles, Mayo Clinic at Rochester, and Whitaker Cardiovascular Institute at Boston University are in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care. The neovascularization capacity of BMCs alone or in cotreatment with 1.0% vitamin E added to the chow, 0.05% vitamin C added to the drinking water, and 6% l-arginine in drinking water (16, 22) was investigated in a murine model of unilateral hindlimb ischemia in male C57BL/6J mice, essentially as described in refs. 8 and 24. Briefly, the proximal portion of the femoral artery including the superficial and deep branches and the distal portion of the saphenous artery were ligated with 7–0 silk suture. All arterial branches between the ligation were obliterated by using an electrical coagulator. The overlying skin was closed with three surgical staples. The mice then were randomized to three interventions: (i) hindlimb ischemia treatment alone (controls, n = 21; the ischemic hindlimbs of these mice were injected with PBS only); (ii) hindlimb ischemia and subsequent BMC therapy (n = 18); or (iii) hindlimb ischemia and BMC therapy plus metabolic intervention (n = 19). After 24 h of ischemia, 1.5 × 106 autologous BMCs diluted in PBS were injected i.v. Usually, the only way to obtain BMCs is by euthanizing the mice (3, 5–7). In contrast, we used an original technique for obtaining BMCs by aspiration from the femur of living mice proposed by Verlinden et al. (25). The technique is simple and efficient and does not disable the animals. Consistent with their data (25), on average 1.8 ± 0.3 × 106 BMCs can be collected from one femur at a time (contralateral to that of hindlimb-ischemia and done at the same time as ligation of the vessels in the ischemic limb), which is sufficient for flow cytometry analysis and the infusion procedure. The cellular composition of the samples obtained by puncture is identical to that of bone marrow harvested by flushing the femur after killing the animals (25). After 2 weeks, ischemic (right)/normal (left) limb blood flow ratio was measured by using a laser Doppler blood flowmeter, as described in refs. 8 and 24. The average perfusion of the ischemic and nonischemic limbs was calculated on the basis of histogram pixels. To minimize variables including ambient light and temperature, perfusion is expressed as the ratio of the ischemic to the nonischemic hindlimb.
Histology and Morphometric Analysis. Limb interstitial fibrosis was morphometrically assessed by using the classical Azan–Mallory staining and expressed as percent of total muscle section. Tissue vascularization was determined in 5-μm frozen sections of the adductor and semimembranous muscles from the ischemic and nonischemic limbs (8). Ischemic and nonischemic muscles were dissected and progressively frozen in isopentane solution cooled in liquid nitrogen. Sections were first incubated for 30 min in PBS containing 5% BSA at room temperature and then 1 h with polyclonal antibody (Ab) directed against total fibronectin (dilution 1:50; Research Diagnostics, Flanders, NJ) or monoclonal Ab directed against CD31 (20 μl/ml; JC/70A, DAKO) to identify capillaries. Capillary densities then were calculated in randomly chosen fields of a definite area and expressed as the number of capillaries per myocyte relative to the individual nonischemic limb (8). T lymphocytes and macrophages were detected by immunostaining with anti-CD3ε monoclonal Ab (Santa Cruz Biotechnology) and the anti-F4/80 monoclonal Ab, respectively (16, 22). Immunohistochemistry was achieved by treating sections with 3% H2O2 and with a biotinylated secondary Ab with a horseradish peroxidase–streptavidin conjugate (dilution 1:50). Morphometric imaging evaluation was computer-assisted, as described in refs. 16 and 22. Finally, immunofluorescence detection of Ki-67 (a proliferation-associated marker) was determined by using the Ki-67 mouse IgG1 (dilution 1:50; DAKO).
Evaluation of Oxidative Stress and Nitrite and Nitrate (NOx) Levels. Blood was collected at the time of killing into Eppendorf tubes containing 1 mM Na2-EDTA. Isoprostane 8-epi-PGF2α, a well recognized index of tissue oxidative stress, purified from plasma samples was measured by using a commercially available immunoassay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions, as described in ref. 22. NO was measured as NOx, which is made up of stable metabolites of NO, and the concentration of NO in the blood was assessed by NOx. NOx levels in the plasma were measured with Griess reagent (Calbiochem) according to the manufacturer's instructions (22).
