Summary
We report a novel X-linked mental retardation (XLMR) syndrome, with characteristic facial dysmorphic features, segregating in a large North Carolina family. Only males are affected, over four generations. Clinical findings in the seven living affected males include a moderate degree of mental retardation (MR), coarse facies, puffy eyelids, narrow palpebral fissures, prominent supraorbital ridges, a bulbous nose, a prominent lower lip, large ears, obesity, and large testicles. Cephalometric measurements suggest that the affected males have a distinctive craniofacial skeletal structure, when compared with normative measures. Obligate-carrier females are unaffected with MR, but the results of cephalometric skeletal analysis suggest craniofacial dysmorphisms intermediate between affected males and normative control individuals. Unaffected male relatives show no clinical or cephalometric resemblance to affected males. The blood-lymphocyte karyotype and the results of DNA analysis for fragile-X syndrome and of other routine investigations are normal. Linkage analysis for polymorphic DNA markers spanning the X chromosome established linkage to Xq26-q27. Maximum LOD scores were obtained at marker DXS1047 (maximum LOD score = 3.1 at recombination fraction 0). By use of haplotype analysis, we have localized the gene for this condition to an 18-cM genetic interval flanked by ATA59C05 and GATA31E08. On the basis of both the clinical phenotype and the mapping data, we were able to exclude other reported XLMR conditions. Therefore, we believe that a unique recessive XLMR syndrome with a distinctive and recognizable phenotype is represented in this family.
Introduction
X-linked mental retardation (XLMR) conditions are common, representing an estimated range of 10%–50% of all cases of mental retardation (MR) (Turner et al. 1970; Lehrke 1972; Turner 1982; Opitz et al. 1986; Kerr et al. 1991). These are genetically heterogeneous, and, for most types, the underlying genetic basis has not yet been identified (Gedeon et al. 1994). At present, the nosology of XLMR can be broadly grouped into two categories: (1) XLMR syndromes that are associated with a specific or characteristic phenotype and (2) those cases of XLMR that do not present with consistent clinical features (Kerr et al. 1991). The latter are more frequent than are the syndromic XLMR conditions.
Characterization of the specific clinical features associated with a syndromic form of XLMR permits identification of individuals in sufficient numbers to enable more-accurate genetic counseling and, ultimately, gene identification (Lubs et al. 1996a). Identification of the underlying genetic basis is necessary to improve nosology, to understand the disease pathogenesis, and to permit development of better diagnostic and management strategies.
Here we report the clinical description and localization of a gene locus for a novel X-linked MR syndrome. The family was ascertained by identification of seven mentally retarded males from a North Carolina family. The results of examination of the pedigree were consistent with an X-linked recessive mode of inheritance. Clinical examination of normal males and males with MR revealed several phenotypic features that appeared to be present only in males with MR. Cephalometric radiographic analysis revealed a unique craniofacial skeletal profile in affected males. Although several of these clinical findings are present in other XLMR conditions, the composite phenotype appears to be unique and has previously been unreported. To evaluate support for linkage to the X chromosome and to localize the responsible gene, genetic-linkage analysis was performed. These analyses localized the gene locus for this condition to an 18-cM region on chromosome Xq26-q27. Although a number of XLMR conditions have been mapped to this region, none appears to be consistent with our findings in this family. Thus, on the basis of both the unique clinical phenotype and the results of our mapping studies, we believe that this represents a hitherto undescribed syndromic form of XLMR.
Case Reports
A 33-year-old woman (individual IV-11; fig. 1) requested genetic consultation for her four mentally retarded brothers after reading an article on fragile-X syndrome. She was also interested in knowing her own risk of having an affected child. A detailed family history was taken. The results of pedigree analysis were consistent with X-linked recessive inheritance. Seven living affected males were identified; all received a complete physical examination. One affected male (individual IV-9; figs. 1 and 2) died in his 40s, after a flulike illness. His photographs show a marked resemblance to the other affected males.
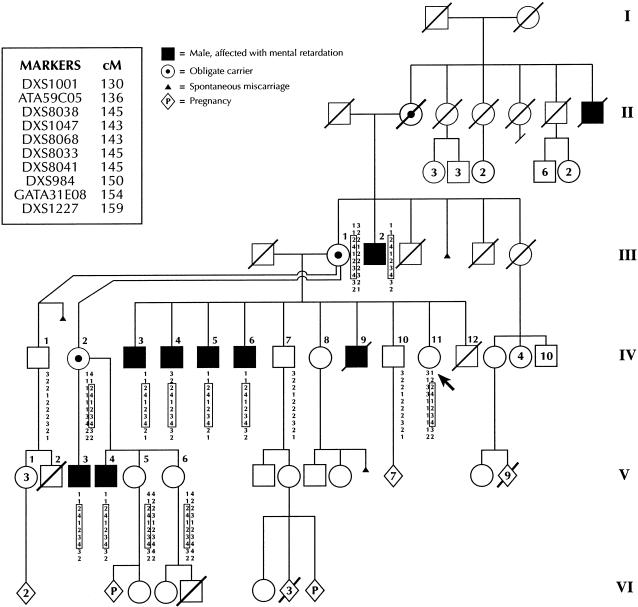
Figure 1.
