Abstract
Overactivity of the dopaminergic system in the brain is considered to be a contributing factor to the development and symptomatology of schizophrenia. Therefore, the GABAergic control of dopamine functions was assessed by disrupting the gene encoding the α3 subunit of the GABAA receptor. α3 knockout (α3KO) mice exhibited neither an obvious developmental defect nor apparent morphological brain abnormalities, and there was no evidence for compensatory up-regulation of other major GABAA-receptor subunits. Anxiety-related behavior in the elevated-plus-maze test was undisturbed, and the anxiolytic-like effect of diazepam, which is mediated by α2-containing GABAA receptors, was preserved. As a result of the loss of α3 GABAA receptors, the GABA-induced whole-cell current recorded from midbrain dopamine neurons was significantly reduced. Spontaneous locomotor activity was slightly elevated in α3KO mice. Most notably, prepulse inhibition of the acoustic startle reflex was markedly attenuated in the α3KO mice, pointing to a deficit in sensorimotor information processing. This deficit was completely normalized by treatment with the antipsychotic D2-receptor antagonist haloperidol. The amphetamine-induced hyperlocomotion was not altered in α3KO mice compared with WT mice. These results suggest that the absence of α3-subunit-containing GABAA receptors induces a hyperdopaminergic phenotype, including a severe deficit in sensorimotor gating, a common feature among psychiatric conditions, including schizophrenia. Hence, agonists acting at α3-containing GABAA receptors may constitute an avenue for an effective treatment of sensorimotor-gating deficits in various psychiatric conditions.
Keywords: haloperidol, sensorimotor gating
Monoaminergic neurons and basal forebrain acetylcholine neurons enable the cortex to exert global executive functions, such as attention, vigilance, mood, and reward (1). These neurotransmitter systems are thought to be of pathophysiological relevance in psychiatric diseases, particularly in schizophrenia. In this disease, the hypothesis of a dopamine hyperfunction is now yielding to a multifactorial view in which other monoamines as well as glutamate and GABA are included (2). The inhibitory control of the dopaminergic and other monoaminergic neurons appears to be of particular relevance for this disease. The GABAergic control of monoaminergic neurons is of special interest, because the α3 GABAA receptor is the main subtype expressed in these neurons (3). A deficit of α3-containing GABAA receptors would, therefore, be expected to affect most profoundly the monoaminergic functions.
A GABAergic hypofunction in schizophrenia has long been postulated (4) and is evident in postmortem studies, with subsets of GABAergic interneurons being dysfunctional in various brain areas (5, 6). In particular, the parvalbumin- (but not calretinin-) containing subpopulation of GABAergic interneurons in the prefrontal cortex is specifically affected in schizophrenia (7). The resulting alteration in perisomatic inhibition of pyramidal cells may contribute to a diminished capacity for the γ-frequency synchronized neuronal activity required for perception and working memory function in schizophrenic patients (8). A second line of evidence supports a developmental GABAergic defect due to a deficit in reelin expression in various brain regions in the postmortem schizophrenic brain (9-12). Reelin is expressed in GABAergic interneurons (13) from where it is secreted into the extracellular matrix and interacts with the dendritic spines of pyramidal neurons. Given the important role of reelin in the laminar formation of the cortex and synaptogenesis, its deficit could account for selective abnormalities in GABAergic neuronal function. Reduced neurotrophic signaling secondary to decreased expression of BDNF (14) might be a further pathogenic mechanism resulting in altered expression of GABA-related genes.
We now provide a genetic basis for a GABAergic hypofunction by demonstrating that GABAA receptor α3 knockout (α3KO) mice show behavioral signs indicative of functional hyperactivity in the midbrain dopamine system, which manifests itself as a deficit in sensorimotor gating. Thus, α3KO mice provide a monogenic animal model of sensorimotor-gating deficiency, a typical endophenotype of schizophrenia (15) and other mental disorders. Hence, drugs selective for α3-containing GABAA receptors may represent a route to dampen not only dopaminergic but also serotonergic transmission and thereby affect both positive and negative symptoms of schizophrenia.
Materials and Methods
Generation of α3KO Mice. Mice lacking the GABAA receptor α3 subunit (α3KO mice) were generated by gene targeting. For details of knockout construction, breeding strategy, Southern blot, RT-PCR, Northern blot, and Western blot analyses, see Supporting Information, which is published on the PNAS web site.
