Summary
Otospondylomegaepiphyseal dysplasia (OSMED) is an autosomal recessive skeletal dysplasia accompanied by severe hearing loss. The phenotype overlaps that of the autosomal dominant disorders—Stickler and Marshall syndromes—but can be distinguished by disproportionately short limbs, severe hearing loss, and lack of ocular involvement. In one family with OSMED, a homozygous Gly→Arg substitution has been described in COL11A2, which codes for the α2 chain of type XI collagen. We report seven further families with OSMED. All affected individuals had a remarkably similar phenotype: profound sensorineural hearing loss, skeletal dysplasia with limb shortening and large epiphyses, cleft palate, an extremely flat face, hypoplasia of the mandible, a short nose with anteverted nares, and a flat nasal bridge. We screened affected individuals for mutations in COL11A2 and found different mutations in each family. Individuals from four families, including three with consanguineous parents, were homozygous for mutations. Individuals from three other families, in whom parents were nonconsanguineous, were compound heterozygous. Of the 10 identified mutations, 9 are predicted to cause premature termination of translation, and 1 is predicted to cause an in-frame deletion. We conclude that the OSMED phenotype is highly homogenous and results from homozygosity or compound heterozygosity for COL11A2 mutations, most of which are predicted to cause complete absence of α2(XI) chains.
Introduction
Otospondylomegaepiphyseal dysplasia (OSMED [MIM 215150]) is characterized by sensorineural hearing loss, enlarged epiphyses, disproportionate shortness of the limbs, and abnormalities in vertebral bodies. Patients have typical facial features, including mid-face hypoplasia with a short upturned nose and depressed nasal bridge. Cleft palate and a small mandible are also common findings (Insley and Astley 1974; Giedion et al. 1982; Kääriäinen et al. 1993; Pihlajamaa et al. 1998; Spranger 1998).
Type XI collagen is a heterotrimer composed of three different α chains, α1(XI), α2(XI), and α3(XI) (Brewton and Mayne 1994). The α3(XI) chain is a more extensively posttranslationally modified product of the COL2A1 gene, which codes for the α1 chain of type II collagen (Eyre and Wu 1987). Type XI collagen is therefore encoded by three distinct genes, COL11A1, COL11A2, and COL2A1 (Ala-Kokko et al. 1995; Vuoristo et al. 1995; Annunen et al. 1999). It is a quantitatively minor fibrillar collagen expressed in hyaline cartilage, the vitreous body of the eye, and the intervertebral disk together with the major cartilage collagen, type II collagen. Type XI collagen interacts with type II collagen fibrils and has been shown to play a role in regulating fibril diameter (Linsenmayer et al. 1993; Cremer et al. 1998).
Because of the close structural and functional relation between the collagen types XI and II, mutations in the genes coding for them result in partially overlapping phenotypes. Type II collagen mutations have been found to cause a variety of cartilage disorders ranging from lethal to mild conditions such as primary generalized osteoarthritis (Ala-Kokko et al. 1990; Spranger et al. 1994; Horton 1996). Identification of COL2A1 premature translation termination codons in one allele of patients with Stickler syndrome (MIM 108300) indicated that the disease was caused by functional haploinsufficiency (Horton 1996; Williams et al. 1996; Annunen et al. 1999). Stickler syndrome and Marshall syndrome (MIM 154780) have also been associated with mutations in COL11A1 (Richards et al. 1996; Griffith et al. 1998; Annunen et al. 1999). Interestingly, none of these mutations cause premature termination of translation. Four mutations have been identified in the COL11A2 gene. A Gly175→Arg substitution was found in a family with recessive OSMED (Vikkula et al. 1995; van Steensel et al. 1997), whereas an individual with Weissenbacher-Zweymüller syndrome (MIM 277610) was heterozygous for a Gly955→Glu substitution (Pihlajamaa et al. 1998). COL11A2 mutations have also been found in two families with nonocular Stickler syndrome (MIM 184840). These mutations constituted a 27-bp deletion in exon 39 (Sirko-Osadsa et al. 1998) and a splicing mutation of exon 60 resulting in a deletion of 18 amino acid residues (Vikkula et al. 1995).
In spite of partial overlap between the Stickler, Marshall, Weissenbacher-Zweymüller, and OSMED phenotypes caused by mutations in the COL2A1, COL11A1, and COL11A2 genes, the absence of eye involvement in patients carrying COL11A2 mutations is worth noting. This is best explained by the finding that the COL11A2 gene is not expressed in the vitreous body and that the a2(V) chain replaces the a2(XI) chain in this tissue (Mayne et al. 1993).
Herein we describe clinical and molecular findings in seven families with OSMED. All affected family members were found to be homozygous or compound heterozygous for COL11A2 mutations, indicating that this disorder is caused by mutations of the COL11A2 gene.