Pedigree of the family, showing an X-linked recessive mode of inheritance and the haplotypes of the members studied. The consultand is indicated by the arrow. Genetic-map distances are derived from the female chromosome X genetic map.
Figure 2.
Composite picture of affected males, showing characteristic facial features. In order, these males are individuals III-2 (A), IV-3 (B), IV-4 (C), IV-5 (D), IV-6 (E), IV-9 (F), V-3 (G), and V-4 (H). Individual IV-9 is deceased. Individual III-2 has recently lost >100 lbs., as a result of dietary restrictions required because of diabetes mellitus.
All seven living affected males share a distinctive facial appearance (fig. 2) that consists of coarse facies, prominent supraorbital ridges, periorbital fullness, narrow palpebral fissures, a bulbous nose, a prominent lower lip, and large ears. They are all obese and have large testes. Determination of obesity was based on calculation of body-mass index (BMI), where BMI = wt (kg)/ht (m2); a BMI >30 was considered to be indicative of obesity (Cole 1991). All affected males have normal secondary sexual characteristics. Testicular volumes were either measured by use of an orchidometer or based on length and width measurements by use of a standard formula (Nielson et al. 1982). Volumes >34 ml were considered to be indicative of macroorchidism. Clinical features of the affected males are summarized in table 1. In addition, individuals IV-4 and IV-6 had cataracts develop during the 4th decade. Individual III-2 had cataracts diagnosed at age 55 years. There is no history of seizures or of progressive neurological deterioration.
Table 1.
Clinical Features of Affected Males
|
Individual |
|||||||
| Feature | III-2 | IV-3 | IV-4 | IV-5 | IV-6 | V-3 | V-4 |
| Age (years) | 71 | 50 | 46 | 44 | 39 | 35 | 21 |
| BMIa | 42 | 38 | 40 | 31 | 40 | 46 | 34 |
| Height (percentile) | <3d | 10th–25th | 3d | <3d | 10th | 90th | 50th |
| OFCb (percentile) | 10th | 50th | 25th | 3d | 25th | 25th | 3d |
| Coarse facies | + | + | + | + | + | + | + |
| Puffy eyelids | + | + | + | + | + | + | + |
| Narrow PFsc | + | + | + | + | + | + | + |
| Prominent supraorbital ridge | + | + | + | + | + | + | + |
| Bulbous nose | + | + | + | + | + | + | + |
| Prominent lower lip | + | + | + | + | + | + | + |
| Large ears | + | + | + | + | + | + | + |
| Large testes | + | + | + | + | +d | + | + |
BMI >30 is indicative of obesity.
OFC = occipitofrontal circumference.
PFs = palpebral fissures.
Right testis undescended.
Formal psychological testing was performed on individuals IV-3 and IV-5; their IQs are assumed to be representative of those of the other affected males, since all are similarly affected. The full-scale IQs were 55 and 52, respectively, on the Wechsler Adult Intelligence Scale. On the Vineland Adaptive Behavior Scales, the composite scores were 27 and 28, respectively, with both scores being close to the age equivalent of 4 years 9 mo. Thus, on the basis of the IQ and adaptive (self-help) scores, both individuals are regarded as being moderately mentally retarded. Individual IV-3 holds a job as a sweeper, but none of the others are able to work. They can care for themselves and can do chores at home.
Unaffected males in the family (see figs. 1 and 3) share no resemblance to their affected male relatives. All three living unaffected males who are at risk (individuals IV-1, IV-7, and IV-10; fig. 1) have received a high-school education and hold jobs. Obligate-carrier females have no abnormal physical findings. Individual III-1, who is an obligate carrier, is reported to have had learning difficulties in school but has been able to cope well with daily-living skills and has raised a large family.
Figure 3.

Photo of unaffected male (individual IV-10), illustrating lack of resemblance to the affected males.
A blood-lymphocyte karyotype and DNA testing for fragile-X syndrome were performed on individual IV-3; the results of both tests were normal. A separate cytogenetic study was performed to look for a fragile X, because of the possibility of fragile X syndrome E–MR; the findings were normal. Complete skeletal radiographs, performed on individual IV-3, did not show any abnormalities. A blood smear for hemoglobin H staining, done on individual IV-4, did not show any inclusions. Thyroid-function tests, which were performed on individual IV-3 because of the individual's coarse facial features, were normal. No abnormalities were evident on magnetic-resonance imaging of the head of individual IV-3.