Electrophysiology. Procedures for preparation of slices and recording from midbrain dopamine neurons were as described in ref. 16. Recording electrodes (2-6 MΩ) contained 110 mM cesium methansulfonic acid, 10 mM cesium chloride, 10 mM Hepes, 0.4 mM EGTA, 2 mM NaCl, 5 mM tetraethylammonium chloride, 2.5 mM MgATP, and 0.25 mM MgGTP (pH 7.2-7.4, 275-285 mOsm). Cells were held at -10 mV for recording whole-cell GABA currents, which were elicited by bath application of GABA (100 μM) for 30 sec, followed by rapid wash-out.
Behavioral Phenotyping of α3KO Mice. For a detailed description of the behavioral phenotyping strategy and the determination of locomotor activity in the open field, prepulse inhibition of the acoustic startle reflex, and anxiety behavior in the elevated-plus-maze test, see Supporting Information.
Briefly, spontaneous locomotor activity was assessed for 60 min by using four 40 × 40-cm arenas under diffused lighting. The locomotor response to systemic amphetamine (2.5 mg/kg i.p. administered immediately before the test) was evaluated by using the same procedures but with an extended period of observation (120 min).
A test of prepulse inhibition (PPI) was conducted by using four San Diego Instruments (San Diego) startle chambers for mice in which the startle reflex was triggered by a pulse stimulus in the form of a 40-ms 120-dB[A] burst of white noise. Inhibition of the pulse-elicited startle reflex was achieved by using a 20-ms prepulse (white noise) stimulus of various intensities (69, 73, 77, and 81 dB[A]) that preceded the pulse stimulus by 100 ms. The test was conducted under a constant 65-dB[A] background noise and comprised a mixture of different trial types, as described in ref. 17. The efficacy of haloperidol (0.2 mg/kg i.p. administered 45 min before the test) to reverse the PPI deficit seen in the α3KO animals was assessed by using the same apparatus and procedures. PPI was indexed by percent inhibition of the startle response obtained in the pulse-alone trials by the following expression: 100% × [1 - (mean reactivity on “prepulse-plus-pulse” trials/mean reactivity on “pulse-alone” trials)], for each subject, and at each of the four possible prepulse intensities (4, 8, 12, and 16 dB[A] above background).
The standard elevated-plus-maze test of anxiety (18) was used as described in ref. 19. Over a period of 5 min, measures of anxiety-related behavior and locomotor activity were obtained. First, we assessed the behavior of α3KO mice. Next, the anxiolytic efficacy of diazepam to modify elevated-plus-maze behavior was evaluated in a dose-response analysis (0, 0.25, 0.5, and 1 mg/kg i.p. administered 30 min before the test).
All behavioral measures were subject to parametric ANOVA with the between-subjects factors genotype (α3KO vs. WT) and sex (male vs. female); additional repeated-measures factors were included whenever appropriate. In addition, restricted analyses and pair-wise post hoc comparisons using Fisher's least significant difference procedure (based on the appropriate pooled error variance from the ANOVAs) were conducted to assist interpretation.
Results
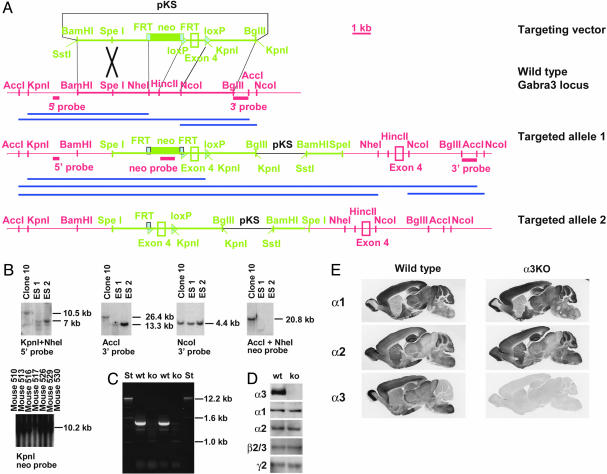
Generation of α3KO Mice. The Gabra3 gene encoding the α3 subunit of the GABAA receptor was inactivated in mice by gene targeting. Because two standard replacement vectors disrupting exons 4 or 8 did not yield any correct gene-targeting event when ≈2,000 clones were analyzed, insertion vector IV-3KO was constructed (Fig. 1A). Homologous recombination with insertion vectors requires a single reciprocal recombination, whereas homologous recombination with replacement vectors requires a double reciprocal recombination, and it has been shown that, at least in some cases, insertion vectors of the type used here may have significantly higher targeting frequencies than do replacement vectors (20). Insertion vector IV-3KO was linearized at a SpeI site within the 5′ homology and completely integrated into the ES cell genome (targeted allele 1), which thus contains a duplication of exon 4, which is predicted to shift the reading frame and, thus, prevent formation of the α3-subunit protein. The presence of a 4.4-kb NcoI-NcoI fragment detected by the 3′ flanking probe confirms that a neomycin-resistance cassette and additional loxP site are not present in the 3′ duplicate (Fig. 1B) (for potential target structures with insertion vectors, see ref. 21). Targeted ES cells were injected into blastocysts, and the neomycin-resistance cassette was eliminated by crossing mice carrying targeted allele 1 with hACTB::Flp mice (22) to yield targeted allele 2. The absence of the neomycin-resistance cassette in the germ line was confirmed in the subsequent generation by Southern blotting (Fig. 1B). To test whether both duplicates of exon 4 are incorporated into the mRNA, RT-PCR was performed, revealing that neither a fragment corresponding to the WT allele (1,346 bp) nor a fragment corresponding to a presence of the duplicated exon 4 (1,567 bp) was obtained, indicating that no normal mRNA is generated from targeted allele 2 (Fig. 1C). This observation might be due to nonsense-mediated RNA decay. Northern blot analysis confirmed that no detectable mRNA is present in the α3KO mice (data not shown).
