Summary
Pancentromeric FISH and X-chromosome painting were used to characterize anaphase aberrations in 2,048 cultured lymphocytes from a healthy 62-year-old woman. Of 163 aberrant anaphases, 66.9% contained either chromosomes or their fragments that lagged behind. Characterization of 200 laggards showed that 49% were autosomes, 33.5% were autosomal fragments, and 17.5% were X chromosomes. The X chromosome represented one-fourth of all lagging chromosomes and was involved much more often than would be expected by chance (1/23). Labeling of the late-replicating inactive X chromosome with 5-bromo-2′-deoxyuridine revealed that both X homologues contributed equally to the laggards. Among 200 micronuclei examined from interphase cells, the proportion of the X chromosome (31%) and autosomal fragments (50%) was higher than among anaphase laggards, whereas autosomes were involved less often (19%). These findings may reflect either selection or the fact that lagging autosomes, which were more proximal to the poles than were lagging X chromosomes, were more frequently included within the main nucleus. Our results suggest that the well-known high micronucleation and loss of the X chromosome in women's lymphocytes is the result of frequent distal lagging behind in anaphase and effective micronucleation of this chromosome. This lagging appears to affect the inactive and active X chromosomes equally.
Aneuploid lymphocytes are increased with age. In women, this effect mainly concerns the loss of the X chromosome (Jacobs et al. 1961; Fitzgerald and McEwan 1977; Richard et al. 1993). The X chromosome is highly overrepresented in the micronuclei of female lymphocytes, and the age-dependent increase of micronuclei in women is due mainly to X-chromosome–positive micronuclei (Guttenbach et al. 1994; Hando et al. 1994; Richard et al. 1994; Catalán et al. 1995; Surrallés et al. 1996a). This is interesting, since the formation of micronuclei is probably an important mechanism leading to chromosome loss (Ford et al. 1988). The only X chromosome in men is also micronucleated in excess, but this occurs at a lower rate than is seen in females, and this difference is not explained by the fact that women have two X chromosomes (Catalán et al. 1998). Thus, it has been suggested that the inactive X chromosome is preferentially affected by micronucleation (Tucker et al. 1996), although another group (Surrallés et al. 1996b) did not find any clear difference in micronucleus formation between the homologues. Since lagging behind in anaphase is considered to be a major source of micronuclei (Ford and Correll 1992; Maney et al. 1998), inspection of anaphases could provide further information about the mechanisms of X-chromosome loss and the possible involvement of the inactive X chromosome. This question has not previously been studied, since the rarity of anaphases in specimens of cultured human lymphocytes makes it very difficult to find a sufficient number of aberrant anaphases for examination.
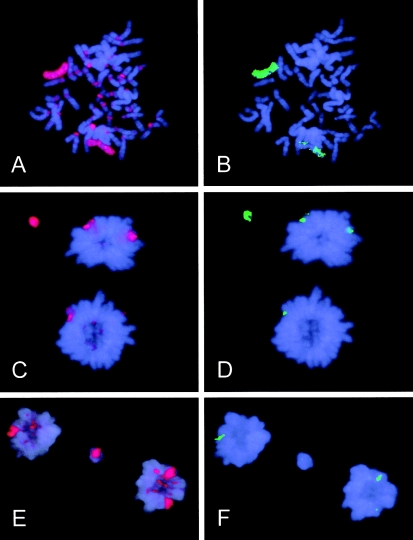
In the present study, we examined >2,000 anaphase-telophase–stage cultured lymphocytes from a 62-year-old healthy woman. Mononuclear leukocytes were isolated and were cultured, in 5-ml cultures at an initial cell density of 1.5 × 106, with the use of phytohemagglutinin but without use of cytochalasin B for 72 hours (see Norppa et al. 1993). We used a pulse treatment with 5-bromo-2′-deoxyuridine (BrdU) for the identification of the late-replicating inactive X chromosome (fig. 1). BrdU (10 μg/ml) was added 7 h before harvest. Anaphase-telophase cells were collected by means of a modification (Lindholm et al. 1991) of the technique of Ford and Congedi (1987). A three-day FISH procedure was simultaneously performed with a biotin-labeled X-chromosome–painting probe (1066-XB; Cambio) and with a biotinylated pancentromeric probe (1141-B; Cambio), according to the manufacturer's instructions. In this way, we were able to distinguish whether the aberration consisted of the X chromosome (painting signal), autosomes (pancentromeric signal), or autosomal fragments (no signals) (fig. 1). The probes were detected and were amplified with the use of rhodamine-conjugated antibodies. The hybridization efficiency of the probes was ascertained by examination of metaphase spreads (see fig. 1A). BrdU-labeled DNA was detected by use of fluorescein isothiocyanate–conjugated antibodies. DNA was stained with 4′, 6-diamidino-2-phenylindole (DAPI). The frequency of aberrant anaphases was evaluated from 2,048 anaphases. A total of 163 aberrant anaphases were characterized for the involvement of the X chromosome and centromeric signals. The scoring of laggards was continued until a total of 200 laggards had been characterized. Fluorescence-microscopy images of all aberrant anaphases were stored in an isis3 in situ imaging system (Metasystems), which allowed us to ascertain the nature (chromatid or chromosome) of each laggard and to measure the distances both between the poles and between each laggard and the nearest pole. In micronuclei analysis, FISH was performed separately for both DNA probes, and BrdU was not detected. In this way, 200 micronuclei were separately characterized for the presence of the X chromosome and centromeres in interphase cells, to assess whether the occurrence of the X chromosome, autosomes, and autosomal fragments in micronuclei corresponded with their involvement in aberrations observed in the preceding anaphase.
Figure 1.
Characterization of anaphase/telophase laggards in female lymphocytes. A, Metaphase in which the X chromosome and centromeres are identified, by means of FISH (red), and (B) the late-replicating inactive X chromosome (uniform green label) is distinguished from the active X chromosome (discontinuous green label), by means of BrdU labeling. C, Anaphase with a lagging X chromatid (red) that represents the inactive X chromosome (D) (green). E, Anaphase with a lagging X chromatid (red) that represents the active X chromosome (F).
Mitotic cells constituted 2% of all cells, and the frequency of anaphases was only .16%; 7.96% (163/2,048) of the anaphases were aberrant (table 1). To accumulate >200 aberrant anaphases, we had to go through >1.5 million cells. Most of the aberrations were laggards (70.6% [66.9% of aberrant anaphases]), in agreement with the findings of earlier studies (Lindholm et al. 1991; Ford and Correll 1992). The rest of the aberrations consisted of autosomal bridges, 19% of which had stretched and broken. One-fourth of the aberrant anaphases (2% of all anaphases) contained two or more aberrations, 76% of which were exclusively laggards (table 1). Such a high proportion of multiple events suggests a common origin for aberrations encountered in a multiaberrant cell.
Table 1.
Aberrations in 2,048 Anaphases in Lymphocytes from a 62-Year-Old Woman
|
Aberrant Anaphases |
||
| Aberration Type and No. | No. | % |
| Laggards: | ||
| One | 74 | 3.61 |
| Two | 20 | .98 |
| Three | 8 | .39 |
| Four | 3 | .15 |
| Total | 105a | 5.13 |
| Bridges: | ||
| One | 48 | 2.34 |
| Two | 6 | .29 |
| Total | 54b | 2.64e |
| One laggard, one bridge: | 4c | .19 |
| Grand Total | 163d | 7.96 |
No. of individual aberrations = 150.
No. of individual aberrations = 60.
No. of individual aberrations = 8.
No. of individual aberrations = 218.
Total = rounded sum of exact figures for bridges one and two.