Subjects and Methods
Subjects
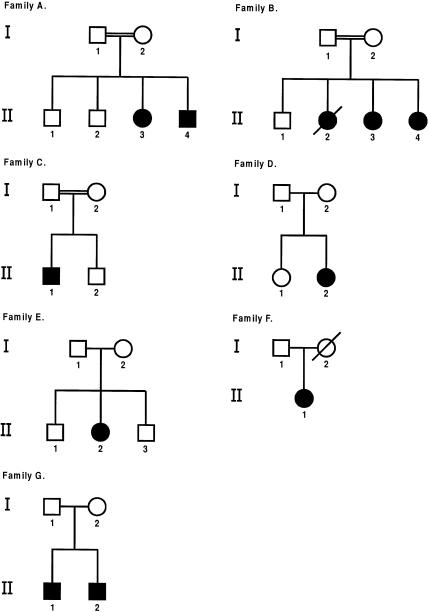
Blood samples were obtained from seven unrelated patients with OSMED and their family members (fig. 1). The families with OSMED originated from Turkey, Morocco, the Netherlands, and northern Europe, and are referred to by letters “A”–“G.” Family members were physically evaluated and the affected individuals also radiologically and by audiometry. Individuals A:I1, A:I2, B:I1, C:I1, and C:I2 refused clinical examination. Radiographs were obtained from the parents of two families (D:I1, D:I2, and E:I2). Audiometry was done to the parents of families B and D. Blood samples from five healthy volunteers, were used as controls in the mutation analysis and RNA analysis. Informed consent was obtained from all the subjects.
Figure 1.
Pedigrees of families with OSMED
Mutation Analysis
Genomic DNA was isolated from EDTA-anticoagulated blood. All 66 exons and the flanking sequences of the COL11A2 gene were amplified by PCR to obtain products of 182–441 bp (Vuoristo et al. 1995; Pihlajamaa et al. 1998). The PCR reactions were done in a volume of 30 μl that contained 100–150 ng genomic DNA, 5–10 pmol of the PCR primers, 1.5 mM of MgCl2, 0.2 mM of dNTPs, and 1 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer). The thermocycling conditions were an initial denaturation at 95°C for 10 min followed by 33 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 40 s, and a final extension at 72°C for 10 min. To generate heteroduplexes for conformation-sensitive gel electrophoresis (CSGE), the samples were denatured at 95°C for 5 min and annealed at 68°C for 30 min. To screen individuals that could be homozygous for mutations, the patients’ PCR products were mixed with an equal amount of PCR products from the control subjects before heteroduplexing. The PCR products were first analyzed for quality and quantity on agarose gel, after which 50–100 ng of heteroduplexed sample was analyzed on CSGE as described earlier (Ganguly et al. 1993; Körkkö et al. 1998).
Sequencing
PCR products that showed previously unidentified patterns in CSGE were sequenced with an ABI prism 377 sequenator and dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer) to reveal the underlying sequence variations. The sequence variations were confirmed by using both forward and reverse primers. In most cases PCR primers were used for sequencing.
RNA Analysis
Total RNA was extracted from Epstein-Barr virus–transformed lymphoblasts (Rapid RNA isolation Kit, Qiagen). The first strand cDNA was synthesized with a Superscript Preamplification System (Gibco BRL) by using random hexameres, and PCR amplification of the cDNA was done by using primers corresponding to exons 21 (5′-GGA CCT CGA GGT CTC CTT GG) and 26 (5′-ACC TTC CTT CCC TGG GTG ACC), and exons 50 (5′-AGT CAG GAT CTC CAG GGA TCC) and 56 (5′-CCT ATA GCG CCA GGA TCT CC) of the α2(XI) cDNA (Vuoristo et al. 1995). Nested primers from exons 22 (5′-GGC GTC CGA GGC ATG GAT GGT C) and 25 (5′-GTC CGT CTG AGC CAG GCA TG), and exons 51 (5′-GGG GCA GCC AGG AGA GCC AG) and 55 (5′-CCT CGG AAC CAG GCG AGC CA) were used for a second PCR amplification. The PCR reactions were done in a volume of 100 μl by using 2 μl of PCR template, 34 pmol of each primer, and 5 U of AmpliTaq Gold DNA polymerase. PCR conditions included one cycle at 95°C for 10 min and 33 cycles at 95°C for 40 s, 60°C for 40 s, 72°C for 45 s followed by one cycle at 72°C for 10 min. The PCR products were analyzed on 2% agarose gel, and the products of the second PCR amplification were sequenced.
Results
Clinical Data
Family A consisted of consanguineous parents of Turkish descent and four children (fig. 1). The first and second pregnancies had ended in spontaneous abortion, after which two healthy children and two affected ones had been born. The clinical data on the patients (table 1) A:II3 and A:II4 have been published elsewhere (Miny and Lenz 1985). Clinical findings of the parents are listed in table 2.
Table 1.
