Abstract
Polymorphisms in TIM-1, a member of the T cell Ig and mucin (TIM) domain family, are associated with relative susceptibility to the development of T helper 2-dominated immune responses such as in allergic asthma. Recent data have also suggested that ligation of TIM-1 can augment T cell activation. We have found that the TIM-1 protein is expressed on CD4+ T cells in vivo after intranasal immunization. Ectopic expression of TIM-1 during T cell differentiation results in a significant increase in the number of cells producing IL-4 but not IFN-γ. Furthermore, TIM-1 expression provides a costimulatory signal that increases transcription from the IL-4 promoter and from isolated nuclear factor of activated T cells/activating protein-1 (NFAT/AP-1) elements. Finally, we provide evidence that TIM-1 can be phosphorylated on tyrosine and that TIM-1 costimulation requires its cytoplasmic tail and the conserved tyrosine within that domain. These results constitute evidence that TIM-1 directly couples to phosphotyrosine-dependent intracellular signaling pathways.
Keywords: costimulation, phosphorylation, asthma, cytokines
Many helper T cell-dependent immune responses skew toward one of two stereotyped profiles. Generally speaking, the T helper (Th) 2 type response has been selected for its ability to eliminate parasitic worm infections, whereas a Th1 response is more effective at dealing with viruses and intracellular bacteria (1, 2). It is still not clear why some individuals respond to certain allergens with strongly polarized Th2 immune responses. Environmental factors likely play a role, but there is strong evidence from both humans and mice that genetic factors also influence the outcome. A number of human linkage analyses have been performed, and the results implicate at least 15 genetic loci in human atopy and asthma (3). One human genetic locus that has received a great deal of attention is 5q23-35. The syntenic region of mouse chromosome 11 has also been implicated in the development of atopic asthma (4) by analysis of congenic mice on the asthma-susceptible BALB/c background with discrete loci from the asthma-resistant strain DBA/2. In one of these lines, termed “HBA,” suppression of asthma susceptibility was mapped to a genetic interval that the authors named tapr (for T cell and airway phenotype regulator). Further analysis implicated two genes in this locus, tim1 and tim3, named for the fact that they encode proteins expressed on T cells and contain immunoglobulin and mucin domains. The T cell Ig and mucin domain proteins (TIMs) are type I transmembrane proteins, with extracellular Ig and mucin domains and intracellular domains of various lengths.
In addition to the genetic linkage to asthma in mice, there are several lines of evidence suggesting that TIM-1 is involved in helper T cell differentiation. First, TIM-1 mRNA is up-regulated by CD4+ T cells within 7 h of stimulation (4), a time when helper T cells are undergoing differentiation to become effector cells. Also, messages for TIM-1 were found at higher levels in Th2 lines derived from patients with multiple sclerosis, compared with TIM-3 message, which was more abundant in Th1 lines (5). In the same study, levels of TIM-1 message correlated positively with levels of message for the Th2-associated cytokine IL-10 and inversely with IFN-γ (5). One hypothesis for how TIM-1 functions in T cells is that it acts as a costimulator to affect T cell activation, either quantitatively or qualitatively. The related molecule TIM-2 has been implicated in the costimulation of murine T cell activation (6, 7) but is most likely not involved in the tapr phenotype, because no polymorphisms were found (4).
Recent studies have examined the expression and function of murine TIM-1 in some detail, by using an anti-TIM-1 antibody (8) or a TIM-1-Ig fusion protein (9). Consistent with the studies cited above, TIM-1 protein was found on activated T cells, with the highest levels seen on Th2 cells (8). Furthermore, addition of the anti-TIM-1 antibody provided a costimulatory signal for T cell activation. The costimulatory function of TIM-1 may be induced by binding to TIM-4, which was shown to be a ligand for TIM-1 (9).
We report here that TIM-1 protein is expressed in vivo on the surface of T cells from lung draining lymph nodes after intranasal immunization. Furthermore, ectopic expression of TIM-1 increases the frequency of IL-4-producing cells when T cells are stimulated under nonpolarizing conditions. We have also found that TIM-1 augments the activation of the IL-4 promoter in a Th2 T cell clone, a costimulatory effect that may occur through increased activation of the nuclear factor of activated T cells (NFAT) transcription factor. Finally, we provide evidence that the cytoplasmic tail of TIM-1 is required for its costimulatory activity, through a mechanism that depends on tyrosine phosphorylation.
Materials and Methods
DNA Constructs. When we commenced these studies, an antibody to murine TIM-1 was not available, so we generated a TIM-1 construct with an extracellular Flag epitope tag. A cDNA clone containing the entire coding sequence of murine TIM-1 (from strain C57BL/6), originally isolated by the Integrated Molecular Analysis of Genomes and their Expression (I.M.A.G.E.) consortium, was purchased from Open Biosystems (Huntsville, AL). The ORF of TIM-1 (excluding the start codon and signal sequence) was amplified from this clone by PCR and ligated into a pCDEF3 expression plasmid containing the human CD8α signal sequence and Flag epitope tag (10). A Flag-TIM-1 construct lacking the cytoplasmic tail (Δ-cyto TIM-1) was generated in an identical fashion, except that the reverse primer was designed around the boundary of the transmembrane and cytoplasmic domains, with a stop codon at the 5′ end of the primer. Tyrosine-276 in the cytoplasmic tail of TIM-1 (BL/6 allele) was mutated to phenylalanine with the QuickChange site-directed mutagenesis kit (Stratagene). All DNA constructs were verified by automated DNA sequencing.
