Summary
We recently reported the absence of significant linkage of phonological coding dyslexia (PCD) to chromosome 6p23-p21.3 in 79 families with at least two affected siblings, even though linkage of dyslexia to this region has been found in four other independent studies. Whereas, in our previous analyses, we used a qualitative (affected, unaffected, or uncertain) PCD phenotype, here we report a reanalysis of linkage to the chromosome 6p region, by use of four quantitative measures of reading disability: phonological awareness, phonological coding, spelling, and rapid-automatized-naming (RAN) speed. The phonological-coding and spelling measures were highly correlated with each other and with the qualitative PCD phenotype, whereas the phonological-awareness and RAN-speed measures were only moderately correlated with the other measures. Using two-point and multipoint quantitative-trait sib-pair linkage analyses and variance-components analyses, we were unable to detect significant evidence for a locus in the 6p23-p21.3 region influencing any of the quantitative reading measures, supporting our previous qualitative linkage results. The most likely explanation for our inability to detect linkage between dyslexia and this region is that families with subtypes of dyslexia linked to this region are underrepresented in our sample, because of either chance or varying ascertainment criteria.
We recently reported an absence of significant linkage of phonological coding dyslexia (PCD) to chromosome 6p23-p21.3 (Field and Kaplan 1998), even though linkage of dyslexia to this region has been found in four other independent studies. Cardon et al. (1994) originally reported evidence for linkage between a composite measure of reading disability and a 2-cM region on chromosome 6p21.3. Grigorenko et al. (1997) reported evidence for linkage between phonological awareness—a core process involved in learning to read—and microsatellite markers that are slightly telomeric to the region suggested by Cardon et al. Recently, the Journal published two papers (Fisher et al. 1999; Gayán et al. 1999) that reported linkage of phonological and orthographic components of reading to chromosome 6p regions, consistent with the linkage findings of previous studies. In contrast, in a sample of 79 families containing at least two affected siblings, we were unable to find evidence for linkage between PCD analyzed as a qualitative (affected, unaffected, or uncertain) phenotype and markers spanning 6p25-p21.3, by use of either LOD-score or affected-sib-pair linkage methods. We were also unable to detect association (linkage disequilibrium) between PCD and markers in this region. Since three of the positive linkage studies used quantitative measures of reading disability, in contrast to our qualitative PCD phenotype, and since the fourth study analyzed single qualitative measures of component reading skills, in contrast to the composite nature of our PCD phenotype, it is possible that use of the PCD phenotype was the cause of our null findings. Here, we present a reanalysis of linkage to the 6p25-p21.3 region in our sample of 79 families, by use of quantitative-trait sib-pair linkage analyses and of variance-components analyses of four measures of reading disability. The results confirm absence of significant linkage to this region in our families.
Families with dyslexic members were ascertained primarily from Calgary-area schools for learning disabled children. All subjects were >8 years of age and gave informed consent in accordance with requirements of the University of Calgary Ethics Review Board. Subjects were given a battery of psychometric tests that assessed four components of reading: phonological awareness, phonological coding, spelling, and rapid automatized naming (RAN). Phonological awareness—the ability to recognize and to manipulate phonemes (the basic sounds that make up language)—was assessed by the Auditory Analysis Test (Rosner and Simon 1971). The results of statistical analyses (SAS 1990) showed that the distribution of scores was skewed toward the maximum raw score of 23 (16% of all subjects scored 23), suggesting the presence of ceiling effects in this test. Scores of 23 were therefore excluded as being unreliable, since the subject may have scored higher if the test had permitted it; in this way, the ceiling effect was somewhat ameliorated. The results of statistical analyses also showed that 7% of the variation in the Auditory Analysis Test in children was the result of variation in age. Raw test scores were therefore age-adjusted for subjects <18 years of age. Phonological coding is the ability to apply grapheme-phoneme correspondence rules to the pronunciation of nonwords. It is a higher-level reading skill than is phonological awareness, and it is thought to be the core deficit in the majority of dyslexics (Field and Kaplan 1998). Phonological-coding skill was tested by use of the Word Attack Subtest of the Woodcock Johnson Psychoeducational Battery–Revised (Woodcock and Johnson 1989). Raw scores were converted into age-adjusted