Abstract
Like acetylcholinesterase, butyrylcholinesterase (BChE) inactivates the neurotransmitter acetylcholine (ACh) and is hence a viable therapeutic target in Alzheimer's disease, which is characterized by a cholinergic deficit. Potent, reversible, and brain-targeted BChE inhibitors (cymserine analogs) were developed based on binding domain structures to help elucidate the role of this enzyme in the central nervous system. In rats, cymserine analogs caused long-term inhibition of brain BChE and elevated extracellular ACh levels, without inhibitory effects on acetylcholinesterase. In rat brain slices, selective BChE inhibition augmented long-term potentiation. These compounds also improved the cognitive performance (maze navigation) of aged rats. In cultured human SK-N-SH neuroblastoma cells, intra- and extracellular β-amyloid precursor protein, and secreted β-amyloid peptide levels were reduced without affecting cell viability. Treatment of transgenic mice that overexpressed human mutant amyloid precursor protein also resulted in lower β-amyloid peptide brain levels than controls. Selective, reversible inhibition of brain BChE may represent a treatment for Alzheimer's disease, improving cognition and modulating neuropathological markers of the disease.
Keywords: anticholinesterase, long-term potentiation, dementia, memory, neurodegeneration
Cholinergic neurons of the basal forebrain play a crucial role in brain, with extensive cortical projections that modulate other neurotransmitters (1). These cholinergic systems are known to be involved in cognitive and behavioral functions that are widely disturbed in Alzheimer's disease (AD). In particular, AD is characterized by a forebrain cholinergic neuron loss and a progressive decline in acetylcholine (ACh) (2, 3).
Activity of ACh in the brain is terminated by the hydrolytic action of cholinesterases (ChEs). Inhibitors of these enzymes have hence been developed to augment the activity of surviving cholinergic neurons in patients with AD. All ChE inhibitors (ChEIs) currently licensed for AD inhibit acetylcholinesterase (AChE, EC 3.1.1.7) and, to a varying extent, butyrylcholinesterase (BChE, EC 3.1.1.8), a second ChE in brain (4). AChE and BChE have numerous physiological functions depending on their localization and time of expression (5).
The classical action of AChE is to catalyze the hydrolysis of ACh within cholinergic synapses of the brain and autonomic nervous system (6). Although BChE shares some of these functions, its role in brain remains unclear. To define its role in brain, selective inhibitors were designed and synthesized based on the x-ray crystallographic structure of the binding sites for ACh that differentiate BChE from AChE (7). These were assessed preclinically based on the following facts.
In healthy human brain, AChE predominates over BChE activity (1), but the latter likely has been previously underestimated (8). Whereas, histochemically, AChE is localized mainly to neurons, BChE is associated primarily with glial cells, as well as to endothelial cells and neurons (9). An important feature distinguishing BChE from AChE is its kinetic response to concentrations of ACh; reflected in their Km values. BChE is less efficient in ACh hydrolysis at low concentrations but highly efficient at high ones, at which AChE becomes substrate inhibited (10). Hence, a possible role for brain BChE, particularly when associated with glia, is for supportive hydrolysis of ACh. Under conditions of high brain activity, local synaptic ACh can reach micromolar levels that approach inhibitory levels for AChE activity. The close spatial relationship of glial BChE would allow synergistic BChE-mediated hydrolysis to assist in the regulation of local ACh levels to permit the maintenance of normal cholinergic function. The survival of AChE knockout mice (8) with normal levels and localization of BChE (11) supports the concept that BChE has a key role that can partly compensate for the action of AChE.
In AD, AChE is lost early by up to 85% in specific brain regions, whereas BChE levels (2, 12), chiefly the G1 form, rise with disease progression. The ratio of BChE to AChE changes dramatically in cortical regions affected by AD from 0.2 up to as much as 11 (1). Clearly, this altered ratio in AD brain could modify the normally supportive role of BChE in hydrolyzing excess ACh only. Selective BChE inhibition may therefore be useful in ameliorating a cholinergic deficit, which likely worsens in AD due to increased activity of BChE.
Histochemical studies show that some cholinergic neurons contain BChE instead of AChE (13). In fact, 10–15% of ChE-positive cells in human amygdala and hippocampus are regulated by BChE independently of AChE (14). Augmenting cholinergic function by inhibiting these pathways may be of clinical value. Indeed, clinical studies with the dual ChEI, rivastigmine (Exelon, Novartis), support a role for the central inhibition of BChE in addition to AChE in AD therapy, based on the high correlation of the former with cognitive improvement (15).
In the AD brain, increasing levels of BChE correlate positively with the development of hallmark cortical and neocortical amyloid-rich neuritic plaques and neurofibrillary tangles (3, 16). The precise role of β-amyloid peptide (Aβ), which accumulates in neuritic plaques, is not well understood. It is cleaved from β-amyloid precursor protein-α (APP) by specific secretases, α-, β-, and γ (17), and Aβ (particularly Aβ42) appears to be toxic to neurons (18). Therefore, reducing Aβ synthesis is a major focus of current AD research (4).
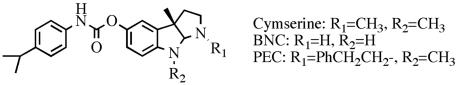
In light of the suggested role of BChE in central cholinergic transmission, its altered expression in AD brain, and probable association with the development of neuropathologic changes, we hypothesized that high BChE activity would be detrimental in AD and that inhibiting this enzyme would be of clinical value. To test this hypothesis, we synthesized selective, reversible BChE inhibitors, cymserine analogs (Fig. 1), and evaluated them in well-described models to define their actions on brain ACh, learning and cognition at synaptic and behavioral levels, as well as on neuropathological markers of AD.
