Abstract
Present strategies for control of herpes genitalis recurrences require multiple daily doses of antiviral medication. Imiquimod, an immune response modifier, induces alpha interferon and interleukin-12; application in the presence of local herpes antigens during a recurrence may augment herpes simplex virus (HSV)-specific cell-mediated immunity. To test this theory, we performed a randomized, double-blind, placebo-controlled study of imiquimod 5% cream to assess safety and efficacy for decreasing recurrences. Patients with six or more recurrences of herpes genitalis per year applied study cream (imiquimod or placebo) to lesions one, two, or three times per week for 3 weeks for each recurrence during a 16-week treatment period. This was followed by a 16-week observation period. Of 124 patients randomized to the study, 103 completed the treatment period and 93 completed the observation period. The median times to first genital herpes recurrence were 53 days for those receiving placebo (n = 30) and 54, 60, and 64 days for those receiving imiquimod one time per week (n = 34), two times per week (n = 32), and three times per week (n = 28), respectively. The median annualized recurrence rates during the treatment period were 3.8, 4.9, 3.2, and 3.1, respectively. There were no statistically significant differences in the time to first recurrence or in the annualized recurrence rate between the imiquimod and placebo groups in either the treatment or the observation period. A trend in increased rates of local adverse events at the application site and a delay in lesion healing with more frequent dosing suggested a pharmacologic effect. Although clinical efficacy has been observed for imiquimod in other conditions in which a TH1-type immune response may be beneficial, including other viral infections such as those caused by human papillomavirus, no apparent effect on the short-term natural history of herpes genitalis recurrences was observed.
Genital herpes represents a significant health problem, as rates of infection with herpes simplex virus (HSV) type 2 (HSV-2), the most common cause of recurrent genital herpes, continue to increase (8, 25). More than 20% of adults in the United States are infected with HSV-2, and in sexually transmitted disease clinics, the seroprevalence rate approaches 50% (3, 8, 11, 12, 16). Because HSV is capable of establishing latency in the sensory ganglion, intermittent reactivation of viral replication can result in both symptomatic and subclinical recurrences. The recurrence rate can be quite variable. In a study of patients with symptomatic genital first-episode infections, 11% of patients had no recurrences, 38% had at least 6 recurrences, and 20% had more than 10 recurrences (3). Subclinical reactivations are also variable, with asymptomatic shedding observed in half of the women with genital herpes in one study and with the time of asymptomatic shedding ranging from 0 to 35% of days sampled (27). Cell-mediated immunity (CMI) is believed to play a major, if not well-understood, role in restricting reactivations (13).
Suppression of genital herpes recurrences focuses on use of nucleoside analogs, such as acyclovir, valacyclovir, and famciclovir, which inhibit viral replication by interfering with HSV DNA polymerase (1). While effective, this approach requires chronic daily dosing to maintain inhibition of the virus, demands good compliance, and is expensive (14, 15, 17, 18, 19). There is no posttreatment efficacy; discontinuation of suppressive treatment results in reversion to the pretreatment recurrence rate (6, 23).
An alternative strategy for control of HSV disease is to enhance the host's ability to control the virus. Although a prophylactic HSV-2 vaccine appears to decrease the risk of infection in women seronegative for both types of HSV (S. Spruance, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L6, 2000), therapeutic genital herpes vaccines have been unsuccessful to date (22, 24).
Imiquimod is a topically active immune response modifier that induces endogenous production of cytokines, including alpha interferon (IFN-α), tumor necrosis factor alpha (TNF-α), and interleukin-12 (IL-12), primarily from cells of the monocyte/macrophage lineage (20). In addition to indirectly inducing gamma interferon (IFN-γ), a pivotal TH1-type cytokine in stimulating CMI, imiquimod enhances antigen presentation. In the recurrent genital herpes model in guinea pigs, administration of imiquimod for 5 days decreased lesion formation but had no posttreatment effect on subsequent lesion formation (10). In contrast, administration for 3 weeks resulted in a continued reduction in lesion formation posttreatment. The effect during administration is probably mediated by induced IFN-α, whereas the posttreatment effect may be mediated via augmentation of HSV-specific CMI (10).