Primary Clinical Features of OSMED Patients in Seven Families
|
Status of Patienta |
||||||||||
| Characteristic | A:II3 | A:II4 | B:II3 | B:II4 | C:II1 | D:II2 | E:II2 | F:II1 | G:II1 | G:II2 |
| Age (years) | 3 | 1.5 | 7 | 3 | 6 | 3 | 25 | 20 | 10 | 10 |
| Height (cm) | 86 | 78 | 115 | 89 | 106 | 83 | 150 | 147 | 122 | 124 |
| Disproportionately short limbs | + | + | + | + | + | + | + | + | + | + |
| Facial features: | ||||||||||
| Cleft palate | +b | +b | + | + | + | + | + | +c | + | + |
| Micrognathia | − | − | + | + | + | + | − | + | + | + |
| Midface hypoplasia | + | + | + | + | + | + | + | + | + | + |
| Flat mala | + | + | + | + | + | + | + | + | + | + |
| Flat nasal bridge | + | + | + | + | + | + | + | + | + | + |
| Short nose | + | + | + | + | + | + | + | + | + | + |
| Anteverted nares | + | + | + | + | + | + | − | + | + | + |
| Long philtrum | + | + | + | + | + | + | + | + | + | + |
| Hypertelorism | + | + | + | + | − | + | − | − | − | − |
| Midfacial hemangioma | + | + | − | − | − | − | − | − | + | + |
| Enlarged joints | + | + | + | + | + | + | +d | + | +e | +e |
| Limited flexion of metacarpophalangeal joints | + | + | + | + | + | − | + | + | ||
| Pectus excavatum | + | + | + | − | − | + | + | |||
| Vertebral body anomalies | + | + | + | + | + | + | +f | +f | + | + |
| Kyphosis | + | + | + | + | + | − | + | + | + | + |
| Lumbar lordosis | − | + | + | + | + | + | + | + | + | + |
| Sensorineural hearing loss (dB) | 80 | 50–70 | 80–90 | 100 | 70 | 60–110 | 90 | 50–90 | 90–95 | 90–95 |
| High myopia | − | − | − | − | − | − | − | + | − | − |
Mental development was normal in all patients.
Cleft of soft palate.
Bifid uvula and submucosal palatal cleft.
Joint pain/stiffness and osteochondrosis in knee joint.
Joint pain/stiffness.
Osteochondrosis-like changes.
Table 2.
Clinical Summary of Phenotypes of Parents
|
Radiography |
||||||
| Parent(Sex) | Height (cm) | Physical Examination | Facial Features | Hearing | Joints Examined | Findingsa |
| A:I1 (M) | 172 | Not done | Normal | Normalb | ||
| A:I2 (F) | 161 | Not done | Normal | Normalb | ||
| B:I1 (M) | 185 | Not done | Normal | Normalc | ||
| B:I2 (F) | 158 | Normal | Dysmorphicd | Normalc | ||
| C:I1 (M) | 176 | Not done | Normal | Normalb | ||
| C:I2 (F) | 162 | Not done | Normal | Normalb | ||
| D:I1 (M) | 182 | Normal | Normal | Deficiente | Hips, knees, ankles, spine | Normal hips, knees, ankles; spinef |
| D:I2 (F) | 178 | Normal | Normal | Normalc | Hips, knees, ankles, spine | Normal hips, knees, and ankles; anomalies in spineg |
| E:I1 (M) | 180 | Normal | Normal | Normalb | ||
| E:I2 (F) | 168 | Normal | Dysmorphich | Normalb | Hips, wrist, hand, knees, spine | Normal hips, wrist, and knees; anomalies in handi and spinej |
| F:I1 (M) | 174 | Not done | Normal | Normalb | ||
| F:I2 (F) | 167 | Not done | Normalk | Normalk | ||
| G:I1 (M) | 175 | Normal | Normal | Normalb | ||
| G:I2 (F) | 155 | Normal | Dysmorphicl | Normalb | ||
No parents complained of pain in the joints.
Not tested.
On the basis of audiogram.
Slightly flat face.
On the basis of audiogram (mild hearing loss—20 dB on the left side and 40 on the right side—considered to be work related).
Slight thoracic kyphosis, mild degenerative changes, one slightly wedge-shaped flattened vertebra, spondylolysis, and grade 1 spondylolisthesis at L5.
Slight thoracic kyphosis, mild degenerative changes.
High palate, crowded teeth, micrognathia, and narrow face.
Narrowing of distal interphalangeal joint space, irregular joint surfaces, a few minor cysts, and some chondrocalsinosis.
Sondylolisthesis at L3.
On the basis of history.
Short nasal spine, small nose, and anteverted nares.