Cephalometric Analyses
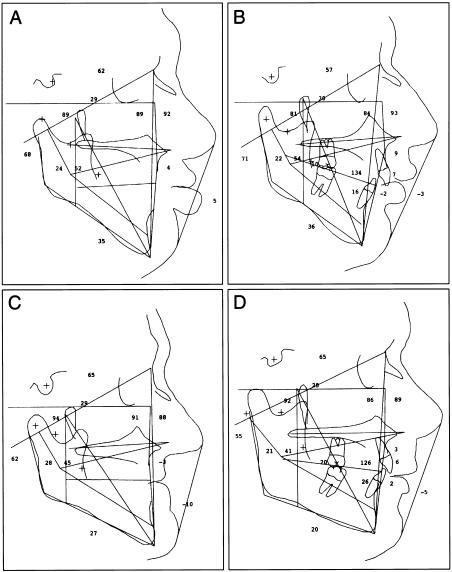
Standard lateral cephalography was performed on individuals IV-3, IV-5, IV-10, and III-1, with use of a B. F. Wehmer cephalostat. The radiographs were digitized and analyzed, by use of the Quick Ceph II computer program (©1986–91, Dr. Gunther Blaseio), and subjected to Steiner analysis (Steiner 1953; Riolo 1974). The affected males showed lip protrusion and a vertical mandibular angle, despite being edentulous. In contrast, the normal male (individual IV-10) showed no lip protrusion and had a horizontal or flat mandibular-plane angle. The results of the same analysis, done on the carrier female (individual III-1), showed the typical pattern seen in an edentulous individual, with collapse of the lips into the face. The angle of the mandible was intermediate, lying between the measurements for the affected males and that for the unaffected male (figure 4). The anterior cranial base in the affected males and the carrier female was 10% shorter than in the normal male.
Figure 4.
Composite picture of cephalometric measurements in affected males IV-3 and IV-5 (A and B, respectively), showing vertical mandibular angle, in comparison with that in unaffected male IV-10 (D). The mandibular angle in carrier female III-1 (C) is between that of the affected males and that of the unaffected male. Note the lip protrusion in the affected males.
DNA and Linkage Analyses
Fifteen family members consented to provide specimens for DNA analysis (fig. 1). Peripheral blood was collected into acid citrate–buffered Vacutainer tubes and, by means of the CytoSoft brush kit (CP-50; Medical Packaging Company), onto buccal swabs. Genomic DNA was extracted from peripheral blood, by use of the QIAmp blood kit (Qiagen). DNA was extracted from the buccal swab by use of 600 μl 50 nM NaOH; this was followed by vortexing and by 5 min in a 95°C water bath. The swab was discarded, and 60 μl Tris, 1 M: pH 8.0 was added. The solution was decanted and was stored at −70°C until use.
Linkage analysis of chromosome X was performed with the use of Weber, version 8A, high-density markers (Research Genetics) (table 2), by use of standard techniques for PCR amplification, with γ[32P] radioactively labeled oligonucleotide primers, as described elsewhere (Weissenbach et al. 1992). After identification of a linkage relationship to DXS1001–DXS1227, a high-density array of 10 short-tandem-repeat polymorphism (STRP) markers, selected from The Cooperative Human Linkage Center chromosome X integrated marker map, version v8c7, was evaluated for linkage, to refine the candidate interval. After PCR amplification, individual samples were separated on a 6% PAGE 7-M urea gel (30/1,500 [w/v]). An M13 sequencing ladder (Sequenase kit; USB) was loaded onto each gel, to permit sizing of individual alleles. After electrophoresis, gels were wrapped in cellophane, were exposed in a phosphorimaging cassette, and were scanned (Molecular Dynamics). Alleles were scored, and genotype data were entered into the pedigree file of the LINKAGE computer package (Lathrop et al. 1984).
Table 2.
Summary of High-Density Chromosome X Screening Markers
| Marker | Distance from Xp Telomere (cM) | Meiotic Recombination | Recombinant Individual(s)a |
| DXS9900 | 0 | Detected | IV-3–IV-5, IV-7 |
| DXS9895 | 9 | Detected | IV-2, IV-4, IV-5, IV-7 |
| DXS9902 | 22 | Uninformative | |
| DXS9896 | 40 | Detected | IV-2, IV-4, IV-5 |
| DSX6810 | 64 | Uninformative | |
| GATA144D04F | 71 | Detected | IV-2, IV-5 |
| DXS7132 | 83 | Detected | IV-2, IV-5 |
| DXS6800 | 93 | Detected | IV-2, IV-5 |
| DXS6789 | 104 | Uninformative | |
| DXS6797 | 113 | Detected | IV-4 |
| GATA172DO5F | 116 | Detected | IV-4 |
| GATA165B12F | 133 | Uninformative | |
| DXS1047 | 143 | Not Detected | None |
| GATA31E08 | 154 | Detected | IV-3, IV-5 |
| DXS1227 | 159 | Detected | IV-3, IV-5 |
| DXS7127 | 165 | Detected | IV-3, IV-5 |
| DXYS154 | 184 | Uninformative |
Individuals of generation V were not tested with the screening markers.