Topical imiquimod is used for the treatment of external anogenital warts, a condition caused by human papillomavirus (7). It was hypothesized that application of imiquimod to herpes lesions would induce IFN-α and IL-12 in the skin, which, in the presence of HSV antigens present during a recurrence, would augment HSV-specific CMI. This enhanced immunity might manifest as a delay in time to the next recurrence or a decrease in the overall recurrence rate. However, as some of the cytokines induced by imiquimod have proinflammatory properties, application in the presence of lesions may also increase the inflammatory response.
Therefore, a pilot, placebo-controlled, dose-frequency-ranging study was performed to determine the safety of application of imiquimod to anogenital herpes lesions and its effect on subsequent recurrences.
MATERIALS AND METHODS
Population.
Men and women between 18 and 65 years of age with a history of recurrent genital herpes (six or more recurrences per year while not on suppressive antiherpes therapy) were enrolled at seven centers in the United States. Prospective study patients were screened by medical history, physical examination, complete blood count, serum chemistries, urinalysis, serology for human immunodeficiency virus infection, and, where applicable, pregnancy testing. Documentation of a prior clinical diagnosis or of HSV-2 seropositivity was required. Eligible patients were required to present to the clinic within 24 h of onset of a genital herpes recurrence for clinical confirmation and randomization into the 16-week treatment period. All patients signed informed consent forms approved by local institutional review boards. Enrollment began in November 1998, and all study procedures were completed by July 2000.
Study procedures.
For each recurrence during the treatment period, the patients applied study cream (imiquimod 5% cream [Aldara; 3M Pharmaceuticals, St. Paul, Minn.] or matching placebo cream) at bedtime (to achieve topical treatment for approximately 8 to 10 h per application) according to their assigned dosing regimen (one, two, or three times per week for 3 weeks). At the completion of the treatment period, patients entered a 16-week observation period to assess recurrences. Patients were required to present to the clinic at scheduled visits and for each recurrence in both periods. Swabs for HSV culture and/or PCR were obtained at the clinic for each recurrence; PCR was performed only on culture-negative samples. Results from the initial recurrence were not required for randomization.
Laboratory.
Routine laboratory tests, human immunodeficiency virus serologies, and HSV cultures were performed by Quintiles Laboratory (Atlanta, Ga.). HSV PCR was performed by the Emory University Virology Laboratory (Atlanta, Ga.). HSV Western blotting was performed by the University of Washington, Seattle (2).
Efficacy analysis.
The primary efficacy parameter was the time to recurrence following treatment. Two time frames were considered: time to next recurrence after the initial treated recurrence (to evaluate the effect of a single treatment cycle) and time to next recurrence after the last treated recurrence (to evaluate a cumulative effect, as multiple recurrences may be treated). These parameters were analyzed for any patient-reported recurrence (primary endpoint), investigator-confirmed recurrence, and virologically confirmed recurrence. Investigator-confirmed recurrences were defined as those that were visually verified in the clinic. Recurrences that had a positive HSV culture or PCR result were defined as virologically confirmed.
Safety.
Safety was determined by clinical laboratory measurements, collection of information regarding adverse events, and lesion assessments (the number of lesions and the size, location, and stage of each lesion). Information on specific local adverse events was collected independently, and adverse events were defined as local signs (erythema, edema, ulceration, vesicles, scabbing or flaking) or local symptoms (pain, numbness or tingling, burning, pruritis).
Statistical analysis.
Data for all placebo patients were pooled regardless of the frequency of application for the analysis. For safety parameters, overall tests comparing all treatment groups were performed. Efficacy was assessed by comparing each imiquimod group with the pooled placebo group. All statistical tests, other than efficacy analyses, were two tailed at a significance level of 0.05. Efficacy analysis type I error was adjusted for multiple comparisons by using the Bonferroni method (three comparisons, P = 0.017).
The distributions of time-to-recurrence variables were estimated by the Kaplan-Meier product limit method and were compared between treatment groups by the Wilcoxon test. Differences between treatment groups after adjusting for covariates were also analyzed by using the Cox proportional hazards regression model, stratified by study center. The numbers of recurrences were normalized for the number of days during the treatment and observation periods and were compared by the Wilcoxon rank-sum test. The Fisher exact test was used to compare the proportions of patients who were recurrence free and the incidence of adverse events across treatment arms.