Family B (fig. 1) originated from Morocco and consisted of consanguineous parents (table 2), three affected children and one unaffected child. The third child, B:II3 had normal body length (48 cm) at birth, but short limbs with enlarged joints and stiff interphalangeal joints. The radiological features included vertebral coronal clefts, square iliac wings and a thick ischium, large metaphyses of the long bones and enlarged epiphyses of the elbows and knees. At age 7 years she was of nearly normal height (115 cm, −1 SD), but disproportionate, and her palate was extremely narrow, with a double row of teeth (table 1). Eye examination revealed slight myopia. The clinical and radiological features of the fourth child, B:II4, were similar to those of her sister, B:II3, but her vision was normal. The family’s second child was stillborn with neonatal rhizomelic dwarfism. In view of the radiographic findings regarding the skeleton, she was considered afterwards to have had OSMED (not shown).
Patient C:II1 was born to healthy consanguineous Turkish parents (family C; see fig. 1 and table 2). The patient was noted to have a flat face with posterior medial palatal clefting and micrognathia immediately after birth, there was insufficient ventilation and the neonate had to be intubated and ventilated for 24 h and given continuous positive airway pressure for another 5 d. Respiratory symptoms were prominent for the first 2 years, with a mixed inspiratory/expiratory obstruction pattern precipitated by minor viral airway infections or by physical exercise. The phenotypic features were similar to those of the other patients (table 1). Kniest dysplasia was considered in the first year of life, but the radiological findings were entirely consistent with OSMED. The trachea was unusually narrow during the first 2 years but grew to low normal dimensions later. The boy is of normal intelligence and socially pleasant and well integrated, albeit handicapped by the hypoacusis and speech defect. He has a younger brother who is clinically unremarkable.
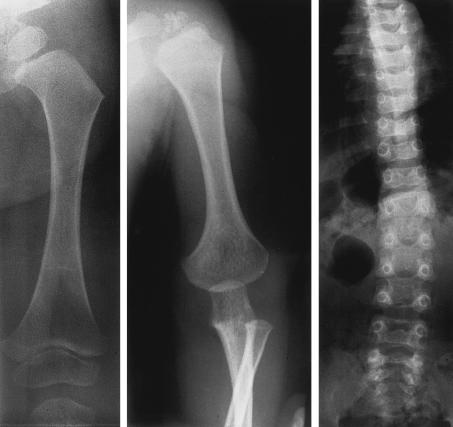
The patient D:II2 (fig. 1) was the second child of healthy Dutch parents (table 2). Genealogical analysis of the parents’ ancestry going back five generations revealed no consanguinity. The patient was born at term after a normal pregnancy. Length at birth was 54 cm. The phenotypical findings were consistent with OSMED (table 1). Radiographs of the skeleton at age 5 mo showed markedly short extremities with pronounced metaphyseal flaring of the long bones, especially the femora, and the vertebral bodies seemed almost fragmented (not shown). The skull showed no apparent abnormalities (not shown). Radiographs at age 16 mo revealed broad metaphyses with metaphyseal flaring of the long bones (fig. 2, left and middle) and disproportionately large epiphyses in the hips and knees and mild platyspondyly with irregularities in the endplates of the vertebrae (fig. 2, right). The phalangeal epiphyses were also large. Ophthalmological assessment revealed no abnormalities. At the time of this study, the patient (age 7 years) had normal intelligence and normal height but still had disproportionately short limbs with large joints and had pain in her hips, knees, and ankles after minor exercise. She has a healthy 9-year-old sister (D:II1).
Figure 2.
Radiographs of femur (left), humerus (middle), and spinal column (right) of patient DII:2 at age 16 mo
Patient E:II2 was the second of three children to nonconsanguineous parents (fig. 1; table 2). The family originated from the Netherlands. A clinical description of the patient has been published earlier (table 1; Kääriäinen et al. 1993; patient 3). In addition to clinical features described in table 1, patient E:II2 had mild to moderate flexion contractures in several joints, extensive osteoporosis (especially in spine), a brachycephalic skull, and a prominent mandible.
Patient F:II1 was the only child of nonconsanguineous parents of Finnish descent (fig. 1; table 2). The clinical description of the patient has been published earlier (Kääriäinen et al. 1993; patient 2). The main clinical features are described in table 1.