Statistical Analysis. Histological analysis was performed in a blinded fashion. Comparisons among groups were analyzed by using ANOVA. Post hoc range tests and pairwise multiple comparisons were performed with the t test (two-sided) with Bonferroni adjustment. Probability values of P < 0.05 were considered statistically significant.
Results
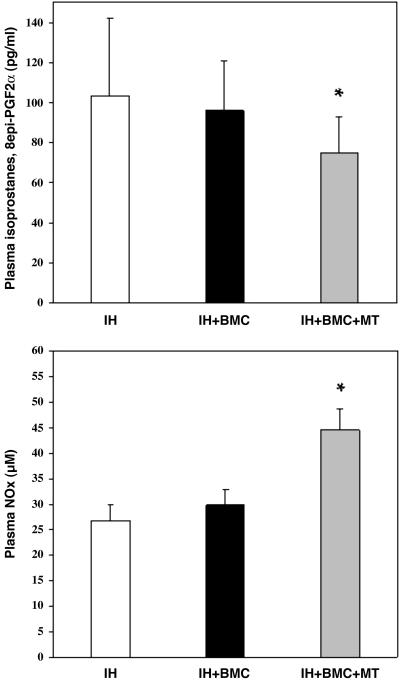
Plasma Parameters. Fig. 1 shows data regarding plasma evaluation of oxidative stress and NOx production in the various study groups. Under the present experimental conditions, untreated mice (controls that were not subjected to hindlimb ischemia) had 83 ± 31 pg/ml of isoprostanes and 23.8 ± 3.5 μM NOx. The combined treatment of BMC therapy and metabolic intervention resulted in a significant improvement of oxidative–redox balance and NO bioactivity in comparison with untreated mice.
Fig. 1.
Plasma oxidative stress (isoprostanes, 8-epi-PGF2α;pg/ml; *, P < 0.05 vs. ischemic hindlimb (IH); Upper) and NO bioactivity production (μM; *, P < 0.01 vs. IH; Lower) in the various study groups. Data are expressed as mean ± SD of single measurement in each mouse of the study group. Each group contains 18–21 animals.
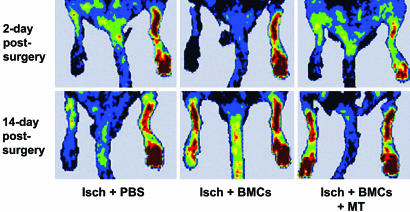
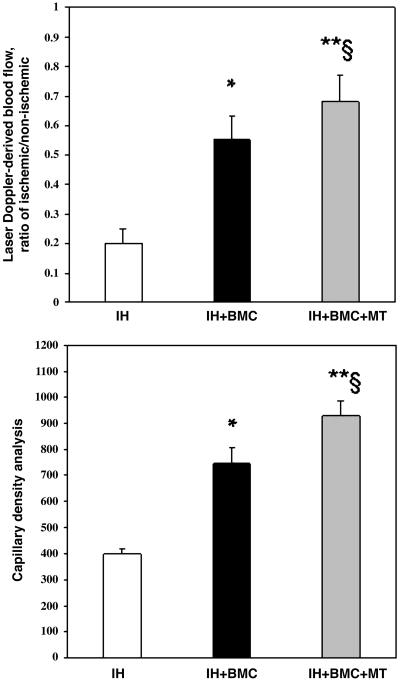
Blood Flow Recovery in Ischemic Hindlimb. As shown in Fig. 2, under the defined experimental conditions, quantitative analysis of the laser Doppler imaging revealed a significant increase in blood flow after the injection of BMCs in the ischemic hindlimb in comparison with controls receiving infusion of PBS. Relative blood flow in ischemic limb measured after 14 days of ischemia was increased by ≈2-fold in BMC-treated ischemic mice. Moreover, the combined treatment with metabolic supplementation further improved blood perfusion (Fig. 2). This result indicated that the restoration of perfusion in the ischemic hindlimbs was accelerated by i.v. BMC treatment alone and even further by combined therapeutic approach compared with infusion of PBS and that the recovery of blood flow in the ischemic hindlimbs could not be accelerated by PBS injection alone. Fig. 3 Upper shows the mean blood flow obtained in cumulative laser Doppler experiments. In preliminary studies, we observed a time-dependent increase in flow after the injection of BMCs alone in the ischemic hindlimb that was maximal after 2 weeks but significant already after 7 days (data not shown).