Fig. 1.
Targeting of the GABAA-receptor α3 subunit (Gabra3) gene and molecular analysis. (A) Scheme of targeting strategy. Thin lines represent genomic sequences not included in the targeting vector. Thick lines represent genomic sequences included in the targeting vector. Dotted lines show pKS vector sequences. The neomycin-resistance marker (neo) is flanked by two FRT sites and the exon 4 by two loxP sites. The targeting vector is linearized with SpeI within the region of homology and fully integrated into the genome (targeted allele 1). Subsequent expression of the Flp transgene in mice eliminates the neomycin-resistance marker (targeted allele 2). (B) Southern blot analysis using 5′-flanking, 3′-flanking, and neo probes. ES1, ES cell line RW4; ES2, ES cell line AB2; clone 10, Gabra3-targeted clone. Mice 510, 513, 516, 517, 526, 529, and 530 were offspring of a mouse harboring both the targeted allele 1 and the hACTB::Flp transgene. Mouse 517 has lost the neomycin-resistance cassette (targeted allele 2). (C and D) Confirmation of the knockout at the mRNA and protein levels. mRNA prepared from brain without cerebellum and cortex was used for RT-PCR analysis (C). Western blotting was performed by using GABAA-receptor subunit-specific antibodies (D). (E) Immunohistochemical analysis of GABAA-receptor subunits with subtype-specific polyclonal antibodies recognizing the α1, α2, and α3 subunits.
Immunohistochemical Analysis. At the protein level, the absence of the α3 subunit in α3KO mice was confirmed by Western blot analysis, whereas the expression levels of other major subunits (α1, α2, β2/3, and γ2) were not altered (Fig. 1D). Immunohistochemically, there was no α3 immunoreactivity detectable in the α3KO mice, whereas the amount and distribution of immunoreactivity for the subunits α1, α2 (Fig. 1E), α5, β2/3, and γ2 (data not shown) was indistinguishable from WT mice. Thus, α3KO mice lack the α3 subunit and show no apparent changes in the expression and distribution of the other benzodiazepine-sensitive GABAA-receptor subunits tested.
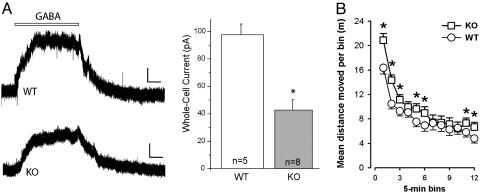
Electrophysiological Analysis. To assess the consequences of the α3KO on dopaminergic neurons at the cellular level, we compared whole-cell GABA currents in midbrain dopamine neurons of WT and α3KO mice. Bath application of GABA (100 μM for 30 sec) elicited inhibitory GABA currents that were significantly diminished in the α3KO animals (WT, 98.2 ± 7.6 pA, n = 5; KO, 43.4 ± 7.7 pA, n = 8, P < 0.05) (Fig. 2A). These results confirm that midbrain dopamine neurons are under inhibitory GABAergic control (23, 24) and demonstrate that inhibition is largely mediated by α3-containing GABAA receptors. The GABA current remaining in midbrain dopamine neurons of α3KO mice is likely mediated by other GABAA receptors (25).
Fig. 2.
