Summary
Fragile X premutations are considered to be a risk factor for premature ovarian failure (POF), which is usually defined as menopause at age <40 years. Since premutations may be inherited from either the mother or the father, we evaluated the influence of the inheritance pattern on the duration of reproductive life in female carriers. The occurrence of POF and age at menopause in women with a paternally inherited fragile X premutation (PIP) were compared to those in women with a maternally inherited fragile X premutation (MIP). We identified 148 women in whom the parental origin of the premutation could be determined. In 109 of these women we were able to establish whether POF had occurred: 82 women had a PIP, and 27 had a MIP. Twenty-three of the women (28%) with a PIP had POF, versus only 1 (3.7%) with a MIP (two -tailed Fisher’s exact test; P=.007). Kaplan-Meier analysis of all 148 premutations showed that the age at menopause was significantly lower in the women with a PIP than in the woman with a MIP (Breslow test in Kaplan-Meier analysis; P=.003). Our data strongly suggest that, when POF occurs in fragile X premutation carriers, a considerable proportion of the premutations are inherited paternally (parent-of-origin effect). We hypothesize that this may be owing to a paternal genomic imprinting effect.
Introduction
The fragile X syndrome (MIM 309550) is an X-linked disorder caused by deficiency of fragile X mental retardation protein (FMRP), a deficiency that is the result of transcriptional silencing of the FMR1 gene by a process of methylation (Imbert et al. 1998). Methylation only occurs when the polymorphic CGG-repeat tract in the promoter (Verkerk et al. 1991) expands into the full mutation range—that is, >200 repeats; normally there are <50 repeats. Alleles with repeat sizes of 50–200 are classified as premutations, which are meiotically unstable and can expand to full mutations only when maternally transmitted. Although FMRP is produced at normal levels by alleles in the premutation range (Feng et al. 1995), there is growing evidence that premutations may have an effect on the normal phenotype.
Recently, an international collaborative study of premature ovarian failure (POF) (Allingham-Hawkins et al. 1999) in fragile X carriers has reported that fragile X premutations are a significant risk factor for POF, defined as complete cessation of periods at age <40 years. This observation is consistent with findings described in other reports (Schwartz et al. 1994; Conway et al. 1998; Murray et al. 1998; Uzielli et al. 1999), as well as with those in our ongoing study. However, Kenneson et al. (1997) screened 33 women with POF and did not find any fragile X premutation.
There is strong evidence that premutation carriers have an increased risk of POF. Nevertheless, the question remains as to why some premutation carriers experience POF whereas others do not. In our ongoing study, we observed that POF was cosegregating with premutations that were inherited paternally. We tested the hypothesis that a parent-of-origin effect might be involved in the increased risk of POF in fragile X–premutation carriers.
Subjects and Methods
Clinical and molecular data were from our study group of families, which previously had been given diagnoses of fragile X syndrome. Female carriers of the fragile X premutation, for whom the parental origin was known, were selected. All the women were age ⩾18 years and had a normal karyotype. They were interviewed personally by the same physician; a complete medical history was taken, including data on the reproductive life span. Whenever possible, the anamnestic data were verified from medical records, with the consent of the participant. The study was approved by the institutional review board.
In the present study, POF was defined as spontaneous menopause at age <40 years. Spontaneous menopause implies either spontaneous cessation of menstruation, with a duration of ⩾1 year, or secondary amenorrhea, with a duration of 6 mo–1 year, with an FSH level of >40 IU/liter and low serum 17β-estradiol concentrations. Spontaneous cessation of menstruation does not apply to cases with termination resulting from hysterectomy, bilateral oophorectomy, chemotherapy, radiotherapy, or ablative medication. If a woman still had menstrual cycles, her ovarian function was assessed on the basis of serum FSH and luteinizing-hormone levels (IMx; Abbott Laboratories) and 17β-estradiol radioimmunoassays, as described elsewhere (Thomas et al. 1977). Women with premenopausal endocrine profiles were categorized as women with proved ovarian function. The age of last menstruation was included for women who were using oral contraceptives. The participants were divided into two groups: women with a paternally inherited premutation (PIP) and women with a maternally inherited premutation (MIP).