standard scores, by use of norms provided in the test protocol. Spelling, which uses phonological skill as well as orthographic skill (recognition of the symbols that represent sounds), was assessed by the Spelling Subtests of the Wide Range Achievement Test (Jastak and Wilkinson 1984). Raw scores were transformed into age-adjusted standard scores, by use of norms provided in the test protocol. RAN is the ability to quickly recall and to verbalize the name of a presented object. During the past 25 years, the results of a number of studies have showed that dyslexic children and adults are slower than control individuals in performing tasks requiring rapid naming of pictured objects, colors, numbers, and letters (see Denckla and Rudel 1976; Wolf et al. 1986; Felton et al. 1990; Bowers and Swanson 1991). The relationship between RAN and phonological deficits in dyslexics is a subject of controversy. However, naming speed contributes variance in reading skill, independent of the variance contributed by phonological-awareness measures (Bowers and Swanson 1991), suggesting that RAN and phonological deficits may be separate factors that contribute to reading difficulty. In the present study, RAN was assessed by use of the orthographically based Rapid Automatized Naming of Numbers Test (Denckla and Rudel 1974), in which subjects quickly recite a list of 50 digits. The number of correct responses for 50 digits (accuracy) and the time required to complete the list, which was then converted into digits recited per second (speed), were recorded. Strong ceiling effects in accuracy were observed across all ages, with the vast majority of subjects obtaining no errors; this resulted in a coefficient of variation for accuracy of only 3%. The accuracy measure was, therefore, not used in the quantitative linkage analyses. The results of statistical analysis showed that RAN speed increased linearly with age ⩽18 years, with 18% of the variation in children resulting from variation in age. Therefore, age adjustment was performed for RAN-speed raw scores, for subjects <18 years of age. Table 1 shows the descriptive statistics of the phonological-awareness, phonological-coding, spelling, and RAN-speed measures (after adjustment for age or conversion to standard scores). Data are given for three samples of subjects: for all members of the 79 families that were used for variance-components analyses and for two sib-pair samples that were used for sib-pair linkage analyses. The variability in each measure was quite large, yielding adequate power to detect linkage. Additional evidence that variability in these quantitative measures was sufficient for detection of linkage is that we have detected strong evidence for linkage to chromosome 6q13-q16.2, by use of these same measures (Petryshen et al. 1999).
Table 1.
Distributions of Reading Measures in All Members of the 79 Families, in the All-Ages Sib-Pair Sample, and in the Sample of Sib Pairs <18 Years of Age[Note]
| Trait | N | Mean | SD | Minimum | Maximum | Skewness | Kurtosis |
| Phonological awareness:a | |||||||
| All family members | 509 | 17.05 | 5.67 | 1 | 28.44b | −.79 | −.07 |
| All-ages sib pairs | 202 | 17.02 | 5.56 | 2.65 | 28.44b | −.48 | −.58 |
| Sib pairs <18 years of age | 163 | 16.96 | 5.75 | 2.65 | 28.44b | −.41 | −.69 |
| Phonological coding:c | |||||||
| All members | 610 | 98.03 | 17.28 | 12 | 149 | −.03 | 1.01 |
| All-ages sib pairs | 223 | 89.69 | 15.11 | 46 | 149 | .53 | 1.14 |
| Sib pairs <18 years of age | 180 | 88.52 | 14.65 | 46 | 129 | .33 | .63 |
| Spelling:c | |||||||
| All members | 599 | 90.85 | 16.33 | 47 | 124 | −.20 | −.90 |
| All-ages sib pairs | 215 | 81.36 | 14.14 | 47 | 115 | .51 | −.29 |
| Sib pairs <18 years of age | 172 | 80.51 | 13.86 | 47 | 115 | .66 | −.02 |
| RAN speed:a | |||||||
| All members | 599 | 3.08 | .62 | 1.09 | 5.00 | .16 | −.13 |
| All-ages sib pairs | 218 | 2.96 | .56 | 1.45 | 4.64 | .23 | .04 |
| Sib pairs <18 years of age | 176 | 2.99 | .56 | 1.59 | 4.64 | .33 | .12 |
Note.—A minority of subjects did not complete all psychometric tests, leading to different sample sizes between traits. For all traits, lower scores indicate greater deficit.
Data are raw scores for adult subjects and age-adjusted scores for subjects <18 years of age.
Age adjustment in subjects <18 years of age occasionally resulted in scores that surpassed the number of test trials of 23.
Data are standard scores, according to test norms (normal population mean = 100, SD = 15).
DNA from each individual was genotyped for the following microsatellite markers: F13A1, D6S89, D6S299, D6S105, TNFB, D6S291, and GLP1R. These markers span a 43-cM region on chromosome 6p25-p21.3. In particular, D6S105 was reported, in the studies by Cardon et al. (1994), Fisher et al. (1999), and Gayán et al. (1999), to be significantly linked to reading disability, whereas, in the study by Grigorenko et al. (1997), D6S299 showed the most significant linkage to phonological awareness. D6S105 and D6S299 are very polymorphic (PIC = .77 and .79, respectively), providing high power to detect linkage.