GABA currents and locomotor activity in α3KO mice. (A) GABA-induced currents of midbrain dopamine neurons. Bath application of GABA elicited significantly smaller whole-cell currents in midbrain dopamine neurons from α3KO animals than from WT littermates (calibration bars, 25 pA, 6 sec). *, P < 0.05. (B) Locomotor activity in the open field was indexed by distance moved per 5-min bin. A slight elevation was revealed in the α3KO mice, regardless of gender, and this effect was most consistently seen in the first half of the test; the effect then subsided before reemerging toward the end of the test session. *, significant difference between α3KO and WT mice at the corresponding bins.
Locomotor Activity Elevated in α3KO Mice. Habituation of locomotor activity was evident in all animals, with a general reduction of distance traveled across successive bins (F11,308 = 101.32, P < 0.0001) (Fig. 2B), regardless of genotype and gender. α3KO mice exhibited a slight but significant elevation of locomotor activity that depended on the time bins of observation. This finding was confirmed by the significant interaction between genotype and bins (F11,308 = 3.88, P < 0.001). As indicated in Fig. 2B, the effect of α3KO was most prominent and consistently seen in the first half of the test session. Female mice were more active overall (F1,28 = 4.48, P < 0.05), but the effect of the mutation did not depend on gender.
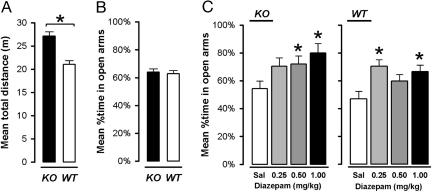
Elevated-Plus-Maze Behavior Unaltered in α3KO Mice. There was no evidence that the α3KO altered the expression of anxiety-related behavior in the elevated-plus-maze test, as indicated by the comparable scores in percent time spent in open arms (Fig. 3B). A2 × 2 (genotype × gender) randomized block ANOVA of both measures yielded no significant difference. In contrast, locomotor activity indexed by total distance traveled revealed a significant difference between α3KO and WT mice (F1,28 = 25.34, P < 0.001) (Fig. 3A), confirming the previous observation in the open field.
Fig. 3.
Elevated-plus-maze test. (A) A difference in locomotor activity (distance moved in the entire maze) was also detected in the elevated-plus-maze test (*, P < 0.05). (B) Anxiety-related behavior in untreated mice, as exemplified by the illustrated measure of percent time spent in the open arms, remained comparable between the two genotypes. (C) Both α3KO mice and the WT controls responded to diazepam, which promoted exploration of the open arms of the elevated-plus-maze test. *, significant difference from the saline (sal) control condition in the respective genotype.
Preservation of the Anxiolytic Effect of Diazepam in α3KO Mice. Because the anxiolytic-like activity of diazepam in the elevated-plus-maze test is mediated by α2-containing GABAA receptors (26), but apparently not by α1-, α3- and α5-containing GABAA receptors (26-29), this drug action was expected to remain undisturbed in the α3KO mice. As illustrated in Fig. 3C, diazepam increased the relative time spent exploring the open arms in the elevated-plus-maze test. This anxiolytic effect was equivalently seen in the α3KO and WT mice. A 2 × 4 (genotype × drug dosage) ANOVA yielded a highly significant main effect of drug [F3,42 = 6.63, P = 0.001] that was confirmed by separate one-way ANOVAs restricted to either the mutant or WT animals (both Ps <0.05). Thus, the anxiolytic-like activity of diazepam is retained in the α3KO mice, in line with the view that the anxiolytic-like activity of diazepam is mediated by GABAA receptors containing α2 subunits (26).
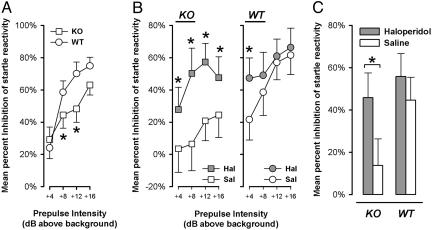
PPI Deficit in α3KO Mice. Sensorimotor gating was assessed by the paradigm of PPI of the acoustic startle response, in which a weak prepulse stimulus suppresses the response to a subsequent startling pulse stimulus. In the pulse-alone trials, startle reactivity of the α3KO mice was not significantly different from WT mice (see Supporting Information) The magnitude of PPI (percent inhibition) increased with increasing intensity of the prepulse stimulus for both WT and α3KO mice (Fig. 4A). Most importantly, a marked attenuation of PPI was seen in the α3KO mice, except at the lowest and highest prepulse intensities. These interpretations were confirmed by a 2 × 2 × 4 (genotype × gender × prepulse levels) ANOVA of percent inhibition, which yielded a significant effect of prepulse intensities (F3,72 = 33.42, P < 0.001) and a significant interaction between genotype and prepulse intensities (F3,72 = 3.32, P < 0.05). No effect of gender was noted.