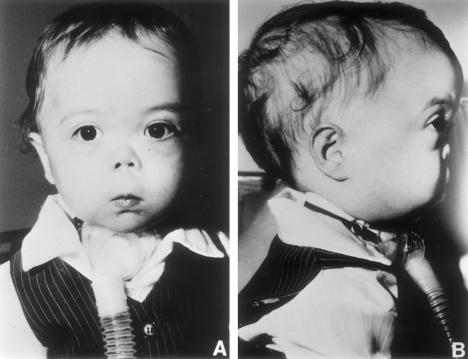
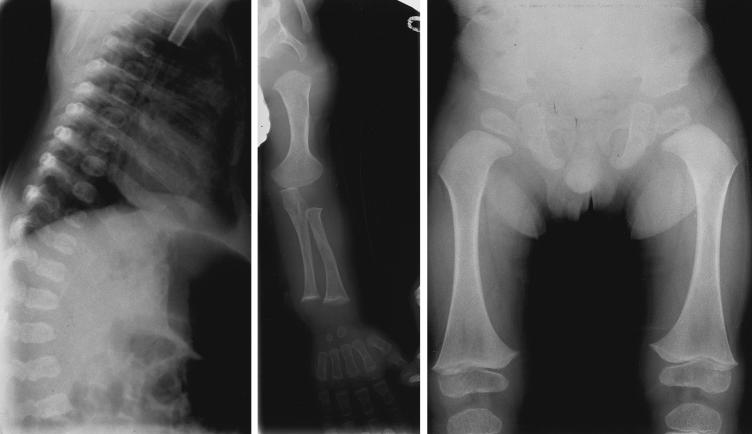
Patients G:II1 (see fig. 3) and G:II2 were male twins of indeterminate zygosity born at 34 wk gestation to nonconsanguineous parents of Northern European descent (fig. 1; table 2). Respiratory distress was apparent in patient G:II1 from birth. Airway obstruction secondary to Robin sequence together with bronchoscopically confirmed laryngomalasia necessitated intubation soon after birth. A tracheostomy was done at age 3 wk. Airway obstruction in G:II2 was initially less severe and tracheostomy was not needed until age 3 mo. Decannulation was accomplished at age 31 mo, in both cases. The phenotypic features of the twins were virtually identical as described in table 1. Radiograph of lateral spine of G:II1 at age 3 mo showed mild platyspondyly and coronal clefting (fig. 4, left). Radiograph of GII:2 at 9 age mo showed shortening of the long bones and quite marked metaphyseal flaring (fig. 4, middle). In the radiograph of pelvis and hips of G:II1 at age 2 years 5 mo were seen short and broad basilar portions of the ilia and relatively horizontal acetabular roofs, as well as coxa vara (fig. 4, right). In addition, the clinical features included a prominent occiput, and mild parietal and frontal bossing and mild generalized brachydactyly.
Figure 3.
Patient G:II1 at age 18 mo
Figure 4.
Left, Radiograph of spine of patient G:II1 at age 3 mo. Middle, Radiograph of left arm of patient G:II2 at age 9 mo. Right, Radiograph of anteroposterior pelvis and hips of patient G:II1 at age 2 years 5 mo.
Mutation Analysis
All 66 exons and the flanking sequences of the COL11A2 gene were amplified from genomic DNA of one affected member of each family. The amplified products were analyzed on CSGE together with the control samples. Also, equal amounts of the PCR products of the affected individuals were mixed with the control samples for heteroduplexing and analysis on CSGE. The analysis identified several unique CSGE patterns (data not shown). The exons with the unique patterns were then analyzed for all available family members. Sequencing indicated that all the patients had COL11A2 mutations (fig. 5 and table 3). The affected individuals in all the consanguineous families (A–C) and in one nonconsanguineous family (D) were homozygous for mutations whereas those in the remaining nonconsanguineous families (E–G) were compound heterozygous for mutations. All the families had different mutations that either generated premature translation termination codons or altered the splicing consensus sequences (table 3). Analysis of samples from all the available parents (12/14) indicated that the mutations were inherited rather than sporadic.
Figure 5.
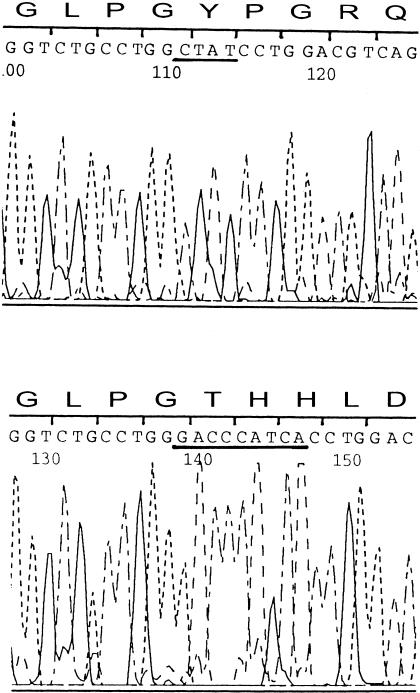
Sequencing of PCR products from a control subject (top) and individual C:II1 (bottom), for exon 31 of the COL11A2 gene. The underlined sequence in the top panel indicates the deletion, whereas the underlined sequence in the bottom panel indicates the insertion.
Table 3.