Fig. 2.
Representative scans of relative blood flow in ischemic limb measured by laser Doppler imaging analysis after 2 and 14 days of ischemia. Isch+PBS, control.
Fig. 3.
Cumulative evaluation of blood flow (laser Doppler-derived blood flow, ratio of ischemic/nonischemic; *, P < 0.01 vs. ischemic hindlimb (IH); **, P < 0.002 vs. IH; §, P < 0.04 vs. IH+BMC therapy; Upper) and capillary density analysis (mean number of fibronectin positive cells per mm2 counted in 10 different fields; *, P < 0.01 vs. IH; **, P < 0.004 vs. IH; §, P < 0.05 vs. IH+BMC therapy; Lower). Data are expressed as mean ± SD. Each group contains 18–21 animals.
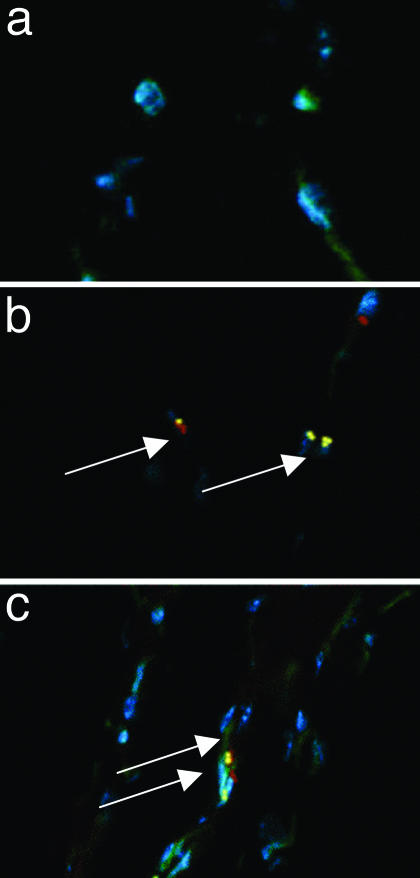
Histological Evaluation of Angiogenesis and Inflammatory Cells. Using an Ab directed against total fibronectin, histological analysis showed that the capillary density in the semimembranous and adductor muscles of the ischemic hindlimb compared with the nonischemic hindlimb was increased after the i.v. infusion of BMCs (Figs. 3 Lower and 4 a–c). Similar results were obtained with CD31 immunostaining to stain specifically endothelial cells (Fig. 4 d–f). Again, the administration of BMCs and metabolic treatment (MT) markedly promoted angiogenesis, and number of capillaries was significantly greater than that in mice receiving BMC therapy alone. We have investigated also the infiltration of inflammatory cells and interstitial fibrosis. The number of T cells was not significantly different among groups (Table 1). However, we have observed that infiltration of macrophages in the ischemic muscle was markedly reduced in the group receiving cotreatment of BMC and metabolic intervention (Table 1). Overall, interstitial fibrosis was increased in PBS-injected ischemic muscle and BMC therapy alone or in combination with MT resulted in a significant reduction of fibrosis (Table 1). Significant expression of Ki-67, a proliferation-associated protein, was detected in muscle sections in CD31-positive cells 2 weeks after BMC therapy, indicating that neoangiogenesis occurs at the late stages of the reparative process (Fig. 5).
Fig. 4.
Original photomicrographs of ischemic muscle sections hybridized with Ab directed against total fibronectin (a–c; capillaries appear in white and myocytes in black) or CD31 Ab (d–f; staining in brown). Isch+PBS, control. (Magnification: ×400.)