Antibodies and Flow Cytometry. Anti-Flag (M2) was from Sigma. Anti-murine TIM-1, rat isotype control, and anti-rat-FITC were from eBioscience (San Diego). Anti-murine CD3 (500A2), CD4, and CD28 (37.52) were obtained from BD Pharmingen or Caltag (Burlingame, CA). Other conjugated secondary and cytokine antibodies were from Caltag. TIM-1-Ig was described in ref. 9. Mouse monoclonal anti-phosphotyrosine 4G10 was from Upstate USA (Charlottesville, VA).
Induction of Airway Tolerance or Inflammation. BALB/cByJ mice were immunized exactly as described in ref. 11.
Transient Transfections and Luciferase Assays. Jurkat or D10 T cells were transfected by electroporation and luciferase assays were performed as described in refs. 12 and 13. Recombinant human IL-2 was obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) from Maurice Gately (Hoffmann-La Roche).
Generation of Recombinant Retrovirus and Infection of Primary T Cells. Flag-TIM-1 was subcloned into the MSCV2.2 retroviral vector, which contains an internal ribosome entry site and GFP ORF downstream of the multiple cloning site. To generate recombinant retrovirus, MSCV-TIM-1 (or empty vector) was transfected into the Phoenix packaging cell line by the calcium phosphate method, along with the ecotropic packaging vector, to increase the efficiency of virus production. Viral supernatant was harvested at 48 h and 72 h after transfection.
T cells were purified from 6-week-old C57BL/6 mice with the murine T cell purification kit from Miltenyi Biotec (Auburn, CA) and stimulated in 24-well plates coated with rabbit anti-hamster IgG (Sigma) and antibodies to CD3 and CD28, at 1 million cells per well. After 28 h, T cells were subjected to two rounds of spin infection, at 4-h intervals, with retroviral supernatant supplemented with IL-2 and Polybrene (8 μg/ml). Cells were expanded 1 day after infection and restimulated the next day with anti-CD3 plus brefeldin A (Sigma), and intracellular cytokine staining was performed after overnight stimulation.
TIM-1 Tyrosine Phosphorylation. D10 or Jurkat cells (20 million) were transfected with empty vector or Flag-TIM-1. Nonidet P-40 cell lysates were made the next day and immunoprecipitated with M2 covalently coupled to agarose beads (Sigma). Immunoprecipitates were separated on SDS/PAGE gels and Western-blotted with M2 and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Pierce). Blots were developed with Super-Signal Pico ECL substrate (Pierce) and digitally imaged on a Kodak Image Station 2000R, with accumulation automatically set to stop at 2,000 gray levels. Blots were then stripped and reprobed with 4G10 and HRP-conjugated anti-mouse IgG. Digital images were exported as JPEG images and final figures were composed in canvas 8.
Results
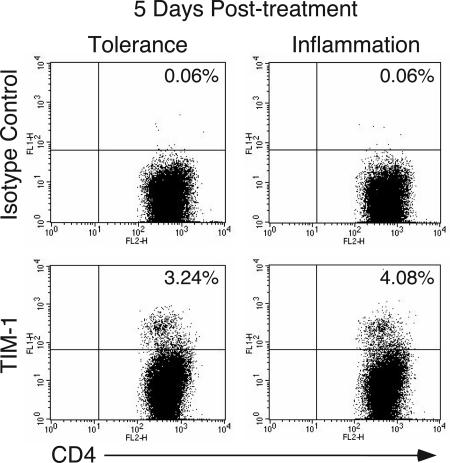
Expression of TIM-1 on CD4+ T Cells in Vivo. The available data suggest that TIM-1 expression is associated with development of Th2 T cell responses. We examined TIM-1 expression on T cells in vivo during a Th2 immune response initiated by intranasal immunization with ovalbumin (OVA) plus cholera toxin (CT) (11, 14-16). As shown in Fig. 1, a significant proportion of T cells isolated from lung-draining lymph nodes after OVA/CT immunization expressed TIM-1 5 days (and also 3 days; data not shown) after treatment (Right). We also assessed the expression of TIM-1 on T cells after treatment with OVA alone, which induces an abortive course of T cell activation and partial Th2 effector development that results in tolerance. As shown in Fig. 1 (Left), this treatment also led to the expression of TIM-1 at days 3 and 5, consistent with up-regulation of TIM-1 expression early after activation, but before the induction of tolerance. No detectable TIM-1 staining was observed on naïve T cells (data not shown). Thus, intranasal immunization of T cells under inflammatory or tolerizing conditions leads to the expression of TIM-1. This finding is a definite demonstration of TIM-1 expression on T cells isolated from a murine model of asthma, a Th2-mediated disease.
Fig. 1.
TIM-1 is expressed on lung-draining lymph node CD4 T cells under conditions of airway tolerance and inflammation. Mice were immunized as described in Materials and Methods, with OVA alone to induce tolerance (Left) or with OVA plus cholera toxin to induce inflammation (Right). Five days after the last intranasal treatment, lung-draining lymph nodes were harvested, and cells were stained with antibodies to CD4 and TIM-1. Results are gated on CD4+ cells.