Fig. 4.
PPI of the acoustic startle response. (A) PPI was indexed by percent inhibition, defined as the percent reduction in reactivity in prepulse-plus-pulse trials relative to pulse-alone trials. Increasing prepulse intensity led to increased magnitude of PPI. PPI was disrupted in α3KO mice, specifically in the middle two prepulse intensities (*, P < 0.05). (B) The PPI deficiency in α3KO mice was restored to WT levels by haloperidol. The drug significantly elevated percent PPI in the mutant across all levels of prepulse, but was effective in the WT mice only at the lowest prepulse intensity (*, P < 0.05). Comparisons between α3KO and WT mice in the saline condition again revealed a significant deficiency of PPI (*, P < 0.05) in the α3KO group at all except the lowest prepulse intensity. (C) Analysis restricted to either the α3KO or the WT mice indicated that a significant drug effect emerged only in the mutant (*, P < 0.05) but not in the WT mice.
Reversal of PPI Deficit in α3KO Mice by Haloperidol. The absence of α3-containing GABAA receptors on dopamine neurons was expected to increase dopamine transmission in various target areas, including the nucleus accumbens (30), which may underlie the deficit in PPI observed. In keeping with this hypothesis, we attempted to reverse the PPI deficit by a blockade of D2 dopaminergic-receptor activation with haloperidol, which is known to be effective against the apomorphine-induced PPI deficit (31).
The startle reactivity observed in pulse-alone trials was not significantly attenuated by haloperidol. The mean (±SEM in arbitrary units) startle magnitude in haloperidol- and saline-treated WT mice were 94.90 ± 30.30 and 161.28 ± 36.04, respectively. The corresponding values in the α3KO mice were 33.58 ± 14.07 and 92.86 ± 33.73, respectively.
Importantly, the PPI deficiency in α3KO mice was restored by haloperidol treatment. In the WT controls, haloperidol showed no significant effect, except at +4 dBA prepulse intensity (Fig. 4B). This restorative effect of haloperidol in the α3KO group was evident at all prepulse levels and yielded a significant genotype × drug × prepulse levels interaction specific to the quadratic trend (F1,39 = 4.20, P < 0.05) (Fig. 4B).
Additional two-way (drug × prepulse intensities) ANOVAs restricted to either α3KO or WT mice confirmed the overall presence of a significant reversal of the PPI deficit in the KO mice (F1,19 = 4.29, P < 0.05) but not in the WT controls (F < 1.0) (Fig. 4C). The differential expression of haloperidol's effect on PPI supports the suggestion of an underlying dopaminergic hyperfunction in the α3KO mice that enhanced their responsiveness to the drug at a sufficiently low dose that was otherwise ineffective in WT controls.
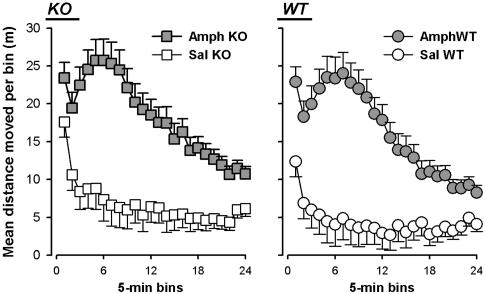
Modulation of Motor Behavior with Amphetamine in α3KO Mice. The increase in open-field locomotor activity of the α3KO mice is likely to be due to the disinhibition of dopamine neurons. To assess the dopaminergic regulation of locomotor activity, the efficacy of amphetamine was analyzed. Amphetamine-treated mice maintained an elevated level of locomotor activity throughout the 120-min test period (Fig. 5). Regardless of genotype or gender, the effect of the drug peaked at a similar time. There was no evidence for a differential response to amphetamine challenge between α3KO and WT mice. A 2 × 2 × 2 × 24 (genotype × sex × drug × 5-min bins) ANOVA of distance traveled confirmed these impressions, with the emergence of a significant main effect of bins (F23,1288 = 39.89, P < 0.0001), drug (F1,56 = 43.67, P < 0.001), and their interaction (F23,1288 = 18.94, P < 0.0001). No effect of sex was noted, and the main effect of genotype also failed to attain significance (F1,56 = 1.46, P = 0.23). However, an ANOVA restricted to the saline condition confirmed again the presence of a genotype effect in the direction of a slight hyperlocomotion, as observed in the first open-field test (F1,28 = 8.76, P < 0.01) also accompanied by a significant interaction with bins (F23,644 = 2.39, P < 0.001). On the other hand, no genotype effect was evident at all in the ANOVA restricted to the amphetamine condition. The amphetamine challenge thus indicates that the response characteristics to this drug were unaltered in α3KO mice.