Table 1. Computer-assisted morphometric analysis of the number of inflammatory cells infiltrating into the ischemic muscle and the degree of muscular interstitial fibrosis in the various study groups.
| Treatment | Macrophages, per mm2 | T cells per mm2 | Interstitial fibrosis, % |
|---|---|---|---|
| IH + PBS | 6.1 ± 0.6 | 4.8 ± 0.5 | 59.5 ± 5.4 |
| IH + BMC | 5.5 ± 0.7 | 4.6 ± 0.6 | 18.9 ± 4.5** |
| IH + BMC + MT | 5.0 ± 0.5* | 4.3 ± 0.5 | 10.5 ± 3.2**§ |
Data are expressed as mean ± SD. Each group contains 18–21 animals. *, P < 0.01 vs. ischemic hindlimb (IH); **, P < 0.001 vs. IH; §, P < 0.05 vs. IH + BMC therapy.
Fig. 5.
Immunofluorescence analysis on representative cryostat sections of ischemic muscle. Proliferation-associated Ki-67 (yellow staining, arrows) was detected in CD31 immunostaining-positive cells (red staining). Nuclear counterstaining was performed by Hoechst dye and is shown in all sections (blue staining). (a) Isch+PBS control group. (b) BMC therapy alone. (c) BMC + MT.
When considering the group receiving BMC treatment alone, a significant positive correlation existed between blood flow and the number of fibronectin positive cells (R = 0.523; P = 0.003). This correlation with blood flow remained even when considering angiogenesis as CD31 positive cells (R = 0.453; P = 0.034). Interestingly, the proliferative marker Ki-67 staining increased with increasing blood flow, although the correlation was not strong (R = 0.294; P = 0.014).
Interestingly, regarding the group receiving the combined BMC treatment and metabolic intervention, the correlation between blood flow and angiogenesis expressed as the number of fibronectin positive cells further increased (R = 0.595; P < 0.002) and remained, and even became stronger, in CD31 immunostaining (R = 0.621; P < 0.001). Finally, in this group the number of macrophages inversely correlated with blood flow (R = -0.550; P = 0.008).
Discussion
The main result of this study is that i.v. autologous BMCs together with metabolic intervention significantly ameliorated ischemia-induced angiogenesis (increased blood flow and capillary density) in the mouse hindlimb. This combined approach was superior to BMC therapy alone. Beneficial effects were associated with reduced systemic oxidative stress as well as macrophage infiltration in ischemic muscle and interstitial fibrosis and increased plasma NO bioactivity. All these phenomena can be exacerbated by oxidation-sensitive mechanisms and, therefore, reduced by antioxidants and l-arginine. Moreover, there is increasing evidence of multiple redox coregulator events affecting the transcription machinery in the arterial wall (26). Much of these mechanisms can influence also the regulation of arteriogenesis and angiogenesis. In addition, because there are obvious signs of inflammation in the ischemic hindlimb (27, 28), some of the beneficial effects seen in the present work can be due to the antiinflammatory action (reduction of macrophage infiltration) elicited by antioxidants and l-arginine. Indeed, many signal transduction pathways involved in vascular inflammation and tissue injury are reduced by these compounds (13–20). Because the major goal of the present study was to investigate recovery of blood flow and neoangiogenesis, our work underscores the detailed role of the inflammatory pathways in the modulation of ischemia-induced angiogenesis. Nevertheless, our results open the way for therapeutic strategies aimed at decreasing oxidation-sensitive mechanisms and vascular inflammation together with activation of the active angiogenic process (high expression of Ki-67 protein) in ischemic tissues elicited by autologous BMCs and metabolic intervention.