RESULTS
This study was conducted in compliance with relevant federal guidelines and local institutional policies.
Patient characteristics.
Of 235 patients screened, 209 entered the 12-week eligibility period and 124 had a qualifying recurrence to be randomized to treatment. There were no significant differences across treatment groups for any baseline characteristics (Table 1). Overall, 65% of patients were female, and the median age was 36 years (age range, 19 to 65 years). The median number of recurrences of herpes genitalis in the past year was 8 (range, 0 to 20 recurrences), and 11% of patients reported having been on oral suppressive therapy prior to study entry. At the baseline, 43% of patients were seropositive for only HSV-2, 52% were positive for both HSV-2 and HSV-1, and 3% were positive for only HSV-1. One patient was seronegative for both HSV-2 and HSV-1, and one had an indeterminate serology.
TABLE 1.
Demographic characteristics of study patients
| Parameter | Placebo | Imiquimod
|
P value | ||
|---|---|---|---|---|---|
| One time/wk | Two times/wk | Three times/wk | |||
| Sex (no. [%] of patients) | |||||
| Female | 22 (73) | 20 (59) | 21 (66) | 17 (61) | 0.647a |
| Male | 8 (27) | 14 (41) | 11 (34) | 11 (39) | |
| Age (yr) | |||||
| Mean | 37.2 | 34.8 | 37.9 | 40.8 | 0.202b |
| SD | 11.74 | 8.70 | 10.59 | 12.69 | |
| Median | 36 | 34 | 36 | 37 | |
| Range | 19-58 | 20-56 | 19-62 | 26-65 | |
| Race (no. [%] of patients) | |||||
| White | 25 (83) | 30 (88) | 29 (91) | 25 (89) | 0.686a,c |
| Nonwhite | 5 (17) | 4 (12) | 3 (9) | 3 (11) | |
| No. of recurrences/yr | |||||
| Median | 6 | 8 | 8 | 8 | 0.203b |
| Range | 0-20 | 6-12 | 2-15 | 6-12 | |
| Time (mo) since diagnosis | |||||
| Median | 64 | 59 | 56 | 110 | 0.621b |
| Range | 8-364 | 10-263 | 8-429 | 1-458 | |
| No. (%) of patients on suppression in past 12 mo | 4 (13) | 5 (15) | 3 (9) | 2 (7) | 0.805a |
| No. (%) of patients with the following serostatus: | |||||
| HSV-2 positive only | 12 (40) | 19 (56) | 15 (47) | 7 (25) | 0.094a |
| HSV-1 positive only | 1 (3) | 1 (3) | 2 (6) | 0 | 0.845a |
| HSV-2 and HSV-1 positive | 17 (57) | 13 (38) | 15 (47) | 20 (71) | 0.061a |
| HSV-2 and HSV-1 negative | 0 | 1 (3) | 0 | 0 | 1.000a |
| HSV-2 indeterminant and HSV-1 positive | 0 | 0 | 0 | 1 (4) | 0.226a |
| No. (%) of patients with history of orolabial herpes | 8 (27) | 10 (29) | 8 (25) | 7 (25) | 0.984a |
Fisher exact test across treatment groups.
Kruskal-Wallis test across treatment groups.
For statistical testing, race was grouped as white versus nonwhite.
Patient-reported herpes locations.
During follow-up, 60 (75%) of the 80 female patients had vulvar lesions and 16 (20%) had perineal lesions. Thirty-eight (86%) of the 44 male patients had penile shaft lesions, and 6 (14%) had penile head or rim lesions. Buttock lesions were present in 22 (28%) female patients and 4 (9%) male patients. Seventy-three percent of the imiquimod patients and 77% of the placebo patients were positive for HSV by culture or PCR at day 1 of the initial treated recurrence. Overall, 110 of the 124 randomized patients (89%) had an HSV culture- or PCR-positive recurrence at some time during the study.
Efficacy.