Fig. 5.
The locomotor response to systemic amphetamine was assessed over 120 min. Amphetamine (2.5 mg/kg i.p.) led to a pronounced increase in activity. The response to the drug was not altered by the mutation. A marginal increase in locomotor activity was again observed.
Discussion
Sensorimotor gating affects the detection and focused attention to behaviorally relevant and novel stimuli at the expense of trivial sensory inputs. A sensorimotor deficit, as tested by PPI, is an endophenotype of schizophrenia and other mental disorders, e.g., schizotypal personality disorder (32), obsessive-compulsive disorder (33), and Huntington's disease (34). Our data provide evidence that a subset of GABAergic neurons is able to modulate sensorimotor gating through α3-containing GABAA receptors.
The major change in phenotypic behavior of α3KO mice was their impaired ability to gate their reaction to a startle-eliciting acoustic stimulus when it was preceded by the prepulse stimulus. The PPI deficiency in the α3KO mice was most severe at prepulse intensities from +8 to +16 dB above background. The further demonstration that this deficiency could be normalized by the D2-receptor antagonist haloperidol at a dose that was insufficient to affect performance in WT controls indicates that the sensorimotor-gating deficit in the α3KO mice is dopamine-dependent, strengthening the view that the deficit in PPI in the α3KO mice stems primarily from a disinhibition of mesolimbic dopamine neurons. This view was substantiated by the markedly reduced sensitivity of midbrain dopamine neurons to GABAergic inhibition in a superfused slice preparation obtained from α3KO mice (Fig. 2 A). A deficit in PPI is known to be induced by hyperdopaminergic activity, as demonstrated by the action of apomorphine or amphetamine (35). However, the α3KO mouse is the first animal model that demonstrates a molecular target for the selective GABAergic regulation of sensorimotor gating, thus opening distinct therapeutic avenues.
α3-containing GABAA receptors represent only 10-20% of all GABAA receptors, distributed in various parts of the brain (3, 36). Nevertheless, α3KO mice displayed a defined hyperdopaminergic phenotype. For instance, the lack of α3 subunits in the reticular nucleus of the thalamus did not change sensory perception, as shown by a lack of a significant difference in the amplitude of the startle response between α3KO and WT mice, indicating that hearing is largely intact in the α3KO mice (see Supporting Information). Thus, the combination of genetic and pharmacological tools allows us to conclude that the impaired GABAergic inhibitory regulation of dopaminergic neurons is responsible for the PPI deficit.
Within the basal ganglia, GABAergic regulation is likely to be reduced mainly through an impact on the substantia nigra, which displays an appreciable level of α3-subunit expression. The reduced inhibition of the nigrostriatal and/or mesolimbic dopaminergic transmission to the striatal complex in the α3KO mice might underlie the slight hyperlocomotion in the open field. A further enhancement of locomotor activity was seen after treatment with amphetamine. Most notably, this amphetamine-induced enhancement of locomotor activity of the α3KO mice did not exceed the level of amphetamine-induced locomotion of WT mice, suggesting that the response characteristics to amphetamine were within the normal range in the α3KO mice. This finding corresponds to the clinical observation that the amphetamine-induced release of dopamine is within the normal range among schizophrenic patients in remission (37), despite the presence of a PPI deficit (38).
Clinically, benzodiazepines have been used as an add-on therapy, particularly in neuroleptic-resistant schizophrenia (39-41). Moreover, the nonselective partial agonist bretazenil was efficacious as monotherapy in 40% of neuroleptic-free patients (42). In line with these clinical results, the α3-containing GABAA-receptor subtype would qualify as a promising target for the development of selective antipsychotics. Interestingly, dynorphinergic GABAergic neurons, which are present in various brain areas and project to dopaminergic neurons, display an enhanced activity induced by both typical and atypical neuroleptic drugs (43), thus providing further support for the idea that the selective modulation of the activity of dopaminergic neurons by α3-containing GABAA receptors will be of therapeutic value. Thus, α3-containing GABAA receptors may serve as valuable targets for novel antipsychotics that would be expected to be nonsedating and free of the extrapyramidal side effects.
Supplementary Material
Acknowledgments
We thank Dr. Susan M. Dymecki (Harvard Medical School, Boston) for providing the hACTB::Flp mice used in the course of this work. This work was supported by the Swiss National Science Foundation.