Fig. 1.
Chemical structure of cymserine analogs.
Materials and Methods
BChE Inhibitors. Cymserine analogs, (-)-N1-phenethylnorcymserine (PEC) and (-)-N1,N8-bisnorcymserine (BNC) were synthesized (7), and their cholinesterase inhibitory activity was determined over a range of 0.3–30,000 nM against freshly prepared human and rat (male Fischer-344 rats, Harlan Sprague–Dawley, Indianapolis, IN) erythrocyte AChE and plasma BChE by Ellman technique (19), as described (7). The concentrations of compounds required to inhibit 50% enzymatic activity (IC50) were determined in triplicate (7).
Log octanol/water partition coefficients for cymserine and analogs were calculated for lipophilicity assessment (20).
Animals. Animals were caged in pairs at 21°C on a 12-h light–dark cycle, with free access to food and water. All experiments complied with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee, or complied with European Community regulations for the care and use of experimental animals (CEE NE86/609).
Pharmacology of BChE Inhibition. Pharmacokinetic and pharmacodynamic studies were performed in anesthetized (isofuorane, Abbott, Chicago) male Fischer-344 rats (21–26 months old, n≥ 3) with femoral artery and vein catheters to administer drug and remove blood samples, respectively. A predrug blood sample was taken to determine resting (no inhibition) AChE and BChE activity. Cymserine and analogs were given at 1.0 mg/kg i.v. for pharmacokinetic studies and at 1.0 and 10 mg/kg i.p. for enzyme inhibition studies. Brain and plasma drug levels were determined by high-pressure liquid chromatography as described for phenserine (20). Plasma, brain, or cerebrospinal fluid (CSF) levels of ChE inhibition were determined as described (20).
Cymserine time-dependent concentration or BChE inhibition data were fitted by nonlinear regression analysis to a single exponential equation: C = Ae-at. Where C is either the cymserine concentration (μg/ml or μg/g) or percent BChE inhibition at time t (min), A is the theoretical zero-time concentration or percent inhibition in a central compartment, and a is the apparent first-order elimination rate constant (min-1). Half-life (T1/2) values were then determined by the formula: T1/2 = 0.693/a. Areas under the concentration-time profiles were calculated by the trapezoidal rule and used to define the brain/plasma time-dependent concentration ratio of the compound after i.v. administration.
To determine a maximum tolerated single dose, compounds were given to male Fischer-344 rats in increasing doses (1–30 mg/kg i.p., n = 3). Acute adverse effects, particularly associated with cholinergic overdrive (e.g., tremor, salivation) were noted so that animals in distress could be killed.
In Vivo Determination of ACh. Microdialysis. Extracellular ACh levels were measured in the parietal cerebral cortex of male Wistar rats (Harlan), 220–250 g weight, by microdialysis (21). Briefly, after anesthesia, a microdialysis tube (AN 69 membrane, Hospal Dasco, molecular mass cutoff > 15 kDa) was inserted transversally into the rat brain at the following coordinates: anteriorposterior -0.5 and dorsoventral -2.5 mm from bregma (22). After recovery, the membrane was perfused with Ringer's solution at a constant flow rate of 3 μl/min. Dialysate was collected over successive 20-min intervals into minitubes preloaded with 5 μl of 0.05 mM HCl to prevent ACh hydrolysis. Animals were given PEC 1.25 and 2.5 mg/kg i.p. 80 min after microdialysis started.
Measurement of ACh. Levels of ACh in dialysates were quantified as described (21). The detection limit for ACh was 40 fmol per injection (40-μl loop). One-way ANOVA followed by Fisher's least significant difference post hoc analysis was used for statistical analysis of ACh release data.
Measurement of Brain AChE and BChE inhibition. A matched separate series of naïve male Wistar rats were administered either PEC (2.5 mg/kg i.p.) or vehicle (n = 4 per group) in a manner similar to animals undergoing microdialysis. They were killed 40 min thereafter, parietal cerebral cortex was removed, and levels of AChE and BChE activity were determined (20).
Ex Vivo Long-Term Potentiation (LTP). A well defined experimental system for inducing LTP (23, 24) was used to investigate effects of BChE inhibition.
Slice Preparation. Studies were performed on transverse slices of rat brain (male Sprague–Dawley–Harlan, 10–25 days old, n = 6). Standard procedures for preparing and maintaining hippocampal slices (350 μm) in artificial CSF (ACSF) were used (25, 26). Slices were allowed to recover for at least 1 h, transferred to a submerged recording chamber, and continuously perfused at 2 ml/min in media (30–32°C).
Electrophysiological Recording. ACSF for LTP experiments contained 25 μM picrotoxin to block GABAA-mediated activity and optimize excitability. Stimuli (30 μs; 0.033 Hz) were delivered through fine bipolar tungsten electrodes to activate Schaffer collateral/commissural afferents (27). The field excitatory postsynaptic potentials (fEPSPs) were recorded in CA1 stratum radiatum with electrodes containing ACSF (impedance of 2–4 MΩ) using an Axopatch-1D amplifier (Axon Instruments). The presence of BChE in the neuropil of rodent hippocampus has been confirmed (11).