COL11A2-Gene Mutations in Families with OSMED
|
Mutationa |
||||
| Subject(s) | Homozygous | Heterozygous | Location | Predicted Consequence(s) |
| A:II3 and A:II4 | 732delCb | Exon 5 | Frameshift stop in E6 | |
| B:II3 and B:II4 | 2492C→Ab | Exon 33 | S345X | |
| C:II1 | 2406–2409del, 2405–2410ins9bp | Exon 31 | Frameshift stop in exon 32 | |
| D:II1 | 4821–4843delb | Exon 64 | Frameshift stop in exon 64 | |
| E:II2 | 1636C→Tc | Exon 17 | R60X | |
| IVS22–2A→Gd | IVS22 | In-frame deletion of exon 23 (54 bp) | ||
| F:II1 | 3032–3033insC | Exon 41 | Frameshift stop in exon 43 | |
| 4750G→T | Exon 63 | G1098X | ||
| G:II1 and G:II2 | 3991C→Td | Exon 55 | R845X | |
| IVS53+5G→Ac | IVS53 | Frameshift stop in 56 | ||
A is from the ATG of the initiator methionine codon and is designated as nucleotide “+1.”
Inherited from both the father and the mother.
Inherited from the mother.
Inherited from the father.
RNA Analysis
To show the consequences of the intronic mutations (IVS22-2A→G and IVS53+5G→A) in patients E:II2, G:II1, and G:II2, total RNA was extracted from EBV-transformed lymphoblasts obtained from mother E:I2, mother G:I2 (fig. 1 and table 2), and a control subject. RNA samples were reverse transcribed and PCR products generated from exon 21 to exon 26 (E:I2 and control) and from exon 50 to exon 56 (G:I2 and control). The second PCR amplification was done by using nested primers. Analysis of the PCR product of E:I2 on agarose gel revealed two bands, of ∼150 bp and ∼200 bp, with an equal intensity, but only one band, of ∼200 bp, in the control sample (data not shown). Sequencing of the shorter product indicated skipping of exon 23, and thus an in-frame deletion of 54 nucleotides (data not shown). Analysis of the PCR products on agarose gel showed no difference between the control and G:I2. Sequencing revealed that the G:I2s mutant allele used a cryptic splice site in exon 53, eliminating 17 bp at the end of the exon. This frameshift results in a premature translation termination codon in exon 56.
Discussion
Analysis of seven families with OSMED for mutations in the COL11A2 gene showed each to have different COL11A2 mutations. The individuals were homozygous for mutations in all three families of consanguineous parents and in one family with nonrelated parents, and compound heterozygous in the other three families with nonrelated parents. Altogether 10 mutations were identified, nine of which led to the generation of a premature translation termination codon because of either deletions or single base substitutions, whereas one altered a consensus sequence for splicing. Thus the data predicts that the affected members of six of the seven families completely lack α2(XI) chains or produce truncated α2(XI) chains, which are unlikely to be incorporated into type XI collagen molecules.
The clinical findings for all the patients described here or described earlier have been very similar (tables 1 and 4). The three siblings of the family that were reported to be homozygous for Gly175→Arg substitution (van Steensel et al. 1997) had a slightly different phenotype from the other patients, as none of the three siblings had the small chin, cleft palate, or bifid uvula typically seen in the patients analyzed here and in the OSMED cases reported earlier (table 4). In addition, the three siblings were reported to have severe osteoarthritis, which has not been reported in the other OSMED cases. The phenotypical variation may reflect the fact that haploinsufficiency and mutations with a dominant negative effect on collagens generally result in different phenotypes or degrees of severity (Kuivaniemi et al. 1997). It is also well known that in addition to the type of a mutation, the location of a mutation in collagens may modify the phenotype or its severity (Spranger et al. 1994; Horton 1996; Kuivaniemi et al. 1997; Spranger 1998). This is also supported by the finding here that the parents with COL11A2 null-allele mutations, as well as the individuals heterozygous for Gly175→Arg substitution, had apparently normal phenotype (van Steensel et al. 1997). However, one parent (E:I2) with a COL11A2 splicing mutation had a mild phenotype consisting mainly of dysmorphic facial features resembling those of the patients with a small in-frame deletion or a splicing mutation in COL11A2 having phenotype of nonocular Stickler syndrome (Vikkula et al. 1995; Sirko-Osadsa et al. 1998).
Table 4.