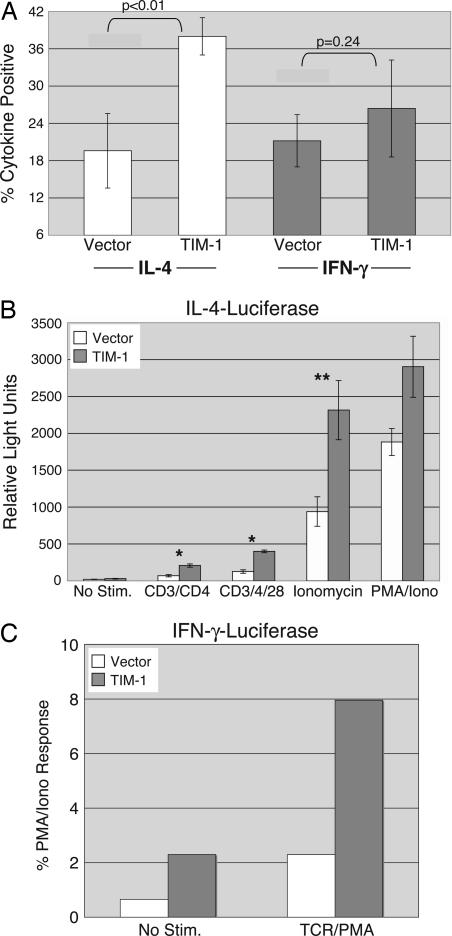
Effects of Ectopic TIM-1 Expression on Helper T Cell Cytokine Production. Expression of the TIM molecules appears to be tightly regulated (7, 17). We wanted to assess directly the effect of enforced TIM-1 expression during T cell activation and differentiation, so we used a widely used retroviral system for expression of exogenous genes in primary T cells (18, 19). Cells infected with control or TIM-1 retrovirus could be followed for expression of GFP, which is also encoded for by the retroviral vector. In addition, cells infected with TIM-1 retrovirus were stained with anti-Flag antibody and analyzed by flow cytometry, to confirm expression of Flag-TIM-1 (Fig. 6A, which is published as supporting information on the PNAS web site). After infection, cells were rested and restimulated with anti-CD3 antibody. Intracellular cytokine staining was performed after restimulation, to assess the number of cells producing IFN-γ and IL-4, the hallmark cytokines of Th1 and Th2 cells. As shown in Fig. 2A, cultures infected with TIM-1 retrovirus contained significantly more (≈2- to 3-fold) cells making IL-4, compared with cells infected with control retrovirus. By contrast, expression of TIM-1 had little or no effect on the number of cells producing IFN-γ in these same cultures. The number of IFN-γ-producing cells could, however, be increased if cells were cultured under Th1-skewing conditions (data not shown). Somewhat unexpectedly, these results were observed in the absence of any active crosslinking of the Flag-TIM-1 protein, and addition of anti-Flag antibody had no further positive or negative effects (data not shown).
Fig. 2.
Costimulation of Th2 differentiation and cytokine transcription by TIM-1. (A) Purified T cells were stimulated in vitro under neutral conditions, then infected with control MSCV-GFP retrovirus (“vector”) or virus encoding Flag-tagged murine TIM-1. Cells were rested and restimulated with anti-CD3 antibody, then stained for intracellular cytokines. Results are the percentage of cytokine-positive cells of the GFP+ population in each case and are the average ± SD of six samples from three experiments. P values were derived from Student's t test analyses. (B) D10 T cells were transfected with an IL-4 promoter luciferase reporter and either an empty vector or Flag-TIM-1. The next day, cells were stimulated as indicated (PMA, phorbol 12-myristate 13-acetate) and analyzed for luciferase activity. Results are presented as relative light units (mean ± SD) of four samples, from two separate experiments. Student's t tests were performed: *, P < 0.05; **, P < 0.01. (C) Jurkat T cells were transfected with a murine IFN-γ promoter luciferase construct, along with either an empty vector or Flag-TIM-1 plasmid. Cells were stimulated as indicated (TCR, T cell receptor) and luciferase activity was determined. Results are the mean response, normalized to PMA/ionomycin (2,522 relative light units for vector; 2,111 for TIM-1) for duplicate points of a single experiment, representative of the three performed.
Costimulation of Inducible Transcription by TIM-1. Because our results suggested that the development of IL-4-producing cells is enhanced in the presence of TIM-1, we wished to determine whether this effect was due, at least in part, to increased IL-4 transcription. We cotransfected an IL-4 promoter luciferase reporter into the D10 Th2 T cell clone (20), along with either an empty vector or Flag-TIM-1 expression plasmid. Flag-TIM-1 was expressed efficiently on the surface of transfected D10 cells (Fig. 6B), and cells expressing TIM-1 responded significantly better to a range of stimuli than cells transfected with empty vector (Fig. 2B). These conditions included stimulation through the T cell receptor (TCR)/CD3 complex, with or without CD28 costimulation, or ionomycin, all of which were enhanced in the presence of TIM-1. By contrast, phorbol 12-myristate 13-acetate (PMA) and ionomycin, which bypass the proximal tyrosine kinases and phospholipase C-γ1, induced a level of stimulation that was not significantly affected by TIM-1 (P = 0.15). In addition, the basal level of IL-4 promoter transcriptional activity was not affected by the transfection of TIM-1.