Slices with a steep input–output curve were selected for study. Stimulation intensity was adjusted to evoke fEPSPs of ≈30% maximum amplitude. The LTP-inducing stimulus consisted of a single train of 100 stimuli at 100 Hz after 10 min of stable baseline recording. The fEPSPs were filtered at 2 KHz, digitized at 4 KHz on a Digidata 1322A interface (Axon Instruments) and recorded by computer. Data were collected and analyzed on or off line by using pclamp 8 software (Axon Instruments).
Drug Administration. PEC (5 mM) was added to the preparation (n = 6) to provide a final concentration of 5 μM. Because PEC was initially dissolved in ethanol and Tween 80 (final volume 0.065% and 0.1%, respectively) to facilitate solubility in ACSF, a vehicle control lacking PEC was used in addition to an untreated control (n = 6 each).
Maze Learning. Male Fischer-344 rats (350–500 g, aged 21–26 months) were assigned randomly to physiological saline (controls, n = 53) or PEC or BNC both at 0.25 and 0.5 mg/kg (n = 8–10 per group). Drugs were freshly prepared daily in physiologic saline (injection volume, 0.1 ml/kg i.p.) and were given twice daily (9 a.m. and 3 p.m.): day 1, injections given and rats immediately returned to their cages; day 2, injections given 30 min before pretraining; days 3 and 4, injections given 30 min before training.
Learning Assessment. The apparatus and procedures for one-way active avoidance pretraining in a straight runway and subsequent acquisition training in a 14-unit T maze have been described (28, 29).
Statistical Analysis. Behavioral measures (errors and runtime) were averaged across four consecutive trials and analyzed over four separate blocks, divided equally between morning and afternoon in the 2-day study. Data were then analyzed by using ANOVA for group, block, and group × block factors. Post hoc group comparisons were made using Fisher's least significant difference test for all data collapsed over the 16 trials.
Neuropathological Markers in Vitro. Human neuroblastoma cells (SK-N-SH), obtained from American Type Culture Collection (Manassas, VA), were incubated with PEC, BNC, and cymserine (0.01–10 μm) or without compound in 0.5% serum media for 16 h (30). Cell viability was assessed by methyl tetrazolium salt metabolic assay (Promega) as well as by sensitive lactate dehydrogenase assay (30, 31). Cell samples and culture supernatant were used to assess intracellular and secreted protein, respectively.
APP Quantification. Conditioned media from each plate were collected, and the cells were centrifuged (800 × g, 10 min) and lysed as described (31). Then, 15 μg of protein from each sample was mixed with Laemmli buffer, boiled for 5 min, loaded on to a 10% Tris-Glycine gel in 1× SDS running buffer (Novex, San Diego), and the proteins were separated at 150 V for 90 min. Gels were transferred on to poly(vinylidene difluoride) at 25 V for 1.5 h. Each blot was probed for 2 h with the mAb 22C11 anti-APP N-terminal antibody (Chemicon) diluted to 2.5 mg/ml, which recognizes all mature forms of APP. Blots were then placed in secondary antibody, anti-mouse or -rabbit IgG conjugated to horseradish peroxidase (Sigma) for 30 min. The samples were detected by chemiluminescence and exposed to film (Amersham Pharmacia). Densitometric quantification of blots was done with a charge-coupled device camera and NIH image (version 4.1). To verify APP quantification with mAb 22C11, mAb 6E10 directed at residues 1–17 of Aβ within APP was used in a selection of samples.
Aβ Quantification. Human Aβ40 levels were quantified by colorimetric ELISA (Biosource, Camarillo, CA). Briefly, a 150-μl sample of culture supernatant was added to the precoated plate with capture antibody (specific for the N terminus of Aβ40) and incubated overnight at 4°C. The secondary (specific for the C terminus of Aβ40) and detection antibodies were added and incubated for 3 h at room temperature. Aβ40 levels were detected at 450 nm against appropriate standards.
Neuropathological Markers in a Transgenic (Tg) Mouse Model. Drug administration. Five-month-old male double-Tg mice (human APP swe+PS1) that overexpress Aβ (32) were given either PEC 3.0 mg/kg i.p. or vehicle (saline) daily for 3 weeks (n = 12 per group).
Aβ quantification. Animals were killed, and brain samples (parietal cerebral cortex) collected and immediately frozen to -70°C. Levels of Aβ40 and Aβ42 were quantified after formic acid extraction using published methods (33).
Results
Pharmacological Profile of Previously Undescribed BChE Inhibitors. Modification of cymserine in the N1 and N8 positions provided potent and selective inhibitors of BChE (Table 1). Lipid solubility predicted high blood–brain barrier permeability, indicated by calculated Log P values of: cymserine, 3.51; PEC, 5.72; and BNC, 1.80.