Comparison of Clinical Findings in the Cases of OSMED
|
Frequency in |
|||
| Characteristic | Present Study | van Steensel et al. (1997) | Other Reported Cases of OSMEDa |
| Disproportionately short limbs | 10/10 | 2/3 | 6/6 |
| Enlarged joints | 10/10 | 3/3 | 6/6 |
| Vertebral body anomalies | 10/10 | 3/3 | 6/6 |
| Small chin | 7/10 | 0/3 | 6/6 |
| Cleft palate/bifid uvula | 10/10 | 0/3 | 6/6 |
| Midfacial hypoplasia | 10/10 | 3/3 | 6/6 |
| Sensorineural hearing loss | 10/10 | 3/3 | 6/6 |
| Prominent supraorbital ridges | 0/10 | 2/3 | Unknown |
| Limited flexion of metacarpophalangeal/interphalangeal joints | 7/8 | 3/3 | 3/6 |
The finding here that the absence of the α2 (XI) chains produces a nonlethal phenotype was surprising because the lack of the other type XI collagen α chains, α1 (XI) or α1 (II) , result in a lethal phenotype in mice (Li et al. 1995a; Li et al. 1995b). This could be explained by other α chains partially compensating for the lack of the α2(XI) chains. In the bovine vitreous body, the α2(V) chain normally substitutes for the α2(XI) chain to form a type V/XI hybrid molecule. Further support for the other α chains partially compensating for the α2(XI) chain’s absence derives from transgenic mice with a targeted disruption of the Col11a2 gene (S.-W. Li, P. Vandenberg, M. Takanosu, M. Arita, Y. Bao, Z-X Ren, R. Mayne, and D. J. Prockop, personal communication). The phenotype of the mice homozygous for Col11a2 null mutation is similar to that of the patients with OSMED. Interestingly, the α1(XI) and α1V) chains are recovered after pepsin digestion of the rib cartilage of these mice, suggesting that α1(XI) or α1(V) chains form triple-helical molecules in the absence of the α2(XI) chains.
Our data suggests that complete absence of α2(XI) collagen chains is not lethal, but results in a characteristic phenotype, which includes facial features, skeletal dyplasia and disproportion, and severe hearing loss. OSMED is an autosomal recessive disorder with a recurrence risk of 25%. This contrasts with the more common autosomal dominant collagen disorders, where it is assumed that de novo mutations account for unaffected parents having an affected child, and therefore the recurrence risk is low. Consequently, early recognition of the OSMED phenotype is important for accurate counseling. Advances in molecular diagnosis now provide an option for prenatal diagnosis in the “at-risk” pregnancies.
Acknowledgments
We would like to thank Dr. G. Eich and Prof. A. Giedion for radiographic diagnoses and Ms. Helena Lindquist for her expert technical assistance. This work was supported by grants from the Academy of Finland (to L.A.-K.), by Swiss National Foundation grant 45401.95 (to A.S.-F.), and by National Institutes of Health grant AR-43827 (to M.L.W.)
Electronic-Database Information
The accession numbers and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.nbci.nlm.nih.gov/Omim (for OSMED [215150], Stickler syndrome types I [108300] and II [184840], Marshall syndrome [154780], and Weissenbacher-Zweymüller syndrome [277610])
References
- Ala-Kokko L, Baldwin CT, Moskowitz RW, Prockop DJ (1990) Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci USA 87:6565–6568 [DOI] [PMC free article] [PubMed]
- Ala-Kokko L, Kvist A-P, Metsäranta M, Kivirikko KI, de Crombrugghe B, Prockop DJ, Vuorio E (1995) Conservation of the sizes of 53 introns and over 100 intronic sequences for the binding of common transcription factors in the human and mouse genes for type II procollagen (COL2A1). Biochem J 308:923–929 [DOI] [PMC free article] [PubMed]
- Annunen S, Körkkö J, Czarny M, Warman ML, Brunner HG, Kääriäinen H, Mulliken JB, et al (1999) Splicing mutations of 54 bp exons in the COL11A1 gene cause Marshall syndrome but other mutations cause overlapping Marshall/Stickler phenotypes. Am J Hum Genet 65:974–983 [DOI] [PMC free article] [PubMed]
- Brewton RG, Mayne R (1994) Heterotypic type II, IX and XI fibrils: comparison of vitreous and cartilage forms. In: Yurchenco PD, Birk DE, Mecham RP (eds) Extracellular matrix assembly and structure. Academic Press, San Diego, pp 129–170 [Google Scholar]
- Cremer MA, Rosloniec EF, Kang AH (1998) The cartilage collagens: a review of their structure, organization, and role in the pathogenesis of experimental arthritis in animals and in human rheumatic disease. J Mol Med 76:275–288 [DOI] [PubMed]
- Eyre D, Wu JJ (1987) Type XI or 1α2α3α collagen. In: Mayne R, Burgeson RE (eds) Structure and function of collagen types. Academic Press, Orlando, pp 261–281 [Google Scholar]
- Ganguly A, Rock MJ, Prockop DJ (1993) Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA 90:10325–10329 [DOI] [PMC free article] [PubMed]