Given the above results, we wanted to determine whether TIM-1 expression also affects transcription of IFN-γ. When an IFN-γ-luciferase reporter was transfected into Jurkat T cells, we noted that cotransfection of TIM-1 led to a significantly higher level of basal luciferase activity, i.e., in the absence of stimulation (Fig. 2C). TIM-1 expression also augmented the stimulation of this reporter by signaling through the TCR. Therefore, at least when expressed in Jurkat T cells, TIM-1 can also augment transcription from the IFN-γ promoter.
Cytokine transcription is controlled by a number of transcription factors (21). Perhaps the best studied of these transcription factors are the NFAT proteins, four of which are expressed by lymphocytes (22). Most NFAT family members are regulated by calcium, their entry into the nucleus occurring after calcineurin-mediated dephosphorylation, which requires increases in intracellular calcium (22). In many promoters, NFAT binds cooperatively to DNA with transcription factors of the AP-1 family (23). The IL-4 promoter contains both cis-acting elements that can bind NFAT alone and sites where NFAT binds cooperatively with activating protein-1 (AP-1) (21). We cotransfected an NFAT/AP-1 luciferase reporter into D10 T cells, along with empty vector or the Flag-TIM-1 expression plasmid. As shown in Fig. 3A, cells expressing TIM-1 and the NFAT/AP-1 reporter responded more robustly to stimulation through the TCR/CD3 complex, compared with cells transfected with empty vector. Consistent with the results shown in Fig. 2B, no increase in basal NFAT/AP-1 reporter activity was observed in TIM-1-transfected D10 cells.
Fig. 3.
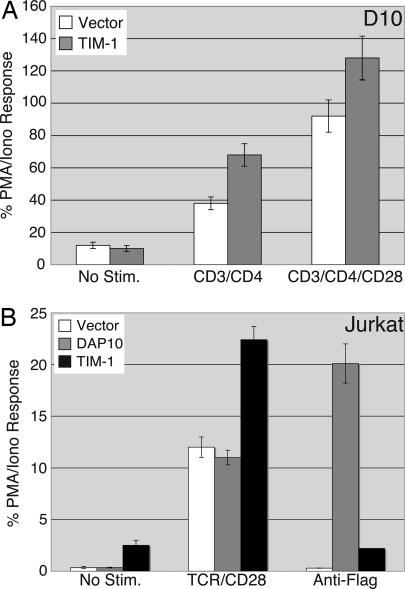
TIM-1 costimulates NFAT/AP-1-dependent transcription. D10 (A) or Jurkat (B) T cells were transfected with an NFAT/AP-1 luciferase reporter and the indicated plasmids. Cells were treated the next day with the indicated stimuli and analyzed for luciferase activity, which is expressed as the percentage (mean ± SD) of the response with PMA/ionomycin from triplicate points of a single experiment. (A) NFAT/AP-1 activity in D10 T cells transfected with empty vector or Flag-TIM-1. Data displayed are representative of three experiments that were performed. PMA/ionomycin values were 2,137 relative light units for vector and 2,603 for TIM-1-transfected cells. (B) NFAT/AP-1 activity in Jurkat cells transfected with empty vector, Flag-DAP10, or Flag-TIM-1. Data displayed are the means of triplicate points from a single experiment, representative of >10 experiments. PMA/ionomycin values were 32,186 relative light units for vector, 59,935 for DAP10, and 59,373 for TIM-1-transfected cells.
We also assessed the ability of TIM-1 to costimulate NFAT/AP-1-dependent transcription in Jurkat T cells. Flag-TIM-1 could be detected on Jurkat T cells after transient transfection (Fig. 6C). When luciferase activity was determined (Fig. 3B), we noted that TIM-1 (black bars) significantly increased the basal activity of the NFAT/AP-1 reporter, in contrast to what was seen in D10 cells (Fig. 3A). Consistent with the IL-4 and NFAT/AP-1 results obtained with D10 cells, TIM-1 could also costimulate transcription induced through the TCR and CD28. Treatment with anti-Flag antibody, however, neither enhanced nor inhibited the basal NFAT/AP-1 activity induced by TIM-1. As a positive control for the anti-Flag treatment, we transfected Jurkat cells with Flag-tagged DAP10, a component of some activating receptors in NK cells (10). Consistent with previous results (L.P.K., unpublished data), crosslinking of this construct could activate transcription from an NFAT/AP-1 reporter but, unlike TIM-1, DAP10 expression alone did not lead to increases in basal or TCR-stimulated NFAT/AP-1 reporter activity (Fig. 3B, gray bars). TIM-1 expression could also costimulate induction of an AP-1 reporter (Fig. 7A, which is published as supporting information on the PNAS web site). However, in contrast to the costimulatory molecule CD28, TIM-1 had no effect on an NF-κB reporter (Fig. 7B).