In our first analysis, women were included when we were able to determine that POF had occurred. We excluded all women age <40 years (in accordance with the definition of POF), all those with a history of nonspontaneous cessation of menstruation at age <40 years, and all those who were using oral contraceptives at the age-cutoff point (40th birthday). The occurrence of POF in the group with a PIP was compared with that in the group with a MIP. Fisher’s exact test was used to evaluate statistically significant differences.
To avoid loss of information on the menstrual status of the women who either had not yet attained age 40 years who had experienced nonspontaneous cessation of menstruation, we also used a Kaplan-Meier survival approach to compare age at menopause in women with a PIP to that in women with a MIP. Age at the time of participation in the study was used for the women with proved ovarian function, whereas the age at cessation was used for the women with nonspontaneous cessation of menstruation. The Breslow test was used to evaluate the age-at-menopause distributions. All analyses were performed with Statistical Package for Social Sciences software, version 8.0, and Statistical Analysis System software, version 6.12.
Results
Relevant data were available on 148 female carriers (age >18 years) from 55 families, with different premutation-repeat sizes; 106 of the women had a PIP and 42 had a MIP. The 106 women with a PIP are daughters of, in total, 48 fathers (average 2.21 daughters, range 1–10) with a premutation, whereas the 42 women with a MIP are daughters of, in total, 29 mothers (average 1.45, range 1–3) with a premutation.
The number of PIPs is higher because premutation males transmit a premutation to all their daughters. Premutation females can transmit either a normal allele to their daughters or a mutated allele that can be a full-mutation allele or a premutation allele. All 148 women were interviewed personally about their menstrual cycles (i.e., whether they were still menstruating), and data were verified on the basis of medical records. Women were subdivided into the following four categories: those with spontaneous menopause at age (1) <40 years or (2) ⩾40 years, (3) those with nonspontaneous cessation of menstruation at age <40 years, and (4) those with proved ovarian function. Subsequently, we investigated the relation between POF (at age < 40 years), and the parental origin of the fragile X premutation. Owing to the age-cutoff point, data on all the women of age <40 years (n=30) were excluded. Three women with a PIP and six women with a MIP were excluded as well, because it could not be established whether POF had occurred (nonspontaneous cessation of menstruation). In the remaining 109 women (82 of whom had a PIP and 27 of whom had a MIP), we determined whether POF had occurred. In the group of women with a PIP, 23 (28%) of 82 had experienced menopause at age <40 years, whereas this had occurred in only 1 (3.7%) of the 27 women in the group with a MIP (table 1). Table 2 shows the distribution of POF among the families with at least one woman with POF. POF is distributed among the kindreds and is not found exclusively in a subset of families. In families with at least two females with POF, differences in age at menopause were found. No correlation was seen between premutation-repeat size and age at menopause.
Table 1.
Female Fragile X Premutation Carriers and Their Menstrual Cycles[Note]
|
No. of Women |
|||
| PIP | MIP | Total | |
| Spontaneous menopause: | |||
| At age <40 years | 23 | 1 | 24 |
| At age ⩾40 years | 36 | 14 | 50 |
| Proved ovarian function | 23 | 12 | 35 |
| Total | 82 | 27 | 109 |
Note.—All women were age ⩾40 years when they participated in the study.
Table 2.
Distribution of POF in Families with at Least One Case of POF
| Daughters, in Birth Ordera |
||||||||||
| Family (Transmission) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1 (Paternal) | + | |||||||||
| 2 (Paternal) | + | |||||||||
| 3 (Paternal) | + | |||||||||
| 4 (Paternal) | + | − | ||||||||
| 5 (Paternal) | + | − | ||||||||
| 6 (Paternal) | + | − | − | |||||||
| 7 (Paternal) | − | + | ||||||||
| 8 (Paternal) | − | + | − | |||||||
| 9 (Paternal) | − | + | − | |||||||
| 10 (Paternal) | − | + | − | − | ||||||
| 11 (Paternal) | − | − | + | |||||||
| 12 (Paternal) | + | + | − | |||||||
| 13 (Paternal) | + | + | − | + | ||||||
| 14 (Paternal) | + | + | + | + | − | + | + | − | + | − |
| 15 (Maternal) | + | |||||||||
A plus sign (+) denotes presence of POF; a minus sign (−) denotes absence of POF.