In the past, it was generally accepted that vasculogenesis was restricted to embryogenesis. However, recent evidence strongly indicated the presence of a population of circulating progenitor cells (CPCs) in adult tissues such as bone marrow and peripheral blood, and that transplanted CPCs could incorporate into sites of angiogenesis after tissue ischemia in the limb contributing to neovascularization in adults (2, 4). CPCs are believed to originate from the common precursor of hematopoietic cells and are characterized by the presence of markers such as CD34 and CD133 (2, 4). The latter effects probably involved the release of proangiogenic factors rather than their functional incorporation into vessels (2, 4, 29). Previous studies in the literature in which exogenous human umbilical cord cells or BMCs/CPCs were given in hindlimb ischemia models provided encouraging results (2–8, 24, 30, 31). To enhance the efficacy of these approaches, it is also possible to stimulate CPCs by growth factors (32, 33) or genetic modifications of the cells before administration (34). In contrast to some previous studies, we did not select BMC subpopulations to promote higher rates of therapeutic neoagiogenesis (1–8). Our approach was based on aspiration of autologous BMCs in living animals and i.v. reinjection of the maximum dose of available cells alone or in cotreatment with a vasculoprotective MT. Although the most efficient BMC subpopulation able to induce beneficial effects in ischemic hindlimb is not known, evidence indicates that CPCs generated from either monocytic or nonmonocytic cells are equally effective and that ex vivo expansion could increase their functional activity to improve neovascularization after hindlimb ischemia (35). Indeed, recent studies suggested high neoangiogenic activity of mesenchymal cells (8, 31) or low dose CD34+KDR+ cells (30).
Therefore, we cannot exclude potential therapeutic effects of unknown BMC subpopulations that would be excluded a priori when selecting a unique population. Additional studies are in progress to address this important pathophysiological problem.
Because data from experimental models cannot be directly extrapolated to humans, a series of human studies need to be conducted to address safety and efficacy. This field of exploration is particularly important for PAD, which is known to be a progressive and sometimes rapid (diabetic patients) disease. BMC-based therapies have been used for several years to treat certain hematological diseases. In contrast, when considering mobilization of CPCs, the potential problems of inducing therapeutic angiogenesis by direct administration or gene transfer of angiogenic growth factors include a risk of neoplasia and plaque angiogenesis (1, 36, 37). Thus, it is critically important to confirm the safety of inducing therapeutic angiogenesis in patients, especially when considering long-term effects. Emerging evidence suggests that another therapeutic approach is feasible in humans, using intra-arterial infusion of autologous CPC isolated from peripheral blood to improve both functional and clinical indices (38). Moreover, exercise training has been shown to improve regional perfusion in ischemic syndromes partially related to a regeneration of diseased endothelium by CPC-derived vasculogenesis [increased vascular endothelial growth factor (VEGF) levels by 310% associated with an increase in CPCs by 440%; both P < 0.05 vs. control] (39). In conclusion, human studies should validate the hypothesis that autologous BMCs together with metabolic intervention could be an effective clinical treatment for PAD. Enhancement of this therapeutic approach should include integration with physical exercise and localization of BMC cell subpopulation(s) particularly effective in vascular regeneration.
Acknowledgments
We thank our colleagues Joseph Loscalzo (Boston University) and Amir Lerman (Mayo Clinic College of Medicine and Foundation) for helpful discussions in the field, Arlene Lising for technical and secretarial assistance, and Francesco P. D'Armiento for his insightful histopathological observations. This work was supported by the Mayo Foundation and National Research Funds from the University of Naples.
Author contributions: C.N. and L.J.I. designed research; S.W.-I., F.d.N., G.d.R., L.O.L., B.F., A.M., G.S., C.B., A.F., R.E.B., D.S., V.S., and L.J.I. performed research; C.N., S.W.-I., F.d.N., G.d.R., L.O.L., B.F., A.M., G.S., C.B., A.F., R.E.B., D.S., V.S., and L.J.I. analyzed data; and C.N., L.O.L., and L.J.I. wrote the paper.
Conflict of interest statement: L.J.I. helped develop and has a financial interest in a commercially available dietary supplement that contains some of the amino acids and antioxidants studied in this work.
Abbreviations: BMC, bone marrow cell; CPC, circulating progenitor cell; MT, metabolic treatment; NOx, nitrite and nitrate; PAD, peripheral arterial disease.