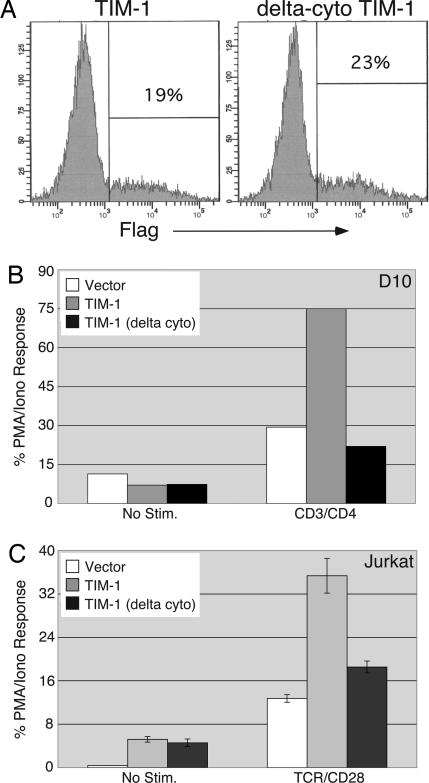
Role of the TIM-1 Cytoplasmic Tail in Costimulation. We next wanted to determine whether the costimulatory signal provided by TIM-1 requires its cytoplasmic tail, analogous to other costimulatory molecules such as CD28. We generated a Flag-TIM-1 construct lacking the cytoplasmic tail and compared its ability to costimulate NFAT-dependent transcription with that of full-length Flag-TIM-1. As shown in Fig. 4A, Δ-cyto Flag-TIM-1 was expressed at the surface of transfected D10 (and Jurkat; data not shown) T cells at levels roughly equivalent to the full-length construct. When the constructs were cotransfected into D10 cells with an NFAT/AP-1 luciferase reporter (Fig. 4B), Δ-cyto TIM-1 displayed an almost complete loss of costimulatory activity. Similarly, the Δ-cyto TIM-1 construct displayed almost no costimulatory activity when transfected into Jurkat T cells (Fig. 4C). For reasons that are still unclear, expression of truncated TIM-1 in Jurkat cells still led to increased levels of basal reporter activity, i.e., in the absence of additional stimulation.
Fig. 4.
The TIM-1 cytoplasmic tail is required for costimulation of transcription. D10 or Jurkat T cells were transfected with the NFAT/AP-1 luciferase reporter and either the empty vector, Flag-TIM-1, or the truncated Flag-TIM-1, lacking the cytoplasmic tail (“delta cyto”). (A) Expression of the Flag-TIM-1 constructs on transfected D10 cells. (B) Luciferase activity of unstimulated or anti-CD3/CD4-stimulated D10 cells transfected with the indicated constructs. Results shown are the mean of duplicate points from a single experiment, representative of four that were performed. (C) Luciferase activity in Jurkat T cells transfected with the same constructs as in B. Luciferase activity is expressed as the percentage (mean ± SD) of the response with PMA/ionomycin from triplicate points of a single experiment, representative of eight that were performed. PMA/ionomycin values were 139,864 relative light units for vector, 49,773 for TIM-1, and 81,540 for Δ-cyto TIM-1-transfected cells.
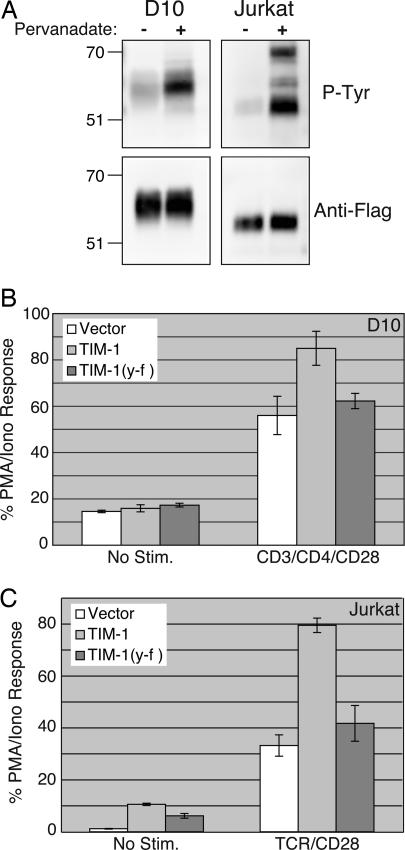
Tyrosine Phosphorylation of TIM-1. Inducible tyrosine phosphorylation is critical for the initiation of TCR/CD28-mediated signal transduction and T cell activation, so we were interested in determining whether TIM-1 could be phosphorylated on tyrosine. As shown in Fig. 5A, TIM-1 expressed in unstimulated Jurkat or D10 cells displayed a low level of basal tyrosine phosphorylation, which was greatly increased after treatment of cells with pervanadate. These results demonstrate that TIM-1 can be tyrosine phosphorylated when expressed in T cells.
Fig. 5.
TIM-1 tyrosine phosphorylation and requirement for tyrosine-276 in costimulation of NFAT. (A) Jurkat or D10 T cells were transfected with Flag-TIM-1. The next day, transfected cells were divided and left unstimulated or stimulated with pervanadate as described in ref. 31. Immunoprecipitations were performed with anti-Flag mAb, then the precipitates were separated by SDS/PAGE and Western-blotted. Blots were probed with anti-Flag (Lower), then stripped and reprobed with anti-phosphotyrosine antibody 4G10 (Upper). Jurkat (B) or D10 (C) cells were transfected with NFAT/AP-1-luciferase plus empty vector, wild type Flag-TIM-1, or Flag-TIM-1 (Y-F). Luciferase activity is expressed as the percentage of the PMA/ionomycin response from triplicate samples (mean ± SD) of a single experiment, representative of five that were performed.