Thus, POF was significantly more common in carriers of a PIP than in carriers with a MIP (two-tailed Fisher’s exact test; P=.007). The odds ratio was estimated to be 10.1 (95% confidence interval 1.3–79.1). This indicates that women with a PIP have a 10-times-higher risk of POF than do women with a MIP.
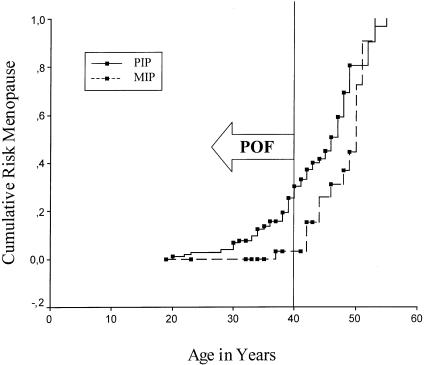
In the first analysis, 39 of 148 women were not included. To avoid loss of information on the menstrual status of these women, we also performed a Kaplan-Meier survival analysis of age at menopause, which included informative data on all the women (n=148). In the women with nonspontaneous menopause, age at intervention was used in the analysis (fig. 1). The Breslow test for equality of survival distributions showed that age at menopause in the group with a PIP was significantly different than that in the group with a MIP (P=.003); female fragile X carriers with a PIP became postmenopausal at a younger age than did those with a MIP. The calculated cumulative probabilities of menopause at various ages (with use of actuarial survival techniques) are given in table 3.
Figure 1.
Cumulative risk of menopause in women with either a PIP (unbroken line) or a MIP (dashed line). Blackened squares denote the censored observations (last included informative data on women with either proved ovarian function or nonspontaneous cessation of menstruation).
Table 3.
Cumulative Percentages of Women with either a PIP or a MIP Who Became Postmenopausal, by Age
|
Cumulative Percentages of Women Who Became Postmenopausal, by Age(years) |
|||||||||
| <25 | <30 | <35 | <40 | <45 | <50 | <55 | <60 | Median Survival(years) | |
| Women with a PIP | 2.85 | 3.81 | 12.7 | 25.7 | 42.2 | 81.8 | 97.0 | 100 | 46.7 |
| Women with a MIP | .00 | .00 | .00 | 3.12 | 26.5 | 46.7 | 100 | 100 | 50.1 |
Discussion
We studied age at menopause and the occurrence of POF in female fragile X carriers with either a PIP or a MIP. The age at menopause was significantly different between the two groups (Breslow’s test; P=.003); women with a PIP became postmenopausal at a younger age. Moreover, we found that 28% of the women with a PIP had become postmenopausal spontaneously at age <40 years and thus satisfied our strictly defined criteria for POF. Only 1 of 27 women with a MIP had POF, a rate that is significantly lower than that in the women with a PIP. Sporadic cases of POF are observed in 1% of the general female population (Coulam et al. 1986). Therefore, it cannot be ruled out that some of our participants (including the woman with a MIP) represented sporadic cases of POF. Nevertheless, our data strongly suggest that female fragile X carriers who inherit the premutation from their fathers have an excess risk for POF.
Table 2 shows that POF was more common in some of our sister sets with premutations (with a maximum of seven cases of POF in one sister set with 10 sisters) than in others. Owing to family relatedness, the occurrence of POF in sister sets might be different from expectations based on a binomial distribution. Consequently, the width of the 95% confidence interval of the odds ratio, as well as the P value of the Fisher's exact test, may be underestimated. To evaluate this potential bias in the variance of our estimates, we performed logistic regression analysis on our data, taking relatedness into account and allowing for extra binomial variation. It appeared that there was no extra binomial variation and that the usual binomial model fitted the data well.