Table 1. Inhibitory activity of cymserine and analogs against human and rat erythrocyte AChE and plasma BChE.
| Human IC50, nM
|
BChE selectivity
|
Rat IC50, nM
|
BChE selectivity
|
|||
|---|---|---|---|---|---|---|
| Compound | AChE | BChE | AChE | BChE | ||
| Cymserine | 760 ± 20 | 50 ± 1 | 15-fold | 800 ± 25 | 38 ± 7 | 21-fold |
| PEC | >30,000 | 6 ± 1 | >5,000-fold | >30,000 | 9 ± 2 | >3,300-fold |
| BNC | 110 ± 15 | 1 ± 0.1 | 110-fold | 150 ± 20 | 1 ± 0.5 | 150-fold |
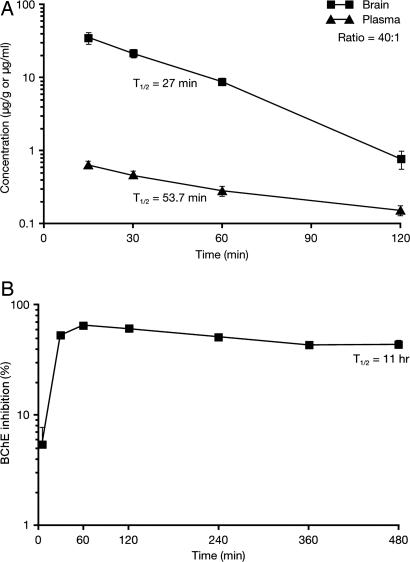
In plasma, cymserine concentration was 0.66 ± 0.02 μg/ml at 15 min, which declined with a T1/2 of 53.7 min. Cymserine readily entered brain tissue, reaching 36.1 ± 6.0 μg/g at 15 min and then declined with a T1/2 of 27 min (Fig. 2A). Brain concentrations were significantly greater than concomitant plasma ones at all times (P < 0.05, Student's t test). The brain to plasma concentration ratio of cymserine was 40:1 (calculated 15–120 min after administration).
Fig. 2.
Time-dependent brain and plasma concentrations of cymserine (±SEM) after 1 mg/kg i.v. (A) and percentage inhibition of plasma BChE by cymserine (±SEM) after 10 mg/kg i.p. (B) in rat. Brain cymserine levels were significantly higher than plasma cymserine levels at all times (P < 0.05, Student's t test).
Administered at 10 mg/kg i.p., cymserine achieved a peak of 65 ± 4% inhibition of plasma BChE at 60 min, which declined with a T1/2 of 11 h (Fig. 2B). In animals receiving a lower dose (1 mg/kg), BChE was maximally inhibited by 30 ± 3% in plasma and 85 ± 5% in CSF, both at 60 min. In all cases, AChE inhibition was <20%. No ChE inhibition was found at 48 h.
No adverse actions of cymserine, BNC, or PEC were observed at doses up to 30 mg/kg i.p. Administration of higher doses was limited by the aqueous solubility of the compounds.
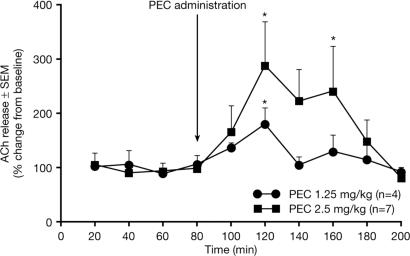
BChE Inhibition Increases Brain ACh Levels. Microdialysis studies showed that i.p. PEC induced dose-dependent increases in endogenous extracellular concentrations of ACh in parietal cortex. The maximal effect was 40 min after 1.25 and 2.5 mg/kg, with a significant increase in ACh of 180% and 290%, respectively, compared with preadministration levels (both P < 0.05) (Fig. 3). The ACh concentration remained high for the next 40 min (P < 0.05 at 160 min with 2.5 mg/kg), finally reaching control levels 2 h after drug administration.
Fig. 3.
Percent changes from baseline in extracellular concentrations of ACh (±SEM) in rat parietal cortex, at steady state and after i.p. PEC. Asterisk indicates a significant ACh elevation vs. baseline (P < 0.05, Fisher's least significant difference).
The degree of AChE and BChE inhibition in parietal cortex was determined at the time of peak ACh elevation. After PEC 2.5 mg/kg i.p., BChE was inhibited by 53 ± 5% at 40 min. In contrast, there was no inhibition of AChE in the same region, supporting the BChE-specific activity of PEC.
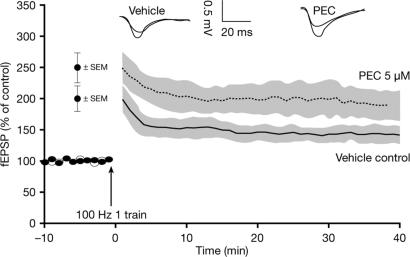
BChE Inhibition Enhanced LTP in the Hippocampus. High-frequency stimulation (HFS) induced LTP with a magnitude of 143.08 ± 13.3% fEPSP in untreated control preparations. In hippocampal slices treated with vehicle alone, HFS induced LTP at a magnitude that was not significantly different from that of untreated controls (150 ± 16.5% fEPSP). In the presence of PEC, LTP was significantly enhanced, with a magnitude of 192.08 ± 23.6% fEPSP (P < 0.05 vs. both controls, Dunnett's t test). Fig. 4 shows the change in LTP with and without PEC at a concentration that achieves selective BChE inhibition (Table 1).
Fig. 4.
Extracellular fEPSPs (±SEM) in CA1 stratum radiatum after induction of LTP in rat hippocampal slices in the presence of PEC (5 μM) and vehicle control. PEC significantly enhanced LTP vs. control (P < 0.05, Dunnett's t test).