References
- 1.Folkman, J. (1998) Circulation 97, 1108-1110. [DOI] [PubMed] [Google Scholar]
- 2.Cao, Y., Hong, A., Schulten, H. & Post, M. J. (2005) Cardiovasc Res. 65, 639-648. [DOI] [PubMed] [Google Scholar]
- 3.Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G. & Isner, J. M. (1997) Science 275, 964-967. [DOI] [PubMed] [Google Scholar]
- 4.Rafii, S. & Lyden, D. (2003) Nat. Med. 9, 702-712. [DOI] [PubMed] [Google Scholar]
- 5.Hamano, K., Li, T. S, Kobayashi, T., Tanaka, N., Kobayashi, S., Matsuzaki, M. & Esato, K. (2001) Surgery 130, 44-54. [DOI] [PubMed] [Google Scholar]
- 6.Shintani, S., Murohara, T., Ikeda, H., Ueno, T., Sasaki, K., Duan, J. & Imaizumi, T. (2001) Circulation 103, 897-903. [DOI] [PubMed] [Google Scholar]
- 7.Iwase, T., Nagaya, N., Fujii, T., Itoh, T., Ishibashi-Ueda, H., Yamagishi, M., Miyatake, K., Matsumoto, T., Kitamura, S. & Kangawa, K. (2005) Circulation 111, 356-362. [DOI] [PubMed] [Google Scholar]
- 8.Miranville, A., Heeschen, C., Sengenès, C., Curat, C. A., Busse, R. & Bouloumié, A. (2004) Circulation 110, 349-355. [DOI] [PubMed] [Google Scholar]
- 9.Aronow, W. S. (2005) Cardiol. Rev. 13, 61-68. [DOI] [PubMed] [Google Scholar]
- 10.Tateishi-Yuyama, E., Matsubara, H., Murohara, T., Ikeda, U., Shintani, S., Masaki, H., Amano, K., Kishimoto, Y., Yoshimoto, K., Akashi, H., et al. (2002) Lancet 360, 427-435. [DOI] [PubMed] [Google Scholar]
- 11.Higashi, Y., Kimura, M., Hara, K., Noma, K., Jitsuiki, D., Nakagawa, K., Oshima, T., Chayama, K., Sueda, T., Goto, C., et al. (2004) Circulation 109, 1215-1218. [DOI] [PubMed] [Google Scholar]
- 12.Dezawa, M., Ishikawa, H., Itokazu, Y., Yoshihara, T., Hoshino, M., Takeda, S., Ide, C. & Nabeshima, Y. (2005) Science 309, 314-317. [DOI] [PubMed] [Google Scholar]
- 13.Becker, L. B. (2004) Cardiovasc. Res. 61, 461-470. [DOI] [PubMed] [Google Scholar]
- 14.de Nigris, F., Lerman, A., Ignarro, L. J., Williams-Ignarro, S., Sica, V., Baker, A. H., Lerman, L. O., Geng, Y. J. & Napoli, C. (2003) Trends Mol. Med. 9, 351-359. [DOI] [PubMed] [Google Scholar]
- 15.Fisher, A. B., Chien, S., Barakat, A. I. & Nerem, R. M. (2001) Am. J. Physiol. Lung Cell Mol. Physiol. 281, L529-L533. [DOI] [PubMed] [Google Scholar]
- 16.de Nigris, F., Lerman, L. O., Ignarro, S. W., Sica, G., Lerman, A., Palinski, W., Ignarro, L. J. & Napoli, C. (2003) Proc. Natl. Acad. Sci. USA 100, 1420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tritto, I. & Ambrosio, G. (1999) Cardiovasc. Res. 42, 600-606. [DOI] [PubMed] [Google Scholar]
- 18.Stokes, K. Y., Cooper, D., Tailor, A. & Granger, D. N. (2002) Free Radic. Biol. Med. 33, 1026-1036. [DOI] [PubMed] [Google Scholar]
- 19.Boak, L. & Chin-Dusting, J. P. (2004) Curr. Vasc. Pharmacol. 2, 45-52. [DOI] [PubMed] [Google Scholar]
- 20.Napoli, C., Sica, V., Pignalosa, O. & de Nigris, F. (2005) Curr. Med. Chem. 12, 1755-1772. [DOI] [PubMed] [Google Scholar]
- 21.Cooke, J. P. & Oka, R. K. (2001) Curr. Atheroscler. Rep. 3, 252-259. [DOI] [PubMed] [Google Scholar]
- 22.Napoli, C., Williams-Ignarro, S., de Nigris, F., Lerman, L. O., Rossi, L., Guarino, C., Mansueto, G., Di Tuoro, F., Pignalosa, O., De Rosa, G., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 8797-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl. Inst. Health, Bethesda), DHHS Publ. No. (NIH) 85-23.