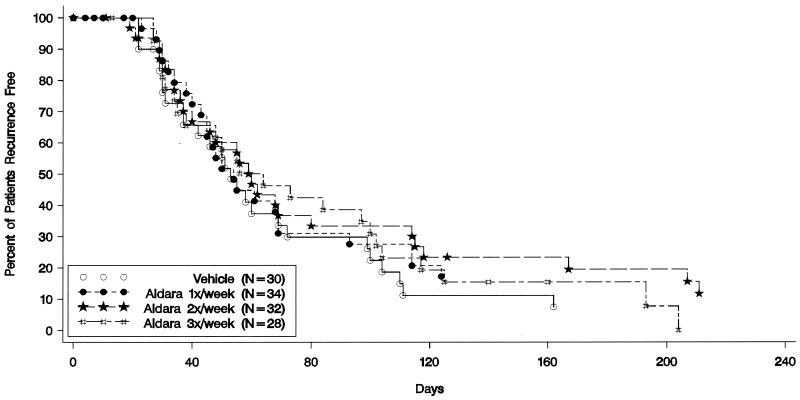
The median times to the next patient-reported recurrence after the initial treated recurrence were 53 days for patients receiving placebo and 54, 60, and 64 days for patients receiving imiquimod at one, two, and three times per week, respectively (Fig. 1). The median times to the next patient-reported recurrence after the last treated recurrence (from day 1 of that recurrence) were 143 days for the patients receiving placebo and 106, 145, and 130 days for the patients receiving imiquimod at one, two, and three times per week, respectively. There were no statistically significant differences or trends when the times to the next recurrence after the initial or last treated recurrence were compared between the imiquimod and placebo groups for patient-reported, investigator-confirmed, or virologically confirmed recurrences. Also, no statistically significant differences in the annualized recurrence rates in either the treatment or the observation period were observed between treatment groups. The proportions of patients who were recurrence free at the end of the treatment period were 10% for the placebo group and 24, 25, and 21% for the patients receiving imiquimod at one, two, and three times per week, respectively; this was not statistically significant.
FIG. 1.
Kaplan-Meier estimate of time to first patient-reported recurrence for each treatment group after the first treated recurrence in the treatment period.
Safety.
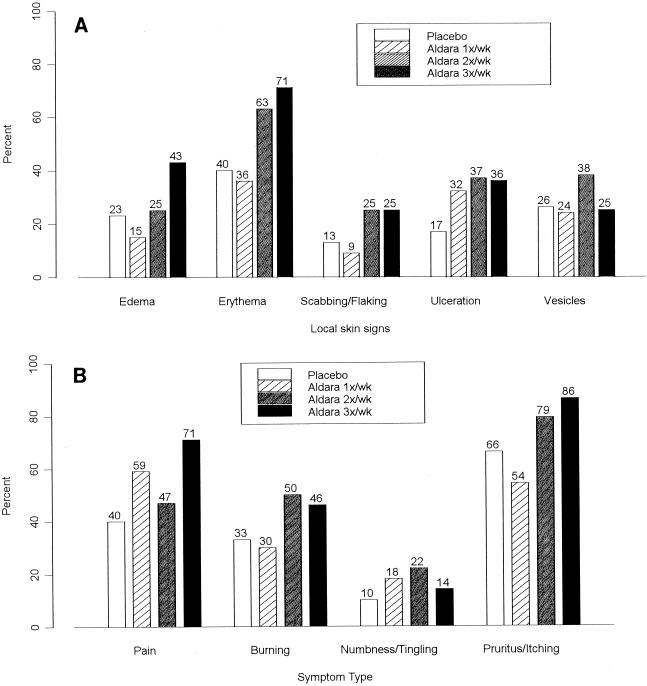
Overall, topical application of imiquimod to genital herpes lesions was reasonably well tolerated. There were no statistically significant differences among the treatment groups in the frequencies of systemic adverse events. More patients in the groups receiving imiquimod two times per week (22%) and three times per week (25%) than patients in the group receiving imiquimod once per week (12%) or placebo (13%) reported adverse events that were considered possibly or probably related to the study medication; however, the differences were not statistically significant, and no trends were observed when the adverse events were analyzed by body system terms, including application-site reactions. Analysis of the maximum severity of each defined local sign and symptom, about which information was collected separately from information on the other adverse events, during the initially treated recurrence did not show differences or trends across treatment groups. As the underlying signs and symptoms of herpes may have obscured differences during the early portion of the 3-week treatment cycle, a subgroup analysis was performed on the defined local skin signs and symptoms from day 8 to day 22. More patients in the groups receiving imiquimod two times per week (any local skin sign, 48%) and three times per week (50%) than patients in the group receiving imiquimod one time per week (15%) or the placebo group (20%) had moderate or severe reactions. Figure 2A provides a comparison of moderate or severe maximum local skin signs by treatment group. A similar trend was observed for the local skin symptoms (Fig. 2B). In addition, fewer patients in the groups receiving imiquimod two times per week (22%) and three times per week (22%) than patients in the group receiving imiquimod one time per week (55%) or placebo (43%) were healed by day 8. Finally, the median maximal lesion size during the initial recurrence was greater for the groups receiving imiquimod two times per week (98 mm2) and three times per week (100 mm2) than for the group receiving imiquimod one time per week (30 mm2) or placebo (42 mm2).