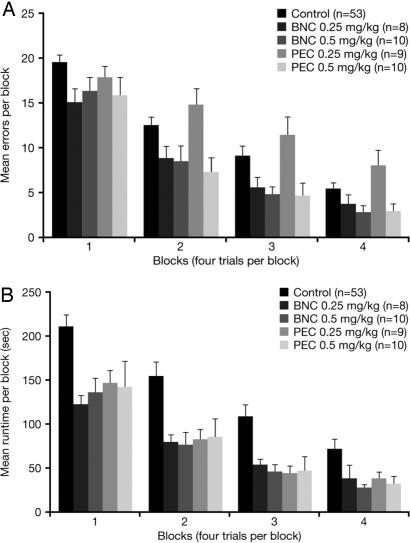
BChE Inhibition Improved Cognitive Performance in Aged Rats. Over successive blocks, rats learned to negotiate the maze with fewer errors (Fig. 5A) and more quickly (Fig. 5B) when administered a BChE inhibitor.
Fig. 5.
Mean (±SEM) number of errors made (P < 0.05 for PEC 0.5, BNC 0.25, 0.5 mg/kg vs. control) (A) and runtime (P < 0.05 for all PEC and BNC doses vs. control) (B) in BNC- and PEC-treated groups of aged rats relative to controls, during acquisition training in the 14-unit T-maze.
For both errors and runtime, ANOVA revealed significant main effects of both group (drug/control) and block, but no significant group × block interaction, permitting all data to be collapsed over the 16 trails. Subsequent post hoc analysis showed that animals receiving PEC (0.5 mg/kg) and BNC (0.25 or 0.5 mg/kg) made significantly fewer errors than controls (P < 0.05) (PEC 0.25 mg/kg was no different from control). Moreover, animals receiving either dose of BNC and PEC had significantly faster runtimes in the maze than controls (P < 0.05).
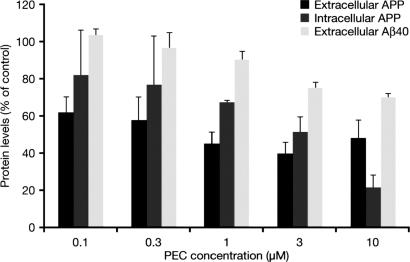
BChE Inhibition Reduced APP and Aβ Levels in Vitro and in Vivo. Administering cymserine and analogs to human neuroblastoma cells significantly reduced levels of intracellular and secreted APP and secreted Aβ40 without altering cellular viability. Fig. 6 shows the effect of PEC on these potential neuropathological markers. At 1.0 μM and above, PEC significantly lowered intracellular and secreted APP and secreted Aβ40 levels compared to controls (P < 0.05, Dunnett's t test).
Fig. 6.
Concentration-dependent reductions in APP and Aβ40 by PEC (expressed as a percent of control values ± SEM, n = 4), in SK-N-SH cells. All doses of PEC significantly lowered extracellular APP, and PEC ≥ 1 μM lowered intracellular APP and extracellular Aβ40 vs. control (P < 0.05, Dunnett's t test).
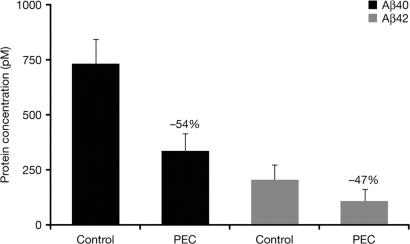
In Tg mice expressing human Aβ (Fig. 7), treatment with PEC led to a significant 54% reduction in Aβ40 and a 47% reduction in Aβ42 from the concentrations seen in controls (P < 0.05, Student's t test).
Fig. 7.
Reductions in brain levels of Aβ40 and Aβ42 in Tg mice overexpressing human Aβ, after daily treatment with PEC or vehicle control (P < 0.05 PEC vs. control, Student's t test).
Discussion
In light of the suggested role of BChE in central cholinergic transmission, its altered expression in the AD brain, and its probable association with the development of neuropathologic changes, we hypothesized that high BChE activity would be detrimental in AD and that inhibiting this enzyme would be of clinical value. Previously, we designed and synthesized ChEIs by matching the three-dimensional structure of the inhibitor to ACh binding domains that distinguish BChE from AChE (7). These agents showed a high level of specificity for BChE over AChE, compared with ChEIs commonly used in AD (34), and their pharmacologic actions were characterized in the present study.
Although inhibition of ACh hydrolysis by ChEIs may have desirable effects in the brain, the same actions within the autonomic nervous system often are dose-limiting for these drugs (1). Hence, cholinergic therapies should optimize central rather than peripheral activity. Among current agents, the claim that greater specificity for AChE vs. BChE would lead to fewer systemic side effects has yet to be borne out in practice (1). More importantly, an ideal ChEI would have high blood–brain barrier permeability and disappear rapidly from the periphery, acting primarily in the brain. After systemic administration in rat, cymserine analogs readily enter the brain, in accord with their log P values, where they produce long-lasting reversible inhibition of BChE. Acute, high doses of cymserine up to 30 mg/kg i.p. in rodents do not result in classical cholinergic toxicity, with no evidence of tremor, increased salivation, or lacrimation as found with high doses of selective AChE inhibitors (20). By comparison, the selective AChE inhibitor, phenserine, would be lethal at this dose (20). Therefore, cymserine and analogs appear to have a favorable pharmacological profile.
The functional importance of BChE was previously underestimated because of its relatively low expression in the normal mammalian brain compared with AChE (8). However, research by Mesulam et al. (11, 35) and others (9, 10) has led to increased interest in defining the role of this “orphan enzyme” within the nervous system and evaluating its importance in coregulating local concentrations of ACh, particularly in conditions such as AD.