Age at menopause is strongly related to the number of ovarian follicles (Ginsburg 1991). Faddy and Gosden (1996) developed a mathematical model that relates follicle depletion to age at menopause in human females. Although POF may be associated with a normal number of oocytes (i.e., the resistant-ovary syndrome), the most likely biological basis for POF is low follicle storage (Gosden and Faddy 1998). Low numbers of oocytes are caused by either deficient oogenesis or increased oocyte loss. A considerable percentage of the women with a PIP experienced POF. These women may have suffered from either abnormalities in oogenesis or increased loss of oocytes during prenatal—or, less likely, postnatal—life.
Mitochondrial function influences the reproductive life span (Dorland et al. 1998; Eichenlaub-Ritter 1998). Since mtDNA is never inherited paternally, we can exclude this as an explanation for POF development in carriers with a PIP. In any case, the oocyte pool is not likely to be small because of mitochondrial abnormalities.
We hypothesize, on the basis of our findings, that the paternal effect in POF in fragile X premutation carriers can be explained by paternal genomic imprinting (genomic imprinting is an epigenetic phenomenon in which there is unequal expression of maternal and paternal genes in the offspring). Expression of the FMR1 gene and other X-linked genes is nonfunctional during normal spermatogenesis (Tamanini et al. 1997), when the X chromosome becomes condensed and transcriptionally inactive (Monesi et al. 1978). Most genes are reactivated during the first few cell divisions after fertilization, whereas two X chromosomes are active in the female morula (Epstein et al. 1978). However, when a PIP is imprinted, there may be a delay in reactivation of the (inactive) paternal X chromosome during early embryonic development; thus, only the maternal allele would be expressed at this critical developmental stage. At the time of X inactivation, either the paternal X or the maternal X is randomly inactivated. Although, in half of the cells. the paternal X is inactive, they will transcribe the normal maternal copy, resulting in normal FMRP expression. But, if the PIP is imprinted on the active X chromosome, this may hinder FMR1-gene transcription and result in reduced or complete absence of FMRP expression. Consequently, only 50% of the cells would have normal production, whereas the other 50% would have either reduced levels or complete absence of FMRP production. Although there is no direct evidence that abnormal FMRP production in oocytes leads to a smaller oocyte pool, it is possible that FMRP plays a role in oogenesis, because FMRP is highly expressed when oogenesis occurs (Bächner et al. 1993). Furthermore, there is evidence that FMRP also plays a role in spermatogenesis (Malter et al. 1997), because FMRP production was found to be increased in type A1 spermatogonia. Our hypothesis predicts decreased FMRP levels during oogenesis, which can be tested in animal models (Dutch-Belgian Fragile X Consortium 1994); the paternally inherited FMR1 gene is knocked out. Paternally inherited FMR1 knockout alleles have resulted in female mice that are fertile. Since mice do not show menopause, we will test whether absence of FMR1-gene expression in the paternal allele results in low numbers of oocytes.
POF is not observed in full-mutation females. Our hypothesis of paternal genomic imprinting also explains why the occurrence of POF in women with a full mutation may be expected to be similar to that in the general population (i.e., 1%). Full mutation is always inherited from the mother (in which case, paternal imprinting will not take place). In early embryogenesis, a full-mutation gene is not methylated (Malter et al. 1997) and might be able to produce FMRP. The timing of inactivation of the fully mutated allele is not known, but it is known from extra embryonal tissue that inactivation of a full mutation occurs much later than does X inactivation (Willemsen et al. 1996).
Examples of X-chromosomal imprinting have been described in humans. It has been suggested that a locus on the maternally inherited X chromosome is imprinted, thereby affecting cognitive function, in Turner syndrome (Skuse et al. 1997). In the mouse, the paternal X chromosome in the extraembryonal tissues is exclusively inactivated (West et al. 1977). In marsupials, the paternal X chromosome is inactivated in most if not all tissues (Cooper 1971).