FIG. 2.
Percentage of patients with moderate or severe maximum severity of specific local skin signs (A) or symptoms (B) at the application site during the initial treated episode.
Twenty-one patients (17%) discontinued from treatment during the treatment period; however, three of these patients continued into the observation period. Four patients discontinued from treatment because of local skin reactions, and one discontinued because of an anxiety attack (the patient had a history of anxiety attacks); all were in the imiquimod groups (two patients were receiving the drug one time per week, one patient was receiving the drug two times per week, and two patients were receiving the drug three times per week). Thirteen of the 106 patients who entered the observation period discontinued. There did not appear to be a difference across treatment groups with respect to discontinuations during the treatment or the observation period.
DISCUSSION
The goal of this study was to determine if topical imiquimod 5% cream, which has evidence of clinical efficacy against genital human papillomavirus infections, would provide a similar benefit for another viral infection, recurrent herpes genitalis. The hypothesis was that treatment with imiquimod, which induces local IFN-α and IL-12 responses, in conjunction with HSV antigens from a recurrence would enhance HSV-specific CMI, resulting in a posttreatment delay in the onset of subsequent recurrences and a reduction in recurrence frequency.
In this study of topical application of imiquimod to lesions of recurrent herpes genitalis, no evidence of posttreatment efficacy was observed with respect to increasing the time to the next recurrence or decreasing the total number of recurrences in either period. This was despite evidence of pharmacologic activity, as manifested by the non-statistically significant increases in local adverse events associated with more frequent imiquimod dosing.
In the guinea pig model of herpes genitalis, imiquimod was effective in decreasing posttreatment lesion formation; however, those studies were not conducted with the imiquimod 5% cream formulation (4, 10). The route (intravaginal route) or the relative dose of imiquimod administered may have provided a more appropriate or greater immunologic stimulus, accounting for the difference. In addition, as a species guinea pigs may also be more sensitive to manipulation of the HSV immune response than humans, and other immune-based therapies with evidence of efficacy in this model have failed to be confirmed in studies with humans (5, 24).
These results are in contrast to those observed in a phase II study involving a related immune response modifier, resiquimod, with a similar patient population (21). Spruance et al. (21) also observed a trend toward increased local adverse events associated with more frequent dosing and higher concentrations of resiquimod gel during the latter portion of a 3-week treatment course. This trend was similar to what was observed in this study and suggestive of a pharmacologic effect from local induction of cytokines. Treatment with topical resiquimod gel, however, was associated with an increase in the median time to first recurrence after the treated recurrence compared to that achieved with placebo (169 versus 57 days; P = 0.006). The median time to the next recurrence after the initial treated recurrence in this study for the imiquimod groups combined was 56 days, nearly identical to that for the placebo group in this study (53 days) and to that in the resiquimod study (57 days).