Our microdialysis study assessed whether selective inhibition of BChE alters the concentration of ACh after synaptic release. The results clearly showed that cortical extracellular ACh levels in rat were dose-dependently elevated after BChE inhibition. Previously, the same microdialysis technique showed that extracellular ACh levels in the cortex and hippocampus are ≈50% lower in aged vs. young rats (21, 36). In a similar study, the unselective ChEI, tacrine, produced marked increases in extracellular ACh levels in both young and aged animals, linked to improved acquisition of a passive avoidance conditioned response in the aged rats, indicating enhanced cognitive function (21). Similarly, metrifonate (an unselective ChEI) led to higher extracellular ACh levels and restored object recognition in aged rats (36), suggesting that elevated ACh in this model is of behavioral relevance.
Thus, we examined the effects of selective BChE inhibition and associated increases in ACh on cognitive function at the molecular and behavioral levels. LTP is widely used to assess mechanisms affecting synaptic connectivity that underlie processes of learning and memory. In LTP, brief periods of intense synaptic activity (as triggered when learning a new task or produced experimentally during acquisition of passive avoidance) can cause a marked and long-lasting strengthening of communication between glutamatergic synapses, and are part of the neurochemical basis of memory engram formation (23, 24). This effect can be replicated experimentally in brain slices, because a test stimulus applied at a specific region before HFS will produce a postsynaptic response of a certain magnitude, whereas the same test applied after HFS will produce a greater response. It is reasonable to postulate that changes in ACh availability would indirectly modulate LTP, because the cholinergic system projects widely in the brain and plays a key role in modulating glutamatergic neurotransmission (1). It is well documented that ACh is involved with hippocampal synaptic plasticity, learning, and memory processes (37–39). Our study substantiates this finding, because a potent inhibitor of BChE enhanced the magnitude of LTP produced by HFS in hippocampal slice preparations compared with controls. Therefore, these results indicate that increased availability of ACh mediated by PEC could have the ability to enhance learning and memory. Although LTP is a classic function of glutamatergic synapses (reviewed by Francis, ref. 40), a recent study suggested that cholinergic neurons may also have similar plasticity. Colgin et al. (25) showed that endogenous activity of cholinergic pathways in the hippocampus (probably within the field CA3) could induce electrophysiological responses that resembled LTP following brief periods of intense activity. Therefore, the role of cholinergic neurons in learning and memory may involve direct effects besides a modulatory role via glutamatergic synapses.
Our data show that, besides enhancing synaptic connectivity, selective BChE inhibition with cymserine analogs also improves cognitive performance of elderly rats in a 14-unit T-maze paradigm. The favorable effect was evident at well below what might be expected to represent a maximum tolerated dose, and the magnitude was similar to that of the AChE inhibitor, phenserine (29). Studies investigating genetic variants of BChE (the K- and atypical-variants: BChE-K and -A) that are associated with a lowered innate ACh hydrolytic activity, suggest that links may exist between specific polymorphisms and AD pathology, but this remains controversial (41). Nevertheless, recent studies in humans support the potential role of BChE in cognition. In patients with dementia, BChE variants correlated with a preserved attentional performance and a reduced rate of cognitive decline (42, 43). Also, in Lewy body dementia, where the cholinergic deficit is considered more profound than in AD (44), a prospectively designed study of autopsy-confirmed cases showed a highly significant association between lower activity of BChE in the temporal cortex and a slower rate of cognitive decline (45).
In AD brain, increasing levels of BChE correlate positively with the presence of amyloid-rich neuritic plaques (3, 16, 17). Despite being characteristic of AD, the precise roles of APP and Aβ associated with plaques remain to be fully defined (4, 17). Recent evidence suggests that small, soluble oligomers of Aβ are potent inhibitors of hippocampal LTP, which has been linked to synaptic failure and memory loss (46, 47). In AD patients given the dual ChEIs tacrine or rivastigmine, there was no significant increase in the CSF levels of Aβ42 with either agent over the 1-year study (48). Therefore, it was worth determining the effects of the BChE inhibitors on the synthesis and secretion of neurotoxic Aβ. In a human neuronal cell line, cymserine analogs reduced levels of secreted Aβ and APP without affecting cell viability. This action translated in vivo, as PEC significantly lowered Aβ levels in Tg mice with accelerated Aβ deposition caused by overexpression of human APP. The mechanisms underpinning this action may involve both cholinergic and noncholinergic actions regulating APP synthesis and processing, as characterized with the AChE selective analog, phenserine, which lowers the translational efficiency of APP mRNA (30). In another study of mice overexpressing APP, there was a positive correlation between Aβ deposition and memory impairment (49).
In summary, selective, reversible inhibition of BChE in the brain by cymserine analogs may represent a treatment for AD, with actions on learning and memory, probably mediated through elevated ACh and lower Aβ levels. A unique and attractive feature of cymserine analogs is that they can selectively silence BChE without apparent adverse cholinergic actions, although results from ongoing formal toxicology studies are required. Indeed, a small percentage of humans have mutations in BChE that render it inactive, and yet lead normal lives (50). The clinical applications of these agents would appear to hold great potential.
Acknowledgments
This work was supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health Grants AG18379 and AG18884 (to D.K.L.) and AG023055-02 (to K.S.), and the Alzheimer's Association and Axonyx, Inc. (D.K.L. and K.S.).