When studying the possible influence of fragile X premutations on age at menopause, we were very careful to comply with an exact definition of POF in our group of patients. This was essential, because menopause can only be established either after 1 year of secondary amenorrhea or when there is secondary amenorrhea with a duration of 6 mo–1 year, with an FSH level of >40 IU/liter. One of the difficulties is that data usually have to be collected retrospectively; thus recall bias may occur. Furthermore, determination of age at menopause will not be reliable if data are not obtained first hand (van Kasteren et al. 1999). Relatives do not know the exact medical background (e.g., the termination of menstruation because of hysterectomy at a young age) of the members of their family, which results in “hearsay” data. Therefore, we interviewed all the women personally, to limit, as much as possible, the risk of misclassification. We also checked the participants’ medical records, to ensure that no concurrent conditions had occurred. Despite such precautions, the definition of POF is a major problem in these studies. Many previously published articles (Schwartz et al. 1994; Partington et al. 1996; Kenneson et al. 1997) on the relation between POF and FMR1-gene mutations have defined POF as menopause at age <40 years. However, it often remains unclear whether the authors of those articles considered that menopause was present at >3, > 6, or >12 mo of secondary amenorrhea, with or without elevated FSH. We would like to emphasize the importance of reaching consensus on the definition of POF. Only then will it be possible to compare the results of the various groups working on this subject.
The cutoff point of age 40 years for POF is based on epidemiological studies. Only 1% of women become postmenopausal prior to that age. However, age 40 years is an arbitrarily defined cutoff point. Women whose menopause occurs just after 40 years of age will generally have an underlying biological basis similar to that of individuals with POF. In this study, women with either a PIP or a MIP were categorized as either POF or not POF. Nevertheless, POF was significantly more common in the women with a PIP than in those with a MIP. In addition, we performed a survival analysis in which the age at menopause did not have to be categorized. In our opinion, this is the best approach to the study of age at menopause. Figure 1 shows that age at menopause is significantly different between the group with a MIP and the group with a PIP and that the whole curve is shifted to the left in the women with a PIP.
In conclusion, the paternal genomic-imprinting hypothesis has important implications not only scientifically, but also for the counseling of premutation carriers. Premutation carriers who inherit the premutation from their mothers have the population-level risk for POF, whereas women with a PIP have an excess risk for POF. Further studies should address the behavior of PIPs during early embryonal and germinal development in the female fetus.
Acknowledgments
We would like to thank all the women who participated in this study, Prof. Hans M. W. M. Merkus for his support, and Meyke Schouten for data processing and statistical assistance. The expert technical assistance of the laboratory technicians and the help of the secretaries are acknowledged. The Zorg Onderzoek Nederland Foundation is acknowledged for financial support. Thanks also to Prof. Han G. Brunner for critically reading the manuscript.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for fragile X syndrome [MIM 309550])
References
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, et al (1999) Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in Fragile X study—preliminary data. Am J Med Genet 83:322–325 [PMC free article] [PubMed]
- Bächner D, Manca A, Steinbach P, Wöhrle D, Just W, Vogel W, Hameister H, et al (1993) Enhanced expression of the murine FMR1 gene during germ cell proliferation suggests a special function in both the male and the female gonad. Hum Mol Genet 2:2043–2050 [DOI] [PubMed]
- Conway GS, Payne NN, Webb J, Murray A, Jacobs PA (1998) Fragile X premutation screening in women with premature ovarian failure. Hum Reprod 13:1184–1187 [DOI] [PubMed]