The cytoplasmic tail of TIM-1 contains two tyrosines, one of which (Y276 in the BL/6 allele) conforms well to a consensus site for src family tyrosine kinase phosphorylation (24). The other tyrosine is only two residues removed from the transmembrane domain and is a poor candidate for phosphorylation. To test the functional relevance of TIM-1 tyrosine phosphorylation, tyrosine-276 in TIM-1 (with a Flag tag) was mutated to phenylalanine. TIM-1(Y-F) is expressed at equivalent levels on the surface of transfected T cells, compared with wild-type Flag-TIM-1 (data not shown). Also, like the Δ-cyto form of TIM-1 (Fig. 4), TIM-1(Y-F) is deficient in costimulation of NFAT transcriptional responses (Fig. 5 B and C). Thus, tyrosine-276 in the cytoplasmic tail of TIM-1 is required for costimulation of NFAT/AP-1-dependent transcription by this molecule.
Discussion
Here we have provided direct evidence that TIM-1 plays a role in T cell activation and differentiation. Thus, we have demonstrated that TIM-1 is expressed on T cells of the lung-draining lymph nodes after intranasal immunization. Furthermore, ectopic retroviral expression of TIM-1 in developing effector T cells increases the number of IL-4-producing cells. This finding is consistent with our observation that expression of TIM-1 in a Th2 T cell clone provides a costimulatory signal for increased transcription from the IL-4 promoter. TIM-1 expression can also augment signaling to NFAT and AP-1, critical transcription factors for Th2 development, and IL-4 production. Finally, we have obtained evidence that the costimulatory function of TIM-1 requires a tyrosine within its cytoplasmic tail, which likely couples the molecule to intracellular signal transduction pathways. We conclude that TIM-1 costimulatory function is mediated at least in part through effects on inducible transcription factors.
We have observed that TIM-1 is expressed on CD4+ effector T cells after immunization protocols that result in either inflammation or tolerance (Fig. 1). Because the induction of tolerance in this model (and others) occurs after some level of T cell activation and effector differentiation (ref. 11 and T.B.O. and A.R., unpublished data), TIM-1 expression most likely occurs before anergy is established. Consistent with a defect downstream of IL-4 receptor signal transduction (11), even the level of IL-4 produced in the presence of TIM-1 is apparently insufficient to overcome the tolerizing block. Intriguingly, using a similar model, Umetsu et al. (8) showed recently that pretreatment of animals with a TIM-1 monoclonal antibody can inhibit tolerance induction. It may be that the TIM-1-expressing cells that we observe after tolerance induction have resulted from bystander activation, rather than being antigen-specific, although further analysis is required to determine whether this is the case.
The effects of TIM-1 on IL-4 production and transcription would appear, at first glance, to be rather modest. However, costimulatory receptors generally do not function in an “all-or-none” fashion. Rather, they modify the initial stimulation conditions such that more cells can surpass the threshold for entry into the cell cycle and differentiation. Absence of a particular costimulatory molecule would therefore be predicted to have modest to severe effects on the ability to develop an immune response, depending on the initial conditions. Th2 immune responses appear to be particularly sensitive to the initial “strength” of stimulation, because full differentiation to the Th2 lineage requires additional rounds of cell division, compared with development of Th1 cells (25, 26). Also, a number of costimulatory and signaling molecules are preferentially required for the generation of Th2, compared with Th1, responses (27-29). It therefore appears that the requirement for further rounds of cell division in their development has made Th2 cells more dependent on costimulation and sustained signaling. This characteristic could be the result of less selection pressure to generate a rapid response because Th2 responses are tailored toward fighting relatively slow-growing extracellular pathogens such as parasitic worms.
The increased number of IL-4-producing cells after TIM-1 expression (Fig. 2 A) is consistent with our findings that transcription from the IL-4 promoter and isolated NFAT/AP-1 elements are also augmented by TIM-1. However, the effects of TIM-1 on inducible transcription cannot completely explain the preferential development of IL-4-producing cells, because we have also observed that ectopic expression of TIM-1 can augment transcription from an IFN-γ luciferase promoter reporter (Fig. 2C). TIM-1 may therefore have a preferential effect on the survival and/or proliferation of Th2 cells, the latter being consistent with the recent finding that TIM-1 ligation can augment TCR-driven proliferation (8). Further investigation will be necessary to determine whether this is the case.