Author contributions: N.H.G., D.K.I., G.P., T.G., K.F., K.S., A.B., and D.K.L. designed research; T.U., Y.W., C.S., Q.-S.Y., J.M., H.W.H., T.G., D.C., K.F., and K.S. performed research; Q.-S.Y. contributed new reagents/analytic tools; N.H.G., T.U., D.K.I., Y.W., G.P., C.S., J.M., H.W.H., T.G., D.C., K.F., K.S., A.B., and D.K.L. analyzed data; and N.H.G. and D.K.L. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AD, Alzheimer's disease; ACh, acetylcholine; ChE, cholinesterase; ChEI, ChE inhibitor; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; Aβ, β-amyloid peptide; APP, Aβ percursor protein; PEC, (-)-N1-phenethylnorcymserine; BNC, (-)-N1,N8-bisnorcymserine; CSF, cerebrospinal fluid; ACSF, artificial CSF; LTP, long-term potentiation; fEPSP, field excitatory postsynaptic potential; Tg, transgenic; HFS, high-frequency stimulation.
References
- 1.Giacobini, E. (2003) Int. J. Geriatr. Psychiatry 18, S1-S5. [DOI] [PubMed] [Google Scholar]
- 2.Perry, E., Perry, R., Blessed, G. & Tomlinson, B. (1978) Neuropathol. Appl. Neurobiol. 4, 273-277. [DOI] [PubMed] [Google Scholar]
- 3.Geula, C. & Mesulam, M. M. (1995) Alz. Dis. Assoc. Dis. 9, Suppl. 2, 23-28. [DOI] [PubMed] [Google Scholar]
- 4.Lahiri, D., Farlow, M., Sambamurti, K., Greig, N., Giacobini, E. & Schneider, L. (2003) Curr. Drug Targets 4, 97-112. [DOI] [PubMed] [Google Scholar]
- 5.Soreq, H. & Seidman, S. (2001) Nat. Rev. Neurosci. 2, 294-302. [DOI] [PubMed] [Google Scholar]
- 6.Taylor, P. (1996) in The Pharmacological Basis of Therapeutics, eds. Hardman, J., Limbird, L., Molinoff, P., Ruddon, R. & Gilman, A. (McGraw–Hill, New York), pp. 161-176.
- 7.Yu, Q., Holloway, H., Utsuki, T., Brossi, A. & Greig, N. (1999) J. Med. Chem. 10, 1855-1861. [DOI] [PubMed] [Google Scholar]
- 8.Li, B., Stribley, J., Ticu, A., Xie. W., Schopfer, L., Hammond, P., Brimijoin, S., Hinrichs, S. & Lockridge, O. (2000) J. Neurochem. 75, 1320-1331. [DOI] [PubMed] [Google Scholar]
- 9.Darvesh, S., Hopkins, D. & Geula, C. (2003) Nat. Rev. Neurosci. 4, 131-138. [DOI] [PubMed] [Google Scholar]
- 10.Silver, A. (1974) The Biology of Cholinesterases (Elsevier/Agricultural Research Council Institute, New York), pp. 426-447.
- 11.Mesulam, M., Guillozet, A., Shaw, P., Levey, A., Duysen, E. & Lockridge, O. (2002) Neuroscience 110, 627-639. [DOI] [PubMed] [Google Scholar]
- 12.Arendt, T., Bruckner, M., Lange, M. & Bigl, V. (1992) J. Neurochem. 21, 381-396. [DOI] [PubMed] [Google Scholar]
- 13.Graybiel, A. & Ragsdale, C., Jr. (1982) Nature 299, 439-442. [DOI] [PubMed] [Google Scholar]
- 14.Darvesh, S., Grantham, D. & Hopkins, D. (1998) J. Comp. Neurol. 393, 374-390. [PubMed] [Google Scholar]
- 15.Giacobini, E., Spiegel, R., Enz, A., Veroff, A. & Cutler, N. (2002) J. Neural Trans. 109, 1053-1065. [DOI] [PubMed] [Google Scholar]
- 16.Guillozet, A., Smiley, J., Mash, D. & Mesulam, M. (1997) Ann. Neurol. 42, 909-918. [DOI] [PubMed] [Google Scholar]
- 17.Sambamurti, K., Greig, N. & Lahiri, D. (2002) Neuromol. Med. 1, 1-31. [DOI] [PubMed] [Google Scholar]
- 18.Turner, P., O'Connor, K., Tate, W. & Abraham, W. (2003) Prog. Neurobiol. 70, 1-32. [DOI] [PubMed] [Google Scholar]
- 19.Ellman, G., Courtney, K., Andres, V. & Featherstone, R. (1961) Biochem. Pharmacol. 7, 88-95. [DOI] [PubMed] [Google Scholar]
- 20.Greig, N., DeMicheli, E., Utsuki, T., Holloway, H., Yu, Q., Perry, T., Brossi, A., Deutsch, J., Ingram, D., Lahiri, D., et al. (2000) Acta. Neurol. Scand. 102, 74-84. [DOI] [PubMed] [Google Scholar]
- 21.Scali, C., Giovannini, M., Prosperi, C., Bartolini, L. & Pepeu, G. (1997) Pharmacol. Res. 36, 463-469. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos, G. & Watson, C. (1982) The Rat Brain in Stereotaxic Coordinates (Academic, New York).