- Giedion A, Brandner M, Lecannellier J, Muhar U, Prader A, Sulzer J, Zweymüller E (1982) Oto-spondylo-megaepiphyseal dysplasia (OSMED). Helv Pediatr Acta 37:361–380 [PubMed]
- Griffith AJ, Sprunger LK, Sirko-Osadsa DA, Tiller GE, Meisler MH, Warman ML (1998) Marshall syndrome associated with a splicing defect at the COL11A1 locus. Am J Hum Genet 62:816–823 [DOI] [PMC free article] [PubMed]
- Horton WA (1996) Molecular genetic basis of the human chondrodysplasias. Endocrinol Metab Clin North Am 25:683–697 [DOI] [PubMed]
- Insley J, Astley R (1974) A bone dysplasia with deafness. Br J Radiol 47:244–251 [DOI] [PubMed]
- Johnston KM, Golabi M, Langer L, Hall BD, Grix A (1987) Oto-spondylo-megaepiphyseal dysplasia (OSMED): differential diagnosis and report of a new case. Proc Greenwood Center 6:155–156 [Google Scholar]
- Kääriäinen H, Barrow M, Hennekam R (1993) Bone dysplasia, midface hypoplasia, and deafness: three new patients and review of the literature. Am J Med Genet 46:223–227 [DOI] [PubMed]
- Körkkö J, Annunen S, Pihlajamaa T, Prockop DJ, Ala-Kokko L (1998) Conformation sensitive gel electroforesis for simple and accurate detection of mutations: comparison with denaturing gradient gel electroforesis and nucleotide sequencing. Proc Natl Acad Sci USA 95:1681–1685 [DOI] [PMC free article] [PubMed]
- Kuivaniemi H, Tromp G, Prockop DJ (1997) Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 9:300–315 [DOI] [PubMed]
- Li S-W, Prockop DJ, Helminen H Fässler R, Lapveteläinen, Kiraly K, Arokoski J, et al (1995a) Transgenic mice with targeted inactivation of the Col2a1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev 9:2821–2839 [DOI] [PubMed]
- Li Y, Lacerda M, Warman ML, Beier DR, Yoshioka H, Nimomiya Y, Oxford JT, et al (1995b) A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell 80:423–430 [DOI] [PubMed]
- Linsenmayer TF, Gibney E, Igoe F, Gordon MK, Fitch JM, Fessler LI, Birk DE (1993) Type V collagen: molecular structure and fibrillar organization of the chicken α1(V) NH2- terminal domain, a putative regulator of corneal fibrillogenesis. J Cell Biol 121:1181–1189 [DOI] [PMC free article] [PubMed]
- Mayne R, Brewton RG, Mayne PM, Baker JR (1993) Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem 268:9381–9386 [PubMed]
- Miny P, Lenz W (1985) Autosomal recessive deafness with skeletal dysplasia and facial appearance of Marshall syndrome. Am J Med Genet 21:317–324 [DOI] [PubMed]
- Pihlajamaa T, Prockop DJ, Faber J, Winterpacht A, Zabel B, Giedion A, Wiesbauer P, et al (1998) Heterozygous glycine substitution in the COL11A2 gene in the original patient with the Weissenbacher-Zweymüller syndrome demonstrates its identity with heterozygous OSMED (nonocular Stickler syndrome). Am J Med Genet 80:115–120 [DOI] [PubMed]
- Richards AJ, Yates JR, Williams R, Payne SJ, Pope FM, Scott JD, Snead MP (1996) A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in α1(XI) collagen. Hum Mol Genet 9:1339–1343 [DOI] [PubMed]
- Salinas CF, de Botero D, Isaza C (1986) Bone dysplasia, deafness and cleft palate syndrome. Presented at the 7th International Congress of Human Genetics, Berlin, September 22–26 [Google Scholar]
- Sirko-Osadsa DA, Murray MA, Scott JA, Lavery MA, Warman ML, Robin NH (1998) Stickler syndrome without eye involvement is caused by mutations in COL11A2 , the gene encoding the α2 (XI) chain of type XI collagen. J Pediatr 132:368–371 [DOI] [PubMed]
- Spranger J (1998) The type XI collagenopathies. Pediatr Radiol 28:745–750 [DOI] [PubMed]
- Spranger J, Winterpacht A, Zabel B (1994) The type II collagenopathies: a spectrum of chondrodysplasias. Eur J Pediatr 153:56–65 [DOI] [PubMed]
- van Steensel MA, Buma P, de Waal Malefijt MC, van den Hoogen FH, Brunner HG (1997) Oto-spondylo-megaepiphyseal dysplasia (OSMED): clinical description of three patients homozygous for a missense mutation in the COL11A2 gene. Am J Med Genet 70:315–323 [DOI] [PubMed]
- Vikkula M, Mariman EC, Lui VCH, Zhidkova NI, Tiller GE, Goldring MB, van Beersum SEC, et al (1995) Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell 80:431–437 [DOI] [PubMed]
- Vuoristo MM, Pihlajamaa T, Vandenberg P, Prockop DJ, Ala-Kokko L (1995) The human COL11A2 gene structure indicates that the gene has not evolved with the genes for the major fibrillar collagens. J Biol Chem 270:22873–22881 [DOI] [PubMed]
- Williams CJ, Ganguly A, Considine E, McCarron S, Prockop DJ, Walsh-Vockley C, et al (1996) A-2→G transition at the 3́ acceptor splice site of IVS17 characterizes the COL2A1 gene mutation in the original Stickler syndrome kindred. Am J Med Genet 63:461–467 [DOI] [PubMed]