Our results are largely in agreement with recent studies (8, 9). Using an anti-TIM-1 antibody, Umetsu et al. (8) provided compelling evidence for a costimulatory role for TIM-1 in T cell activation and differentiation. Also, Meyers et al. (9) showed that TIM-1 costimulation of T cell activation can occur as a consequence of binding to the TIM family protein TIM-4 on antigen-presenting cells. However, for reasons that are still unclear, we have not observed any effect of crosslinking the Flag-TIM-1 construct. Our experiments were carried out with purified T cells and T cell lines, making it unlikely that TIM-4 was present during our analyses, based on the finding that TIM-4 is expressed on neither naïve nor activated T cells (9). The effects of TIM-1 that we have reported here may have resulted in part from interaction with a ligand distinct from TIM-4. Indeed, we have obtained evidence for a TIM-1 ligand on Jurkat, but not D10, T cells (Fig. 8A, which is published as supporting information on the PNAS web site). Thus far, however, we have observed only a partial decrease in basal levels of NFAT/AP-1 reporter activity when using TIM-1-Ig to block interactions with this putative ligand (Fig. 8B).
It is also possible that TIM-1 may homodimerize (at least in our experiments with ectopic expression) in a fashion regulated by its heavily glycosylated mucin domain, perhaps in a manner similar to CD45 (30). This model is particularly intriguing in light of the fact that polymorphisms in both murine and human TIM-1 associated with relative asthma susceptibility are found in the mucin domain. We do not believe that the Flag epitope tag has an influence on the activity of TIM-1 in our system, because expression of a construct lacking the Flag epitope tag can also costimulate the NFAT/AP-1 reporter (data not shown). Also, expression of a Flag-tagged version of the NK-activating DAP10 molecule does not lead to constitutive NFAT activity, but rather requires crosslinking (Fig. 3). Differential glycosylation of TIM-1 may occur in the two main cell types that we have used in these studies, the Jurkat and D10 T cell lines. Thus, when we examined TIM-1 phosphorylation (Fig. 5A), we noted that TIM-1 runs at different apparent molecular weights when expressed in Jurkat or D10 cells.
Virtually nothing is known about how TIM family proteins connect to intracellular signaling pathways. Consistent with our findings (Fig. 5A), a previous report showed that TIM-2 can be tyrosine phosphorylated after interaction with a putative ligand, when expressed in a fibroblast cell line (7). The tyrosine at residue 276 in TIM-1 appears to be an ideal site for phosphorylation by a src family kinase. Many of the residues surrounding this tyrosine are conserved between rodent and primate orthologues of TIM-1, consistent with the likely importance of this motif.
In summary, we have demonstrated in this report that TIM-1 can provide a costimulatory signal that influences effector T cell differentiation and TCR-dependent activation of the IL-4 promoter and NFAT/AP-1 transcription factors. It will be of interest to determine how TIM-1 costimulatory signals intersect with those originating at the TCR/CD3 complex and the wider consequences of this costimulatory activity for T cell function.
Supplementary Material
Acknowledgments
We thank J. Wu and L. Lanier for the Flag-DAP-10 vector, S. Szabo and L. Glimcher for the IL-4-luciferase reporter, and P. Morel and V. Shapiro for critical reading of the manuscript. We also thank V. Kuchroo for providing TIM-1-Ig. Research was supported by startup funds and a Competitive Medical Research Fund award from the University of Pittsburgh School of Medicine (to L.P.K.) and by National Institutes of Health Grant AI48927 (to T.B.O. and A.R.).
Author contributions: A.J.d.S., T.B.O., A.R., and L.P.K. designed research and analyzed data; A.J.d.S., T.B.O., K.J.O., and L.P.K. performed research; and L.P.K. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: AP-1, activating protein-1; NFAT, nuclear factor of activated T cells; OVA, ovalbumin; PMA, phorbol 12-myristate 13-acetate; TCR, T cell receptor; Th, T helper; TIM, T cell Ig and mucin.
References
- 1.Glimcher, L. H. & Murphy, K. M. (2000) Genes Dev. 14, 1693-1711. [PubMed] [Google Scholar]
- 2.Murphy, K. M. & Reiner, S. L. (2002) Nat. Rev. Immunol. 2, 933-944. [DOI] [PubMed] [Google Scholar]
- 3.Steinke, J. W., Borish, L. & Rosenwasser, L. J. (2003) J. Allergy Clin. Immunol. 111, S495-S501. [DOI] [PubMed] [Google Scholar]