LOD scores were generated, with the assumption of X-linked recessive inheritance with complete penetrance in males. Carrier females were assumed to be clinically unaffected. The affected-allele frequency was .0001. Marker-allele frequencies were assumed to be distributed uniformly. Two-point linkage analysis was performed by use of MLINK, version 5.1, from the LINKAGE computer program (Lathrop et al. 1984). Haplotype analysis was used both for error elimination during linkage scan and for the determination of the critical linkage segment. Haplotype construction was performed by means of the CRIMAP program with the CHROMPIC option (Lander and Green 1987).
Results
Two-point linkage analysis of the entire X chromosome either failed to support or rejected the linkage hypothesis for all STRP loci tested, except for those in the interval flanked by GATA172DO5F and GATA31E08, a genetic interval of 38 cM (table 2). The seven affected males all have alleles for these STRP markers—a finding that makes it unlikely that a submicroscopic deletion could be responsible for the phenotype. Linkage analysis of a high-density panel of STRP loci spanning this interval permitted us to refine the candidate interval to ∼18 cM, flanked by ATA59C05 and GATA31E08 (fig. 1). This genetic interval contains loci for ⩾10 X-linked MR conditions, and it also contains a number of potential candidate genes for XLMR (Yntema et al. 1998).
Discussion
XLMR conditions are subdivided into two categories: syndromic and nonspecific (Kerr et al. 1991; Gedeon et al. 1994). In syndromic XLMR, affected males exhibit characteristic phenotypic or neurological manifestations that result in a unique and recognizable condition. In contrast, in nonspecific XLMR, there is no consistent phenotypic manifestation other than MR. Advances in mapping of both syndromic and nonspecific forms of XLMR (Lubs et al. 1996a, 1996b) have helped to increase our understanding of XLMR. Although most syndromic forms of XLMR are rare (Gedeon et al. 1994), clinical reports and mapping studies enable clinicians to recognize and to accurately counsel other families. We report a hitherto undescribed form of recessive XLMR with a distinctive phenotype that conforms to a recognizable syndrome.
The salient clinical features in the affected males (fig. 2) are MR, coarse facies, puffy eyelids with narrow palpebral fissures, prominent supraorbital ridges, a prominent lower lip, large ears, and large testes. As is shown in figure 3, these features are not seen in unaffected male relatives. Although all the affected males are obese, it is possible that the obesity could be either exogenous or a family trait unrelated to the MR, since several other members of the family are obese, including carrier females and an unaffected male (individual IV-10). Onset of obesity in the affected males has been variable, with a range from early childhood to the 3d decade. Since macroorchidism can be an inherited trait independent of MR, we tried to obtain testicular measurements of the unaffected males; however, we were unsuccessful because of reasons of embarrassment and modesty. However, none of the unaffected males stated that they have large testicles. Early onset of cataracts (i.e., during the 4th decade) was seen in individuals IV-4 and IV-6 but was not seen in the other affected males, which makes it difficult to definitively link this to the MR. Obligate-carrier females show no phenotypic abnormalities, but learning difficulties reportedly have been experienced by some of them. The results of such investigations as blood-lymphocyte karyotyping, DNA analysis for fragile-X syndrome, skeletal radiography, and magnetic-resonance imaging of the head were normal. The results of cephalometric analysis of affected males, an unaffected male, and an obligate-carrier female showed a distinctive craniofacial profile in the affected males. The carrier female had mild abnormalities.