- 24.Silvestre, J. S., Mallat, Z., Duriez, M., Tamarat, R., Bureau, M. F., Scherman, D., Duveger, N., Branellec, D., Tedgui, A. & Levy, B. I. (2000) Circ. Res. 87, 448-452. [DOI] [PubMed] [Google Scholar]
- 25.Verlinden, S. F., van Es, H. H. & van Bekkum, D. W. (1998) Exp. Hematol. 26, 627-630. [PubMed] [Google Scholar]
- 26.de Nigris, F., Lerman, L. O. & Napoli, C. (2002) Int. J. Cardiol. 86, 153-168. [DOI] [PubMed] [Google Scholar]
- 27.Couffinhal, T., Silver, M., Zheng, L. P., Kearney, M., Witzenbichler, B. & Isner, J. M. (1998) Am. J. Pathol. 152, 1667-1679. [PMC free article] [PubMed] [Google Scholar]
- 28.Arras, M., Ito, W. D., Scholz, D., Winkler, B., Schaper, J. & Schaper, W. (1997) J. Clin. Invest. 101, 40-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehman, J., Li, J., Orschell, C. M. & March, K. L. (2003) Circulation. 107, 1164-1169. [DOI] [PubMed] [Google Scholar]
- 30.Madeddu, P., Emanueli, C., Pelosi, E., Salis, M. B., Cerio, A. M., Bonanno, G., Patti, M., Stassi, G., Condorelli, G. & Peschle, C. (2004) FASEB J. 18, 1737-1739. [DOI] [PubMed] [Google Scholar]
- 31.Iwase, T., Nagaya, N., Fujii, T., Itoh, T., Murakami, S., Matsumoto, T., Kangawa, K. & Kitamura, S. (2005) Cardiovasc. Res. 66, 543-551. [DOI] [PubMed] [Google Scholar]
- 32.Minamino, K., Adachi, Y., Okigaki, M., Ito, H., Togawa, Y., Fujita, K., Tomita, M., Suzuki, Y., Zhang, Y., Iwasaki, M., et al. (2005) Stem Cells 23, 347-354. [DOI] [PubMed] [Google Scholar]
- 33.Li, X., Tjwa, M., Moons, L., Fons, P., Noel, A., Ny, A., Zhou, J. M., Lennartsson, J., Li, H., Luttun, A., et al. (2005) J. Clin. Invest. 115, 118-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi, J. H., Hur, J., Yoon, C. H., Kim, J. H., Lee, C. S., Youn, S. W., Oh, I. Y., Skurk, C., Murohara, T., Park, Y. B., et al. (2004) J. Biol. Chem. 279, 49430-49438. [DOI] [PubMed] [Google Scholar]
- 35.Urbich, C., Heeschen, C., Aicher, A., Dernbach, E., Zeiher, A. M. & Dimmeler, S. (2003) Circulation 108, 2511-2516. [DOI] [PubMed] [Google Scholar]
- 36.Sensebe, L., Deschaseaux, M., Li, J., Herve, P. & Charbord, P. (1997) Stem Cells 15, 133-143. [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner, I. (2000) Curr. Cardiol. Rep. 2, 24-28. [DOI] [PubMed] [Google Scholar]
- 38.Lenk, K., Adams, V., Lurz, P., Erbs, S., Linke, A., Gielen, S., Schmidt, A., Scheinert, D., Biamino, G., Emmrich, F., et al. (2005) Eur. Heart J. 26, 1903-1909. [DOI] [PubMed] [Google Scholar]
- 39.Sandri, M., Adams, V., Gielen, S., Linke, A., Lenk, K., Krankel, N., Lenz, D., Erbs, S., Scheinert, D., Mohr, F. W., et al. (2005) Circulation 111, 3391-3392. [DOI] [PubMed] [Google Scholar]