- 23.Wheal, H., Lancaster, B. & Bliss, T. (1983) Brain Res. 272, 247-253. [DOI] [PubMed] [Google Scholar]
- 24.Staubli, U. & Lynch, G. (1987) Brain Res. 435, 227-234. [DOI] [PubMed] [Google Scholar]
- 25.Colgin, L., Kubota, D. & Lynch, G. (2003) Proc. Natl. Acad. Sci. USA 100, 2872-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Y., Rowan. M. & Anwyl, R. (1998) J. Physiol. (London) 513, 467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson G., Wang Y. & Alger, B. (2002) Nat. Neurosci. 5, 723-724. [DOI] [PubMed] [Google Scholar]
- 28.Ingram, D., Shimada, A., Spangler, E., Ikari, H., Hengemihle, J., Kuo, H. & Greig, N. (1996) Ann. N.Y. Acad. Sci. 786, 348-361. [DOI] [PubMed] [Google Scholar]
- 29.Ikari, H., Spangler, E., Greig, N., Pei, X, Brossi, A., Speer, D., Patel, N. & Ingram, D. (1995) NeuroReport 6, 481-484. [DOI] [PubMed] [Google Scholar]
- 30.Shaw, K., Utsuki, T., Rogers, J., Yu, Q., Sambamurti, K., Brossi, A., Ge, Y., Lahiri, D. & Greig, N. (2001) Proc. Natl. Acad. Sci. USA 98, 7605-7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahiri, D., Farlow, M., Hintz, N., Utsuki, T. & Greig, N. (2000) Acta Neurol. Scand. 102, 60-67. [DOI] [PubMed] [Google Scholar]
- 32.Borchelt, D., Ratovitski, T., van Lare, J., Lee, M., Gonzales, V., Jenkins, N., Copeland, N., Price, D. & Sisodia, S. (1997) Neuron 19, 939-945. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, N., Cheung, T., Cai, X., Odaka, A., Otvos, L., Jr., Eckman, C., Golde, T. & Younkin, S. (1994) Science 264, 1336-1340. [DOI] [PubMed] [Google Scholar]
- 34.Greig, N., Sambamurti, K., Yu, Q., Perry, T., Holloway, H., Haberman, F., Brossi, A., Ingram, D. & Lahiri, D. (2003) in Butyrylcholinesterase: Its Function and Inhibitors, ed. Giacobini, E. (Martin Dunitz, London), pp. 69-90.
- 35.Mesulam, M., Guillozet, A., Shaw, P. & Quinn, B. (2002) Neurobiol. Dis. 9, 88-93. [DOI] [PubMed] [Google Scholar]
- 36.Scali, C., Giovannini, M., Bartolini, L., Prosperi, C., Hinz, V., Schmidt, B. & Pepeu, G. (1997) Eur. J. Pharmacol. 325, 173-180. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton, S., Hardouin, S., Anagnostaras, S., Murphy, G., Richmond, K., Silva, A., Feigl, E. & Nathanson, N. (2001) Life Sci. 68, 2489-2493. [DOI] [PubMed] [Google Scholar]
- 38.Anagnostaras, S., Murphy, G., Hamilton, S., Mitchell, S., Rahnama, N., Nathanson, N. & Silva, A. (2003) Nat. Neurosci. 6, 51-58. [DOI] [PubMed] [Google Scholar]
- 39.Ji, D., Lape, R. & Dani, J. A. (2001) Neuron 31, 131-141. [DOI] [PubMed] [Google Scholar]
- 40.Francis, P. (2003) Int. J. Geriatr. Psychiatry 18, Suppl. 1, S15-S21. [DOI] [PubMed] [Google Scholar]
- 41.Ballard, C., Greig, N., Guillozet-Bongaarts, A., Enz, A. & Darvesh, S. (2005) Cur. Alz. Res. 2, 307-318. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien, K., Saxby, B., Ballard, C., Grace, J., Harrington, F., Ford, G., O'Brien, J., Swan, A., Fairbairn, A., Wesnes, K., et al. (2003) Pharmacogenetics 13, 231-239. [DOI] [PubMed] [Google Scholar]
- 43.Holmes, C., Ballard, C., Lehmann, D., Smith, A., Beaumont, H., Day, I., Nadeem Khan, M., Lovestone, S., McCulley, M., et al. (2005) J. Neurol. Neurosurg. Psychiatry 76, 640-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Court, J. & Perry, E. (1991) Pharmacol. Ther. 52, 423-443. [DOI] [PubMed] [Google Scholar]
- 45.Perry, E., McKeith, I. & Ballard, C. (2003) Neurology 60, 1852-1853. [DOI] [PubMed] [Google Scholar]
- 46.Walsh, D., Klyubin, I., Fadeeva, J., Rowan, M. & Selkoe, D. (2002) Biochem. Soc. Trans. 30, 552-557. [DOI] [PubMed] [Google Scholar]
- 47.Rowan, M., Klyubin, I., Cullen, W. & Anwyl, R. (2003) Philos. Trans. R. Soc. London B 358, 821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefanova, E., Blennow, K., Almkvist, O., Hellstrom-Lindahl, E. & Nordberg, A. (2003) Neurosci. Lett. 338, 159-163. [DOI] [PubMed] [Google Scholar]
- 49.Westerman, M., Cooper-Blacketer, D., Mariash, A., Kotilinek, L., Kawarabayashi, T., Younkin, L., Carlson, G. A., Younkin, S. & Ashe, K. (2002) J. Neurosci. 22, 1858-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soreq, H. & Zakut, H. (1993) Human Cholinesterases and Anticholinesterases (Academic, New York).