We generated two lists of differential diagnoses; one was based on the clinical characteristics, and the other was based on the results of the linkage studies. Given the clinical features identified in the affected males, several well-known XLMR syndromes were considered in the differential diagnosis (table 3). Coffin-Lowry syndrome (CLS) (Coffin et al. 1966; Lowry et al. 1971; Young 1988) is characterized by MR, a coarse facial appearance, hypertelorism, a bulbous nose, and protuberant lips. Although our patients have some of these features, their facial appearance is distinctive, and they do not have the characteristic tapering of the fingers or skeletal abnormalities (the tufted-drumstick appearance of the distal phalanges) seen in CLS. CLS has been mapped to chromosome Xp22 (Hanauer et al. 1988), with mutations within the ribosomal protein S6 kinase 2 gene (RSK2) being causative (Trivier et al. 1996). Our studies established linkage to Xq26-q27—a finding that suggests that this syndrome is not CLS. Our patients do have some features—such as MR, coarse facies, prominent supraorbital ridges, large ears, and obesity—that are seen in Börjeson-Forssman-Lehmann syndrome (BFLS [MIM 301900]); however, they do not have microcephaly, hypogonadism with gynecomastia, and/or poor sexual development, which are seen in all males with BFLS (Börjeson et al. 1962; Weber et al. 1978; Robinson et al. 1983; Dereymaeker et al. 1986; Turner et al. 1989). Seizures, reported in ⩾50% of individuals with BFLS, are not a feature in our patients. In addition, other common features, such as short stature, nystagmus, and radiographic changes (e.g., steep radiocarpal angle and small femoral and humeral heads) (Robinson et al. 1983), are absent in our patients. Although the gene for BFLS has not yet been identified (Gedeon et al. 1996), it has been mapped to Xq26-q27 (fig. 5) (Mathews et al. 1989; Turner et al. 1989). The candidate gene SOX3, an SRY-like gene in this region, was found to have no mutations in patients with BFLS (Gedeon et al. 1996). The candidate interval for BFLS overlaps with our area of linkage, but the differences in clinical features suggest that these are distinctive conditions. Males with Simpson-Golabi-Behmel syndrome (SGBS [MIM 312879]) have MR, overgrowth (adult height >97th percentile), macrocephaly, upward-slanting palpebral fissures, an upturned nose, a prominent chin, large dysplastic kidneys, postaxial polydactyly of the hands, cardiac conduction defects, and typical radiological manifestations (e.g., flared iliac wings and narrow sacroiliac notches) (Simpson et al. 1975; Golabi and Rosen 1984: Behmel et al. 1988; Hughes Benzie et al. 1992; Chen et al. 1993; Terespolsky et. al. 1995). None of these features are seen in our patients. In addition, our patients have features not seen in SGBS (table 3). Mutations within the glypican 3 (GPC3) gene, which is localized to Xq26, are responsible for SGBS (Pilia et al. 1996). Although GPC3 is contained within our candidate region, the phenotypic differences suggest that our patients have another distinct condition. X-linked alpha-thalassemia MR syndrome (ATR-X [MIM 300032]) (Gibbons et al. 1991, 1995) is unlikely in this family, since none of the affected males are anemic and since the results of HbH staining in individual IV-4 were normal. In addition, ATR-X is caused by mutations in the X-linked helicase-2 (XH2) gene localized to Xq13, whereas our studies show linkage to Xq26-q27. An XLMR syndrome described elsewhere (Atkin et al. 1985) was included in the differential diagnosis. Our patients could be distinguished from those in the study by Atkin et al., on the basis of absence of macrocephaly, hypertelorism, short stature, broad and short hands, diastema, and microdontia, all of which are features seen in Atkin et al.’s report (table 3). Clark and Baraitser (1987) described XLMR with macrocephaly, obesity, and macroorchidism, and, thereafter, additional cases were reported elsewhere (Baraitser et al. 1995; de Pina-Neto and de Molfetta 1998). The clinical features of this condition, which are listed in table 3, are quite distinct from those of the patients in the present study, although there are some similarities. Ultimately, the nosology of XLMR will be established when the individual causative genes are identified; however, after careful comparison of the clinical features in this family with those of the XLMR conditions described above, we are confident that our patients do not conform to other well-known syndromic forms of X-linked MR.
Table 3.
Clinical Manifestations of Some Well-Known X-Linked MR Syndromes, Compared with Those Seen in the Present Study
|
Findings in the Present Study and Characteristics of X-Linked MR Syndromes |
||||||
| Clinical Manifestation | Present Report | BFLS | Coffin-Lowry | Simpson-Golabi | Atkin | Baraitser |
| MR | Moderate | Severe | Severe | Variable | Moderate-severe | Moderate |
| Head size | Normal | Microcephalic | Microcephalic/normal | Macrocephalic | Macrocephalic | Macrocephalic |
| Hypertelorism | Absent | Absent | Present | Present | Present | Absent |
| Diastema | Absent | Absent | Absent | Absent | Present | Present |
| Narrow eyes | Present | Absent | Absent | Absent | Absent | Absent |
| Supraorbital ridges | Prominent | Prominent | Prominent | Not prominent | Prominent | Prominent |
| Thick lower lip | Present | Absent | Present | Variable | Present | Present |
| Large ears | Present | Present | Absent | Absent | Present | Present |
| Obesity | Present | Present | Absent | Absent | Present | Present |
| Heart defect | Absent | Absent | Present | Present | Absent | Absent |
| Testes | Large | Undescended/small | Undescended/small | Undescended/small | Large | Large |
| Stature | Normal | Short | Short | Normal to tall | Short | Normal |
| Skeletal radiographs | Normal | Abnormal | Abnormal | Abnormal | Normal | Not available |
| Mapping | Xq26 | Xq26-q27 | Xp22.1-p22.2 | Xq26 | Unknown | Unknown |
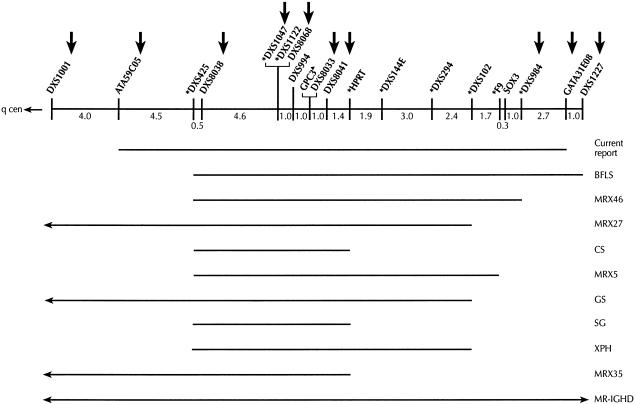
Figure 5.