A closer characterization of 200 laggards (table 2) showed that half (49%) were autosomes, whereas acentric autosomal fragments and the X chromosome were responsible for 33.5% and 17.5% of laggards, respectively. The X chromosome represented one-fourth (35/133) of all lagging chromosomes and was clearly involved more often than was expected by chance (1/23); this indicates that the X chromosome is more prone to be lost than are the autosomes. Both X homologues contributed equally to the X laggards. Although based on a small number of laggards, this finding supports the hypothesis of similar micronucleation of the inactive and active X chromosomes of elderly women (Surrallés et al. 1996b). Five aberrant anaphases showed lagging of both X chromosomes—a phenomenon that was noted elsewhere (Ford and Correll 1992). Thus, if there is a higher loss of the inactive X chromosome (Abruzzo et al. 1985; Tucker et al. 1996), then the process does not seem to be the result of its preferential lagging in anaphase.
Table 2.
Characterization of Anaphase Laggards and Micronuclei in Lymphocytes from a 62-Year-Old Woman
| Characterization | No. (%) of Anaphase Laggards (n=200) | No. (%) of Micronuclei(n=200)a |
| Fragment | 67 (33.5%) | 100 (50.0%) |
| Autosome | 98 (49.0%) | 38 (19.0%) |
| X chromosome | 35 (17.5%) | 62 (31.0%) |
| Active | 15 | |
| Inactive | 17 | |
| Activity status uncertain | 3b |
No. characterized per probe.
Since the cultures were not synchronized (to avoid chemical interference with normal chromosome segregation), the activity status of the X chromosome could not always be ascertained.
The results of the micronuclei analyses are shown in table 2. In comparison with laggards, there was a clear increase in the contribution of autosomal fragments (50%) and the X chromosome (31%), whereas autosomes were found in micronuclei at a lower rate (19%), with the differences being statistically significant (P<.01, χ2 test). A possible explanation is that chromosomes lagging at anaphase will be reincorporated in either of the daughter nuclei, whereas their fragments will tend to be left out and form micronuclei. However, in such a case, one could also expect a decreased incidence of contribution of the X chromosome. It may be that autosomal laggards form micronuclei at a low efficiency because they are more proximal to the daughter nuclei than are the lagging X chromosomes. In fact, the relative distance to the closer pole (the distance to the closer pole divided by distance between poles) was significantly higher for X-chromosome laggards (0.18) than for autosomal (0.13) laggards (P<.05, Fisher least-significant difference). In previous investigations, X chromosome–positive micronuclei frequently lacked a signal when labeled with antikinetochore antibodies (Hando et al. 1994). Absence of a functional centromere may delay sister-chromatid separation, which could result in lagging of the whole duplicated chromosome (Kirsch-Volders et al. 1998; Maney et al. 1998). In fact, we found a significantly higher prevalence (P<.01, by χ2 test) of laggards with both sister chromatids among lagging X chromosomes (68%) than among lagging autosomes (23%). Another explanation for the low proportion of autosomes in micronuclei may be selection against cells that have lost autosomes, which carry genes that are important for cell survival (Marshall et al. 1996). Such cells (especially if multiaberrant) could die before expression of micronuclei or during interphase. Selection against cells lacking the active X chromosome could also explain the possible preferential loss of the inactive X chromosome which is not needed for cell survival (Hando et al. 1994). Finally, breakage of bridges, which was classified separately, is also expected to increase the yield of micronuclei with fragments (Stopper 1997).
In conclusion, the well-known high micronucleation and the loss of the X chromosome in women's lymphocytes are probably the result of frequent lagging behind of the X chromosome during anaphase. This phenomenon appears to affect the inactive and active X chromosomes alike. The distal location of lagging X chromosomes in anaphase further seems to favor micronucleus formation.
Acknowledgments
We thank Dr. Jordi Surrallés, for advice on the labeling of the late-replicating X chromosome, and Ms. Hilkka Järventaus, for technical assistance. The Human Capital and Mobility Programme of the Commission of the European Communities contract ERBCHBGCT940537 supported J.C.