- 4.McIntire, J. J., Umetsu, S. E., Akbari, O., Potter, M., Kuchroo, V. K., Barsh, G. S., Freeman, G. J., Umetsu, D. T. & DeKruyff, R. H. (2001) Nat. Immunol. 2, 1109-1116. [DOI] [PubMed] [Google Scholar]
- 5.Khademi, M., Illes, Z., Gielen, A. W., Marta, M., Takazawa, N., Baecher-Allan, C., Brundin, L., Hannerz, J., Martin, C., Harris, R. A., et al. (2004) J. Immunol. 172, 7169-7176. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarti, S., Sabatos, C. A., Xiao, S., Illes, Z., Cha, E. K., Sobel, R. A., Zheng, X. X., Strom, T. B. & Kuchroo, V. K. (2005) J. Exp. Med. 202, 437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumanogoh, A., Marukawa, S., Suzuki, K., Takegahara, N., Watanabe, C., Ch'ng, E., Ishida, I., Fujimura, H., Sakoda, S., Yoshida, K. & Kikutani, H. (2002) Nature 419, 629-633. [DOI] [PubMed] [Google Scholar]
- 8.Umetsu, S. E., Lee, W.-L., McIntire, J. J., Downey, L., Sanjanwala, B., Akbari, O., Berry, G. J., Nagumo, H., Freeman, G. J., Umetsu, D. T. & DeKruyff, R. H. (2005) Nat. Immunol. 6, 447-454. [DOI] [PubMed] [Google Scholar]
- 9.Meyers, J. H., Chakravarti, S., Schlesinger, D., Illes, Z., Waldner, H., Umetsu, S. E., Kenny, J., Zheng, X. X., Umetsu, D. T., DeKruyff, R. H., et al. (2005) Nat. Immunol. 6, 455-464. [DOI] [PubMed] [Google Scholar]
- 10.Wu, J., Song, Y., Bakker, A. B., Bauer, S., Spies, T., Lanier, L. L. & Phillips, J. H. (1999) Science 285, 730-732. [DOI] [PubMed] [Google Scholar]
- 11.Oriss, T. B., Ostroukhova, M., Seguin-Devaux, C., Dixon-McCarthy, B., Stolz, D. B., Watkins, S. C., Pillemer, B., Ray, P. & Ray, A. (2005) J. Immunol. 174, 854-863. [DOI] [PubMed] [Google Scholar]
- 12.Kane, L. P., Shapiro, V. S., Stokoe, D. & Weiss, A. (1999) Curr. Biol. 9, 601-604. [DOI] [PubMed] [Google Scholar]
- 13.Kane, L. P., Mollenauer, M. N. & Weiss, A. (2004) J. Immunol. 172, 5441-5449. [DOI] [PubMed] [Google Scholar]
- 14.Akbari, O., DeKruyff, R. H. & Umetsu, D. T. (2001) Nat. Immunol. 2, 725-731. [DOI] [PubMed] [Google Scholar]
- 15.Akbari, O., Freeman, G. J., Meyer, E. H., Greenfield, E. A., Chang, T. T., Sharpe, A. H., Berry, G., DeKruyff, R. H. & Umetsu, D. T. (2002) Nat. Med. 8, 1024-1032. [DOI] [PubMed] [Google Scholar]
- 16.van Rijt, L. S., Vos, N., Willart, M., Kleinjan, A., Coyle, A. J., Hoogsteden, H. C. & Lambrecht, B. N. (2004) J. Allergy Clin. Immunol. 114, 166-173. [DOI] [PubMed] [Google Scholar]
- 17.Sabatos, C. A., Chakravarti, S., Cha, E., Schubart, A., Sanchez-Fueyo, A., Zheng, X. X., Coyle, A. J., Strom, T. B., Freeman, G. J. & Kuchroo, V. K. (2003) Nat. Immunol. 4, 1102-1110. [DOI] [PubMed] [Google Scholar]
- 18.Kane, L. P., Andres, P. G., Howland, K. C., Abbas, A. K. & Weiss, A. (2001) Nat. Immunol. 2, 37-44. [DOI] [PubMed] [Google Scholar]
- 19.Pear, W. S., Nolan, G. P., Scott, M. L. & Baltimore, D. (1993) Proc. Natl. Acad. Sci. USA 90, 8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaye, J., Porcelli, S., Tite, J., Jones, B. & Janeway, C. A., Jr. (1983) J. Exp. Med. 158, 836-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li-Weber, M. & Krammer, P. H. (2003) Nat. Rev. Immunol. 3, 534-543. [DOI] [PubMed] [Google Scholar]
- 22.Hogan, P. G., Chen, L., Nardone, J. & Rao, A. (2003) Genes Dev. 17, 2205-2232. [DOI] [PubMed] [Google Scholar]
- 23.Macian, F., Lopez-Rodriguez, C. & Rao, A. (2001) Oncogene 20, 2476-2489. [DOI] [PubMed] [Google Scholar]
- 24.Songyang, Z. & Cantley, L. C. (1995) Trends Biochem. Sci. 20, 470-475. [DOI] [PubMed] [Google Scholar]
- 25.Bird, J. J., Brown, D. R., Mullen, A. C., Moskowitz, N. H., Mahowald, M. A., Sider, J. R., Gajewski, T. F., Wang, C. R. & Reiner, S. L. (1998) Immunity 9, 229-237. [DOI] [PubMed] [Google Scholar]
- 26.Gett, A. V. & Hodgkin, P. D. (1998) Proc. Natl. Acad. Sci. USA 95, 9488-9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salek-Ardakani, S., Song, J., Halteman, B. S., Jember, A. G., Akiba, H., Yagita, H. & Croft, M. (2003) J. Exp. Med. 198, 315-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salek-Ardakani, S., So, T., Halteman, B. S., Altman, A. & Croft, M. (2004) J. Immunol. 173, 6440-6447. [DOI] [PubMed] [Google Scholar]
- 29.Fowell, D. J., Shinkai, K., Liao, X. C., Beebe, A. M., Coffman, R. L., Littman, D. R. & Locksley, R. M. (1999) Immunity 11, 399-409. [DOI] [PubMed] [Google Scholar]
- 30.Xu, Z. & Weiss, A. (2002) Nat. Immunol. 3, 764-771. [DOI] [PubMed] [Google Scholar]
- 31.O'Shea, J. J., McVicar, D. W., Bailey, T. L., Burns, C. & Smyth, M. J. (1992) Proc. Natl. Acad. Sci. USA 89, 10306-10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.