Author contributions: B.K.Y., H.M., and U.R. designed research; B.K.Y., R.K., L.v.B., R.S., D.B., N.H., Y.D., J.-M.F., H.B., and U.R. performed research; B.K.Y., R.K., L.v.B., R.S., D.B., N.H., Y.D., R.C.M., J.-M.F., J.F., H.M., and U.R. analyzed data; and B.K.Y., R.C.M., J.-M.F., J.F., H.M., and U.R. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: α3KO, α3 knockout; PPI, prepulse inhibition.
References
- 1.Robbins, T. W. (2000) Exp. Brain Res. 133, 130-138. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson, A., Waters, N., Holm-Waters, S., Tedroff, J., Nilsson, M. & Carlsson, M. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 237-260. [DOI] [PubMed] [Google Scholar]
- 3.Fritschy, J. M. & Möhler, H. (1995) J. Comp. Neurol. 359, 154-194. [DOI] [PubMed] [Google Scholar]
- 4.Roberts, E. (1972) Neurosci. Res. Program Bull. 10, 468-482. [PubMed] [Google Scholar]
- 5.Lewis, D. A., Volk, D. W. & Hashimoto, T. (2004) Psychopharmacology 174, 143-150. [DOI] [PubMed] [Google Scholar]
- 6.Benes, F. M. & Berretta, S. (2001) Neuropsychopharmacology 25, 1-27. [DOI] [PubMed] [Google Scholar]
- 7.Lewis, D. A., Hashimoto, T. & Volk, D. W. (2005) Nat. Rev. Neurosci. 6, 312-324. [DOI] [PubMed] [Google Scholar]
- 8.Spencer, K. M., Nestor, P. G., Perlmutter, R., Niznikiewicz, M. A., Klump, M. C., Frumin, M., Shenton, M. E. & McCarley, R. W. (2004) Proc. Natl. Acad. Sci. USA 101, 17288-17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Impagnatiello, F., Guidotti, A. R., Pesold, C., Dwivedi, Y., Caruncho, H., Pisu, M. G., Uzunov, D. P., Smalheiser, N. R., Davis, J. M., Pandey, G. N., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 15718-15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti, A., Auta, J., Davis, J. M., Di-Giorgi-Gerevini, V., Dwivedi, Y., Grayson, D. R., Impagnatiello, F., Pandey, G., Pesold, C., Sharma, R., et al. (2000) Arch. Gen. Psychiatry 57, 1061-1069. [DOI] [PubMed] [Google Scholar]
- 11.Fatemi, S. H., Earle, J. A. & McMenomy, T. (2000) Mol. Psychiatry 5, 654-663. [DOI] [PubMed] [Google Scholar]
- 12.Knable, M. B., Barci, B. M., Webster, M. J., Meador-Woodruff, J. & Torrey, E. F. (2004) Mol. Psychiatry 9, 609-620. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez, M. A., Caruncho, H. J., Costa, E., Pesold, C., Liu, W. S. & Guidotti, A. (2002) J. Comp. Neurol. 451, 279-288. [DOI] [PubMed] [Google Scholar]
- 14.Weickert, C. S., Hyde, T. M., Lipska, B. K., Herman, M. M., Weinberger, D. R. & Kleinman, J. E. (2003) Mol. Psychiatry 8, 592-610. [DOI] [PubMed] [Google Scholar]
- 15.Braff, D. L. & Geyer, M. A. (1990) Arch. Gen. Psychiatry 47, 181-188. [DOI] [PubMed] [Google Scholar]
- 16.Dong, Y., Saal, D., Thomas, M., Faust, R., Bonci, A., Robinson, T. & Malenka, R. C. (2004) Proc. Natl. Acad. Sci. USA 101, 14282-14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauser, J., Rudolph, U., Keist, R., Möhler, H., Feldon, J. & Yee, B. K. (2005) Mol. Psychiatry 10, 201-207. [DOI] [PubMed] [Google Scholar]
- 18.Lister, R. G. (1987) Psychopharmacology 92, 180-185. [DOI] [PubMed] [Google Scholar]
- 19.Yee, B. K., Hauser, J., Dolgov, V. V., Keist, R., Möhler, H., Rudolph, U. & Feldon, J. (2004) Eur. J. Neurosci. 20, 1928-1936. [DOI] [PubMed] [Google Scholar]