A number of possibilities may account for the different observations in these studies. In the resiquimod study, patients applied unit doses of 100 or 200 mg of gel, while in the present study patients only used sufficient cream to cover the lesion(s), potentially resulting in underdosing. While there are similarities in dose frequency-related late local adverse events, suggesting that a pharmacologic effect was achieved, these events may be more a consequence of inflammatory effects qualitatively distinct from the immunologic stimulus required for effective stimulation of HSV-specific CMI. In addition, there are qualitative differences between imiquimod and resiquimod. Resiquimod induces more IL-12 and TNF-α directly and more IFN-γ indirectly compared to the levels of induction of these cytokines caused by imiquimod (26). In addition, resiquimod appears to be more effective in activating dendritic cells, which may be crucial in HSV antigen presentation in the skin (9).
Despite evidence of some pharmacologic response, imiquimod 5% cream, dosed in the regimens studied, did not reduce the number of subsequent herpes recurrences or prolong the interval between recurrences. The hypothesis that combining an immune-response modifier with host-provided HSV antigen, a form of “therapeutic vaccination,” might control HSV recurrences may still prove valid in future studies with resiquimod.
Acknowledgments
This study was funded by 3M Pharmaceuticals.
REFERENCES
- 1.Alrabiah, F. A., and S. L. Sacks. 1996. New antiherpesvirus agents: their targets and therapeutic potential. Drugs 52:17-32. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, R. L., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedetti, J., L. Corey, and R. Ashley. 1994. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann. Intern. Med. 121:847-854. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, D. I., R. L. Miller, and C. J. Harrison. 1993. Effects of therapy with an immunomodulator (imiquimod, R-837) alone and with acyclovir on genital HSV-2 infection in guinea-pigs when begun after lesion development. Antivir. Res. 20:45-55. [DOI] [PubMed] [Google Scholar]
- 5.Corey, L., A. G. M. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 6.Douglas, J. M., C. Critchlow, J. Benedetti, G. J. Mertz, J. D. Connor, M. A. Hintz, A. Fahnlander, M. Remington, C. Winter, and L. Cory. 1984. A double-blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. N. Engl. J. Med. 310:1551-1556. [DOI] [PubMed] [Google Scholar]
- 7.Edwards, L., A. Ferenczy, L. Eron, D. Baker, M. L. Owens, T. L. Fox, A. J. Hougham, K. A. Schmitt, et al. 1998. Self-administered topical 5% imiquimod cream for external anogenital warts. Hum. Papilloma Virus. Arch. Dermatol. 134:25-30. [DOI] [PubMed] [Google Scholar]
- 8.Fleming, D. T., G. M. McQuillan, R. E. Johnson, A. J. Nahmias, S. O. Aral, F. K. Lee, and M. E. St. Louis. 1997. Herpes simplex virus type 2 in the United States, 1976-1994. N. Engl. J. Med. 337:1105-1111. [DOI] [PubMed] [Google Scholar]
- 9.Gaspari, A. A., R. P. Burns, S. Kondo, A. Nasir, A. Kurup, D. Mlodynia, D. Sauder, and R. K. Barth. 1998. Characterization of the altered cutaneous reactivity of transgenic mice whose keratinocytes overexpress B7-1. Clin. Immunol. Immunopathol. 86:259-270. [DOI] [PubMed] [Google Scholar]
- 10.Harrison, C. J., R. L. Miller, and D. I. Bernstein. 1994. Posttherapy suppression of genital herpes simplex virus (HSV) recurrences and enhancement of HSV-specific T-cell memory by imiquimod in guinea pigs. Antimicrob. Agents Chemother. 38:2059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koutsky, L. A., R. L. Ashley, K. K. Holmes, C. E. Stevens, C. W. Critchlow, N. Kiviat, C. M. Lipinski, P. Wolner-Hanssen, and L. Corey. 1990. The frequency of unrecognized type 2 herpes simplex virus infection among women: implications for the control of genital herpes. Sex. Transm. Dis. 17:90-94. [DOI] [PubMed] [Google Scholar]