- Cooper DW (1971) Directed genetic change model for X chromosome inactivation in eutherian mammals. Nature 230:292–294 [DOI] [PubMed]
- Coulam CB, Adamson SC, Annegers JF (1986) Incidence of premature ovarian failure. Obstet Gynecol 67:604–606 [PubMed]
- Dorland M, van Kooij RJ, te Velde ER (1998) General aging and ovarian aging. Maturitas 30:113–118 [DOI] [PubMed]
- Dutch-Belgian Fragile X Consortium (1994) Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78:23–33 [PubMed]
- Eichenlaub-Ritter U (1998) Genetics of oocyte ageing. Maturitas 30:143–169 [DOI] [PubMed]
- Epstein CJ, Smith S, Travis B, Tucker G (1978) Both X chromosomes function before visible X-chromosome inactivation in female mouse embryos. Nature 274:500–503 [DOI] [PubMed]
- Faddy MJ, Gosden RG (1996) A model confirming the decline in follicle numbers to the age of menopause in women. Hum Reprod 11:1484–1486 [DOI] [PubMed]
- Feng Y, Lakkis L, Devys D, Warren ST (1995) Quantitative comparison of FMR1 gene expression in normal and premutation alleles. Am J Hum Genet 56:106–113 [PMC free article] [PubMed]
- Ginsburg J (1991) What determines the age at the menopause? the number of ovarian follicles seems to be the most important factor. Br Med J 302:1288–1289 [Google Scholar]
- Gosden RG, Faddy MJ (1998) Biological bases of premature ovarian failure. Reprod Fertil Dev 10:73–78 [DOI] [PubMed]
- Imbert G, Feng Y, Nelson DL, Warren ST, Mandel JL (1998) FMR1 and mutations in fragile syndrome: molecular biology, biochemistry, and genetics. In: Wells RD, Warren ST (eds) Genetic instabilities and hereditary neurological disorders. Academic Press, New York, pp 27–53 [Google Scholar]
- Kenneson A, Cramer DW, Warren ST (1997) Fragile X premutations are not a major cause of early menopause. Am J Hum Genet 61:1362–1369 [DOI] [PMC free article] [PubMed]
- Malter HE, Iber JC, Willemsen R, de Graaff E, Tarleton JC, Leisti J, Warren ST, Oostra BA (1997) Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet 15:165–169 [DOI] [PubMed]
- Monesi V, Geremia R, D’Agostino A, Boitani C (1978) Biochemistry of male germ cell differentiation in mammals: RNA synthesis in meiotic and postmeiotic cells. Curr Top Dev Biol 12:11–36 [DOI] [PubMed]
- Murray A, Webb J, Grimley S, Conway G, Jacobs P (1998) Studies of FRAXA and FRAXE in women with premature ovarian failure. J Med Genet 35:637–640 [DOI] [PMC free article] [PubMed]
- Partington MW, Moore DY, Turner GM (1996) Confirmation of early menopause in fragile X carriers. Am J Med Genet 64:370–372 [DOI] [PubMed]
- Schwartz CE, Dean J, Howard-Peebles PN, Bugge M, Mikkelsen M, Tommerup N, Hull C, et al (1994) Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet 51:400–402 [DOI] [PubMed]
- Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, et al (1997) Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature 387:705–708 [DOI] [PubMed]
- Tamanini F, Willemsen R, van Unen L, Bontekoe C, Galjaard H, Oostra BA, Hoogeveen AT (1997) Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet 6:1315–1322 [DOI] [PubMed]
- Thomas CM, Corbey RS, Rolland R (1977) Assessment of unconjugated oestradiol and progesterone serum levels throughout pregnancy in normal women and in women with hyperprolactinaemia, who conceived after bromocriptine treatment. Acta Endocrinol (Copenh) 86:405–414 [DOI] [PubMed]
- Uzielli ML, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, Biondi C, et al. (1999) Premature ovarian failure (POF) and fragile X premutation females: from POF to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. Am J Med Genet 84:300–303 [PubMed]
- van Kasteren YM, Hundscheid RD, Smits AP, Cremers FP, van Zonneveld P, Braat DD (1999) Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod 14:2455–2459 [DOI] [PubMed]
- Verkerk AJMH, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DPA, Pizutti A, Reiner O, et al (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914 [DOI] [PubMed]
- West JD, Frels WI, Chapman VM, Papaioannou VE (1977) Preferential expression of the maternally derived X chromosome in the mouse yolk sac. Cell 12:873–882 [DOI] [PubMed]
- Willemsen R, Oosterwijk JC, Los FJ, Galjaard H, Oostra BA (1996) Prenatal diagnosis of fragile X syndrome. Lancet 348:967–968 [DOI] [PubMed]