Schematic localization of a unique XLMR syndrome, as implied by the current report, in relation to other reported XLMR conditions in close proximity. Vertical arrows indicate DNA markers tested in the present study. Asterisks (*) indicate markers that have previously been linked to other XLMR conditions. Mutations within the GPC3 gene have been found in SBGS. CS = Cowchock syndrome, GS = Gustavson syndrome, SG = SGBS , XPH = X-linked recessive panhypopituitarism, MR-IGHD = mental retardation with isolated growth hormone deficiency, and MRX = nonspecific XLMR.
The mapping studies in this family support linkage to an 18-cM genetic interval, flanked by ATA59C05 and GATA31E08 (maximum LOD score = 3.1 at θ=0, at DXS1047), on Xq26-q27. This interval is 20 Mb. On the basis of this genetic localization, we considered other XLMR syndromes in close proximity (fig. 5). Gustavson syndrome is characterized by microcephaly, severe MR, optic atrophy with visual impairment, hearing loss, spasticity, seizures, and restricted joint mobility (Gustavson et al. 1993; Malgrem et al. 1993). These features are clearly different from those seen in the family that we studied. Hereditary motor-sensory neuropathy, deafness, and MR occur in Cowchock syndrome (Cowchock et al. 1985), which has been mapped between DXS425 and HPRT. In addition to having features not seen in Cowchock syndrome, our patients do not have neuropathy and deafness, which are seen in Cowchock syndrome. Lesch-Nyhan syndrome, a disorder of purine metabolism (MIM 308000), is caused by mutations in the hypoxanthine phosphoribosyltransferase (HPRT) gene on chromosome Xq26 (Bland JH 1968; Davidson et al. 1991; Huang et al. 1991). The clinical manifestations (except for MR)—consisting of choreoathetosis with onset in infancy, loss of milestones, self-injurious behavior later in childhood, MR, and gouty symptoms—are undoubtedly different from those found in the present study. A few nonspecific X-linked MR conditions have been mapped to this interval, as can be seen in figure 5 (Yntema et al. 1998). The family that we studied has, along with MR, a unique phenotype that distinguishes this entity from nonspecific XLMR.
In conclusion, we have described a new form of syndromic XLMR that maps to chromosome Xq26-q27. As part of the differential diagnoses, we have considered conditions with a similar phenotype and, also, those XLMR conditions that have been mapped in close proximity. We did not find the features in this family to be consistent with those seen in any of the previously delineated forms of XLMR syndromes. Although reports such as this one describe a single family, they permit clinicians to recognize similar families. This could lead to better understanding of the condition and could also enable identification of the gene, permit understanding of the pathogenesis, help in molecular diagnostics, and enable clinicians to provide more-accurate genetic counseling for families.