Electronic-Database Information
The URL for data in this article is as follows:
- Center for Molecular Genetics and Toxicology, http://www.swan.ac.uk/cget/ejgt/article4.htm (for Stopper [] reference article)
References
- Abruzzo MA, Mayer M, Jacobs PA (1985) Aging and aneuploidy: evidence for the preferential involvement of the inactive X chromosome. Cytogenet Cell Genet 39:275–278 [DOI] [PubMed]
- Catalán J, Autio K, Kuosma E, Norppa H (1998) Age-dependent inclusion of sex chromosomes in lymphocyte micronuclei of man. Am J Hum Genet 63:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán J, Autio K, Wessman M, Lindholm C, Knuutila S, Sorsa M, Norppa H (1995) Age-associated micronuclei containing centromeres and the X chromosome in lymphocytes of women. Cytogenet Cell Genet 68:11–16 [DOI] [PubMed]
- Fitzgerald PH, McEwan CM (1977) Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet 39:329–337 [DOI] [PubMed]
- Ford JH, Congedi MM (1987) Rapid induction of anaphase in competent cells by hypotonic treatment. Cytobios 51:183–192 [PubMed]
- Ford JH, Correll AT (1992) Chromosome errors at mitotic anaphase. Genome 35:702–705 [DOI] [PubMed]
- Ford JH, Schultz CJ, Correll AT (1988) Chromosome elimination in micronuclei: a common cause of hypoploidy. Am J Hum Genet 43:733–740 [PMC free article] [PubMed]
- Guttenbach M, Schakowski R, Schmid M (1994) Aneuploidy and ageing: sex chromosome exclusion into micronuclei. Hum Genet 94:295–298 [DOI] [PubMed]
- Hando JC, Nath J, Tucker JD (1994) Sex chromosomes, micronuclei and aging in women. Chromosoma 103:186–192 [DOI] [PubMed]
- Jacobs PA, Court Brown WM, Doll R (1961) Distribution of human chromosome counts in relation to age. Nature 191:1178–1180 [DOI] [PubMed] [Google Scholar]
- Kirsch-Volders M, Cundari E, Verdoodt B (1998) Towards a unifying model for the metaphase/anaphase transition. Mutagenesis 13:321–335 [DOI] [PubMed]
- Lindholm C, Norppa H, Hayashi M, Sorsa M (1991) Induction of micronuclei and anaphase aberrations by cytochalasin B in human lymphocyte cultures. Mutat Res 260:369–375 [DOI] [PubMed]
- Maney T, Hunter AW, Wagenbach M, Wordeman L (1998) Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol 142:787–801 [DOI] [PMC free article] [PubMed]
- Marshall RR, Murphy M, Kirkland DJ, Bentley KS (1996) Fluorescence in situ hybridization with centromere-specific centromeric probes: a sensitive method to detect aneuploidy. Mutat Res 372:233–245 [DOI] [PubMed]
- Norppa H, Renzi L, Lindholm C (1993) Detection of whole chromosomes in micronuclei of cytokinesis-blocked human lymphocytes by antikinetochore staining and in situ hybridization. Mutagenesis 8:519–525 [DOI] [PubMed]
- Richard F, Aurias A, Couturier J, Dutrillaux AM, Flüry-Hérard A, Gerbault-Sereau M, Hoffschir F, et al (1993) Aneuploidy in human lymphocytes: an extensive study of eight individuals of various ages. Mutat Res 295:71–80 [DOI] [PubMed]
- Richard F, Muleris M, Dutrillaux B (1994) The frequency of micronuclei with the X chromosome increases with age in human females. Mutat Res 316:1–7 [DOI] [PubMed]
- Stopper H (1997) Genotoxicity of not directly DNA damaging compounds. Eur J Genet Toxicol (see Electronic-Database Information) [Google Scholar]
- Surrallés J, Falck G, Norppa H (1996a) In vivo cytogenetic damage revealed by FISH analysis of micronuclei in uncultured human T lymphocytes. Cytogenet Cell Genet 75:151–154 [DOI] [PubMed]
- Surrallés J, Jeppesen P, Morrison H, Natarajan AT (1996b) Analysis of loss of inactive X chromosomes in interphase cells. Am J Hum Genet 59:1091–1096 [PMC free article] [PubMed]
- Tucker JD, Nath J, Hando JC (1996) Activation status of the X chromosome in human micronucleated lymphocytes. Hum Genet 97:471–475 [DOI] [PubMed]