- 12.Koutsky, L. A., C. E. Stevens, K. K. Holmes, R. L. Ashley, N. B. Kiviat, C. W. Critchlow, and L. Corey. 1992. Underdiagnosis of genital herpes by current clinical and viral-isolation procedures. N. Engl. J. Med. 326:1533-1539. [DOI] [PubMed] [Google Scholar]
- 13.Lopez, C. 1981. Resistance to herpes simplex virus-type 1 (HSV-1). Curr. Top. Microbiol. Immunol. 92:15-24. [DOI] [PubMed] [Google Scholar]
- 14.Mertz, G. J., C. C. Jones, J. Mills, K. H. Fife, S. M. Lemon, J. T. Stapleton, E. L. Hill, and L. G. Davis. 1988. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. JAMA 260:201-206. [PubMed] [Google Scholar]
- 15.Mertz, G. J., M. O. Loveless, M. J. Levin, S. J. Kraus, S. L. Fowler, D. Goade, S. K. Tyring, et al. 1997. Oral famciclovir for suppression of recurrent genital herpes simplex virus infection in women. A multicenter, double-blind, placebo-controlled trial. Arch. Intern. Med. 157:343-349. [PubMed] [Google Scholar]
- 16.Oliver, L., A. Wald, M. Kim, J. Zeh, S. Selke, R. Ashley, and L. Corey. 1995. Seroprevalence of herpes simplex virus infections in a family clinic. Arch. Fam. Med. 4:228-232. [DOI] [PubMed] [Google Scholar]
- 17.Patel, R., N. J. Bodsworth, P. Wooley, B. Peters, G. Vejlsgaard, S. Saari, and J. Robinson. 1997. Valaciclovir for the suppression of recurrent genital HSV infection: a placebo controlled study of once daily therapy. Genitourin. Med. 73:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry, C. M., and A. J. Wagstaff. 1995. Famciclovir. A review of its pharmacological properties and therapeutic efficacy in herpesvirus infections. Drugs 50:396-415. [DOI] [PubMed] [Google Scholar]
- 19.Reitano, M., S. Tyring, W. Lang, C. Thoming, A. M. Worm, S. Borelli, L. O. Chambers, J. M. Robinson, L. Corey, et al. 1998. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. J. Infect. Dis. 178:603-610. [DOI] [PubMed] [Google Scholar]
- 20.Slade, H. B., M. L. Owens, M. A. Tomai, and R. L. Miller. 1998. Imiquimod 5% cream (Aldara™). Exp. Opin. Investig. Drugs 7:437-450. [DOI] [PubMed] [Google Scholar]
- 21.Spruance, S. L., S. K. Tyring, M. H. Smith, and T. C. Meng. 2001. Application of a topical immune response modifier, resiquimod gel, to modify the recurrence rate of recurrent genital herpes: a pilot study. J. Infect. Dis. 184:196-200. [DOI] [PubMed] [Google Scholar]
- 22.Stanberry, L. R., A. L. Cunningham, A. Mindel, L. L. Scott, S. L. Spruance, F. Y. Aoki, and C. J. Lacey. 2000. Prospects for control of herpes simplex virus disease through immunization. Clin. Infect. Dis. 30:549-566. [DOI] [PubMed] [Google Scholar]
- 23.Straus, S. E., H. E. Takiff, M. Seidlin, S. Bachrach, L. Lininger, J. J. Di Giovanna, K. A. Western, H. A. Smith, S. N. Lehrman, T. Creagh-Kirk, et al. 1994. Suppression of frequently recurring genital herpes. A placebo-controlled double-blind trial of oral acyclovir. N. Engl. J. Med. 310:1545-1550. [DOI] [PubMed] [Google Scholar]
- 24.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. Langenberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 25.Tao, G., W. J. Kassler, and D. B. Rein. 2000. Medical care expenditures for genital herpes in the United States. Sex. Transm. Dis. 27:32-38. [DOI] [PubMed] [Google Scholar]
- 26.Wagner, T. L., C. L. Ahonen, A. M. Couture, S. J. Gibson, R. L. Miller, R. M. Smith, M. J. Reiter, J. P. Vasilakos, and M. A. Tomai. 1999. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell. Immunol. 191:10-19. [DOI] [PubMed] [Google Scholar]
- 27.Wald, A., J. Zeh, S. Selke, T. Warren, A. J. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes simplex virus 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844-850. [DOI] [PubMed] [Google Scholar]