Acknowledgment
The authors are deeply grateful to Dr. William Wilson, University of Virginia, for his thoughtful comments and ideas.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Cooperative Human Linkage Center, The http://www.chlc.org/ (for chromosome X integrated marker map, version v8c7)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for BFLS [MIM 301900], SGBS [MIM 312879], ATR-X [MIM 300032], and Lesch-Nyhan syndrome [MIM 308000])
References
- Atkin JF, Flaitz K, Patil S, Smith W (1985) A new X-linked mental retardation syndrome. Am J Med Genet 21:697–705 [DOI] [PubMed]
- Baraitser M, Reardon W, Vijeratnam S (1995) Nonspecific X-linked mental retardation with macrocephaly and obesity: a further family. Am J Med Genet 57:380–384 [DOI] [PubMed]
- Behmel A, Plochl E, Rosenkranz W (1988) A new X-linked dysplasia gigantism syndrome: follow-up in the first family and report on a second Austrian family. Am J Med Genet 30:275–285 [DOI] [PubMed]
- Bland JH (1968) Proceedings of seminars on the Lesch-Nyhan syndrome. Fed Proc 27:1017–1112 [Google Scholar]
- Börjeson M, Forssman H, Lehmann O (1962) An X-linked recessively inherited syndrome characterized by grave mental deficiency, epilepsy and endocrine disorder. Acta Med Scand 171:13–21 [DOI] [PubMed] [Google Scholar]
- Chen E, Johnson JP, Cox VA, Golabi M (1993) Simpson-Golabi-Behmel syndrome: congenital diaphragmatic hernia and radiologic findings in two patients and follow-up of a previously reported case. Am J Med Genet 46:574–578 [DOI] [PubMed]
- Clark RD, Baraitser M (1987) A new X-linked mental retardation syndrome. Am J Med Genet 26:13–15 [DOI] [PubMed]
- Coffin GS, Siris E, Wegienka LC (1966) Mental retardation with osteocartilaginous anomalies. Am J Dis Child 112:205–213 [Google Scholar]
- Cole TJ (1991) Weight-stature indices to measure underweight, overweight and obesity. In: Himes JH (ed) Anthropometric assessment of nutritional status. Wiley & Sons, New York, pp 83–112 [Google Scholar]
- Cowchock FS, Duckett SW, Streletz LJ, Graziani LJ, Jackson LG (1985) X-linked motor-sensory neuropathy type-II with deafness and mental retardation: a new disorder. Am J Med Genet 20:307–315 [DOI] [PubMed]
- Davidson BL, Tarle SA, Van Antwerp M, Gibbs DA, Watts RW, Kelley WN, Palella TD (1991) Identification of 17 independent mutations responsible for human hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency. Am J Hum Genet 48:951–958 [PMC free article] [PubMed]
- de Pina-Neto JM, de Molfetta GA (1998) The X-linked mental retardation, macrosomia, macrocephaly and obesity syndrome (Baraitser syndrome): a Brazilian case. Clin Dysmorphol 7:233–234 [DOI] [PubMed]
- Dereymaeker AM, Fryns JP, Hoefanagels M, Heremans G, Marien J, van den Berghe H (1986) The Börjeson-Forssman-Lehmann syndrome: a family study. Clin Genet 29:317–320 [PubMed]
- Gedeon AK, Donnelly AJ, Mulley JC, Kerr B, Turner G (1996) How many X-linked genes for nonspecific MR (MRX) are there? Am J Med Genet 64:158–162 [DOI] [PubMed]
- Gedeon AK, Kozman HM, Robinson H, Pilia G, Schlessinger D, Turner G, Mulley JC (1996) Refinement of the background genetic map of Xq26-q27 and gene localisation for Börjeson-Forssman-Lehmann syndrome. Am J Med Genet 64:63–68 [DOI] [PubMed]
- Gibbons RJ, Picketts DJ, Villard L, Higgs DR (1995) Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome). Cell 80:837–845 [DOI] [PubMed]
- Gibbons RJ, Wilkie AO, Weatherall DJ, Higgs DR (1991) A newly defined X-linked mental retardation syndrome associated with alpha thalassemia. J Med Genet 28:729–733 [DOI] [PMC free article] [PubMed]
- Golabi M, Rosen L (1984) A new X-linked mental retardation-overgrowth syndrome. Am J Med Genet 17:345–358 [DOI] [PubMed]
- Gustavson KH, Anneren G, Malmgren H, Dahl N, Ljunggren CG, Backman H (1993) A new X-linked syndrome with severe mental retardation, severely impaired vision, severe hearing defect, epileptic seizure, spasticity, restricted joint motility, and early death. Am J Med Genet 45:654–658 [DOI] [PubMed]
- Hanauer A, Alembik Y, Gilgenkrantz S, Mujica P, Nivelon-Chevallier A, Pembrey ME, Young ID, et al (1988) Probable localisation of the Coffin-Lowry locus (CLS) in Xp22.2-p22.1 by multipoint linkage analysis. Am J Med Genet 30:523–530 [DOI] [PubMed]
- Huang THM, Hejtmancik JF, Edwards A, Pettigrew AL, Herrera CA, Hammond HA, Caskey CT, et al (1991) Linkage of the gene for an X-linked mental retardation disorder to a hypervariable (AGAT)n repeat motif within the human hypoxanthine phosphoribosyltransferase (HPRT) locus (Xq26). Am J Hum Genet 49:1312–1319 [PMC free article] [PubMed]