- 20.Hasty, P., Crist, M., Grompe, M. & Bradley, A. (1994) Mol. Cell. Biol. 14, 8385-8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudolph, U., Brabet, P., Hasty, P., Bradley, A. & Birnbaumer, L. (1993) Transgenic Res. 2, 345-355. [DOI] [PubMed] [Google Scholar]
- 22.Dymecki, S. M. (1996) Proc. Natl. Acad. Sci. USA 93, 6191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrobak, J. J. & Napier, T. C. (1993) J. Neural Transm. 93, 123-143. [DOI] [PubMed] [Google Scholar]
- 24.Brudzynski, S. M., Wu, M. & Mogenson, G. J. (1993) Can. J. Physiol. Pharmacol. 71, 394-406. [DOI] [PubMed] [Google Scholar]
- 25.Okada, H., Matsushita, N. & Kobayashi, K. (2004) J. Neurochem. 89, 7-14. [DOI] [PubMed] [Google Scholar]
- 26.Low, K., Crestani, F., Keist, R., Benke, D., Brunig, I., Benson, J. A., Fritschy, J. M., Rulicke, T., Bluethmann, H., Möhler, H. & Rudolph, U. (2000) Science 290, 131-134. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph, U., Crestani, F., Benke, D., Brunig, I., Benson, J. A., Fritschy, J. M., Martin, J. R., Bluethmann, H. & Möhler, H. (1999) Nature 401, 796-800. [DOI] [PubMed] [Google Scholar]
- 28.McKernan, R. M., Rosahl, T. W., Reynolds, D. S., Sur, C., Wafford, K. A., Atack, J. R., Farrar, S., Myers, J., Cook, G., Ferris, P., et al. (2000) Nat. Neurosci. 3, 587-592. [DOI] [PubMed] [Google Scholar]
- 29.Crestani, F., Keist, R., Fritschy, J. M., Benke, D., Vogt, K., Prut, L., Bluthmann, H., Möhler, H. & Rudolph, U. (2002) Proc. Natl. Acad. Sci. USA 99, 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisman, J. E. & Grace, A. A. (2005) Neuron 46, 703-713. [DOI] [PubMed] [Google Scholar]
- 31.Russig, H., Spooren, W., Durkin, S., Feldon, J. & Yee, B. K. (2004) Psychopharmacology 175, 143-147. [DOI] [PubMed] [Google Scholar]
- 32.Cadenhead, K. S., Geyer, M. A. & Braff, D. L. (1993) Am. J. Psychiatry 150, 1862-1867. [DOI] [PubMed] [Google Scholar]
- 33.Swerdlow, N. R., Benbow, C. H., Zisook, S., Geyer, M. A. & Braff, D. L. (1993) Biol. Psychiatry 33, 298-301. [DOI] [PubMed] [Google Scholar]
- 34.Swerdlow, N. R., Paulsen, J., Braff, D. L., Butters, N., Geyer, M. A. & Swenson, M. R. (1995) J. Neurol. Neurosurg. Psychiatry 58, 192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yee, B. K., Russig, H. & Feldon, J. (2004) Neuropsychopharmacology 29, 240-248. [DOI] [PubMed] [Google Scholar]
- 36.Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W. & Sperk, G. (2000) Neuroscience 101, 815-850. [DOI] [PubMed] [Google Scholar]
- 37.Laruelle, M., Abi-Dargham, A., Gil, R., Kegeles, L. & Innis, R. (1999) Biol. Psychiatry 46, 56-72. [DOI] [PubMed] [Google Scholar]
- 38.Parwani, A., Duncan, E. J., Bartlett, E., Madonick, S. H., Efferen, T. R., Rajan, R., Sanfilipo, M., Chappell, P. B., Chakravorty, S., Gonzenbach, S., et al. (2000) Biol. Psychiatry 47, 662-669. [DOI] [PubMed] [Google Scholar]
- 39.Chouinard, G., Annable, L., Turnier, L., Holobow, N. & Szkrumelak, N. (1993) Can. J. Psychiatry 38, Suppl. 4, S114-S121. [PubMed] [Google Scholar]
- 40.Lingjaerde, O. (1983) in The Benzodiazepines: From Molecular Biology to Clinical Practice, ed. Costa, E. (Raven, New York), pp. 369-381.
- 41.Pecknold, J. C. (1993) J. Psychiatry Neurosci. 18, 82-84. [PMC free article] [PubMed] [Google Scholar]
- 42.Delini-Stula, A. & Berdah-Tordjman, D. (1996) J. Psychiatr. Res. 30, 239-250. [DOI] [PubMed] [Google Scholar]
- 43.Ma, J., Ye, N., Lange, N. & Cohen, B. M. (2003) Neuroscience 121, 991-998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.