- Hughes-Benzie RM, Hunter AGW, Allanson JE, Mackenzie AE (1992) Simpson-Golabi-Behmel syndrome associated with renal dysplasia and embryonal tumor: localization of the gene to Xqcen-q21. Am J Med Genet 43:428–435 [DOI] [PubMed]
- Kerr B, Turner G, Mulley J, Gedeon A, Partington M (1991) Non-specific X linked mental retardation. J Med Genet 7:378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Green P (1987) Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci USA 84:2363–2367 [DOI] [PMC free article] [PubMed]
- Lathrop GM, Lalouel JM (1984) Easy calculations of LOD scores and genetic risks on small computers. Am J Hum Genet 6:460–465 [PMC free article] [PubMed]
- Lehrke RG (1972) A theory of X-linkage of major intellectual traits. Am J Ment Defic 76:611–619 [PubMed]
- Lowry RB, Miller JR, Fraser FC (1971) A new dominant gene mental retardation syndrome: association with small stature, tapering fingers, characteristic facies and possible hydrocephalus. Am J Dis Child 121:496–500 [PubMed]
- Lubs HA, Chiurazzi P, Arena JF, Schwartz C, Tranebjaerg L, Neri G (1996a) XLMR genes: update 1996. Am J Med Genet 64:147–157 [DOI] [PubMed]
- Lubs HA, Schwartz CE, Stevenson RE, Arena JF (1996b) Study of X-linked mental retardation (XLMR): summary of 61 families in the Miami/Greenwood study. Am J Med Genet 64:169–175 [DOI] [PubMed]
- Malgrem H, Sundvall M, Dahl N, Gustavson KH, Anneren G, Wadelius C, Steen-Bondeson ML, et al (1993) Linkage mapping of a severe X-linked mental retardation syndrome. Am J Hum Genet 52:1046–1052 [PMC free article] [PubMed]
- Mathews KD, Ardinger HH, Nishimura DY, Buetow KH, Murray JC, Bartley JA (1989) Linkage localization of Börjeson-Forssman-Lehmann syndrome. Am J Med Genet 34:470–474 [DOI] [PubMed]
- Nielson KB, Tommerup N, Dyggve HV, Schou C (1982) Macroorchidism and fragile X in mentally retarded males: clinical, cytogenetic, and some hormonal investigations in mentally retarded males, including two with the fragile site at Xq28,fra(X)(q28). Hum Genet 61:113–117 [DOI] [PubMed]
- Opitz JM (1986) On the gates of hell and a most unusual gene. Am J Med Genet 23:1–10 [DOI] [PubMed]
- Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, et al (1996) Mutations in GPC3, a glypican gene cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 12:241–247 [DOI] [PubMed]
- Riolo ML, Moyers RE, McNamara JA Jr, Hunter WS (1934) Craniofacial growth series. Monograph 2: An atlas of craniofacial growth. University of Michigan Center for Human Growth and Development, Ann Arbor [Google Scholar]
- Robinson LK, Jones KL, Culler F, Nyhan WL, Sakati N, Jones KL (1983) The Börjeson-Forssman Lehmann syndrome. Am J Med Genet 15:457–468 [DOI] [PubMed]
- Simpson JL, Landey S, New M, German J (1975) A previously unrecognized X-linked syndrome of dysmorphia. Birth Defects 11:18–24 [PubMed]
- Steiner CC (1953) Cephalometrics for you and me. Am J Orthod 39:729–755 [Google Scholar]
- Terespolsky D, Farrell SA, Siegel-Bartelt J, Weksberg R (1995) Infantile lethal variant of Simpson-Golabi-Behmel syndrome associated with hydrops fetalis. Am J Med Genet 59:329–333 [DOI] [PubMed]
- Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, Mandel JL, et al (1996) Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 384:567–570 [DOI] [PubMed]
- Turner G (1982) X-linked mental retardation. Psychol Med 12:471–473 [DOI] [PubMed]
- Turner G, Gedeon A, Mulley J, Sutherland G, Rae J, Power K, Arthur I (1989) Börjeson-Forssman-Lehmann syndrome: clinical manifestations and gene localization to Xq26-27. Am J Med Genet 34:463–469 [DOI] [PubMed]
- Turner G, Turner B, Collins E (1970) Renpenning’s syndrome-X linked mental retardation. Lancet 2:365–366 [DOI] [PubMed]
- Weber FT, Frias JL, Julius RL, Felman AH (1978) Primary hypogonadism in the Börjeson-Forssman-Lehmann syndrome. J Med Genet 15:63–66 [DOI] [PMC free article] [PubMed]
- Weissenbach J, Gyapay G, Dib C, Vignal A, Morissette J, Millasseau P, Vayseix G, et al (1992) A second-generation linkage map of the human genome. Nature 359:794–801 [DOI] [PubMed]
- Yntema HG, Hamel BCJ, Smits AP, van Roosmalen T, van den Helm B, Kremer H, Ropers HH, et al (1998) Localization of a gene for non-specific X-linked mental retardation (MRX46) to Xq25-26. J Med Genet 35:801–805 [DOI] [PMC free article] [PubMed]
- Young ID (1988) The Coffin-Lowry syndrome. J Med Genet 25:344–348 [DOI] [PMC free article] [PubMed]