Two-point quantitative sib-pair linkage analyses were performed by means of the SIBPAL program, version 3.1, in the SAGE package (SAGE 1997). With use of this program, testing for genetic linkage of quantitative-trait loci (QTL) is done by means of traditional Haseman-Elston linear regression of the squared sib-pair trait difference on the estimated proportion of alleles shared identical-by-descent (IBD) by the sib pair, for each marker locus. Multiple sib pairs within each nuclear family are accommodated by the use of a modified t-test with reduced degrees of freedom based on the effective sample size (the number of sibs minus one, summed over all the families). Multipoint quantitative sib-pair linkage analyses were performed with use of the MAPMAKER/SIBS program, version 2.0 (Kruglyak and Lander 1995). This program infers the IBD distribution across the marker region for each sib pair, after which QTL mapping can be performed by use of Haseman-Elston regression, maximum-likelihood variance estimation, and nonparametric methods. Analyses were performed with the use of all possible sib pairs from each sibship, with either weighting or no weighting of sibships with more than two members. The marker map employed was derived from published genetic-marker maps (Field and Kaplan 1998). For the two-point and multipoint sib-pair analyses, we selected one nuclear family (generally consisting of at least two siblings with PCD plus their parents and other available siblings with an affected, unaffected, or uncertain diagnosis) from each of the 79 families, many of which were extended pedigrees. Two samples of sib pairs from the nuclear families were analyzed: a broad sample of 241 sib pairs of all ages (144 independent pairs) and a restricted sample of 165 sib pairs <18 years of age (112 independent pairs). Table 2 indicates the numbers of nuclear families of various sibship sizes, in both the all-ages sib-pair sample and the sample of sib pairs <18 years of age. The large majority of nuclear families consisted of two or three siblings, with only a few larger five- and seven-sibling nuclear families that were not likely to distort the linkage results. For the sample of sib pairs <18 years of age, separate analysis was performed, since ceiling effects occurred in adults in the measures of phonological coding and spelling. Thus, these measures may not be as reliable in adults as they are in children. Marker-allele frequencies for use in the sib-pair linkage analyses were determined by counting alleles in the nuclear-family parents; however, 95% of the parents were genotyped, so the sib-pair linkage analyses were not heavily dependent on marker-allele frequencies to estimate the proportion of IBD alleles shared by sib pairs.
Table 2.
Distributions of Nuclear Families of Various Sibship Sizes, in the All-Ages Sib-Pair Sample and in the Sample of Sib Pairs <18 Years of Age
|
No. of Nuclear Families in Samples of Sibships of |
||
| Sibship Size | All Ages | Age <18 Yearsa |
| Two sibs | 37 | 32 |
| Three sibs | 25 | 29 |
| Four sibs | 13 | 6 |
| Five sibs | 3 | 1 |
| Seven sibs | 1 | 0 |
| Total | 79 | 68 |
Restriction to sibs <18 years of age resulted in the exclusion of 11 nuclear families; therefore, the total number of nuclear families in this sample was 68, and the distribution of sibship sizes in the remaining nuclear families changed.
Linkage was also assessed by means of variance-components analyses, with use of the GENEHUNTER program, version 2.0 (Kruglyak et al. 1996). With this method, evidence of QTL is detected by determining whether a significant amount of the genetic variance of a trait can be attributed to a QTL at each marker position. Specifically, maximum-likelihood estimates of variance components are calculated for major QTL, unlinked polygenic, and environmental effects at each marker. The significance of the QTL effects is tested by a comparison of this maximum-likelihood model with a model in which the QTL variance components are constrained to equal zero (no linkage). The GENEHUNTER program, version 2.0, can handle pedigrees of moderate size. Thus, all members of the 79 families (including all members of extended pedigrees) were used in this analysis, although it was necessary to divide five large pedigrees into smaller manageable subpedigrees. As for sib-pair linkage analyses, the marker map used was derived from published genetic maps, and allele frequencies were calculated from the nuclear-family parents. However, 86% of the subjects in this sample were genotyped; thus, the analyses were not heavily dependent on marker-allele frequencies.
The qualitative PCD phenotype used in our previously reported study (Field and Kaplan 1998) was primarily based on data from the Word Attack Subtest of the Woodcock Johnson Psychoeducational Battery–Revised and on a second word attack test with an age-equivalent scoring system that does not permit direct comparisons across different age groups. Therefore, only the results from the first word attack test were used for the analyses in this study. The results of the Auditory Analysis Test and the Spelling Subtests of the Wide Range Achievement Test, along with those of an eight-item structured interview used to assess reading history in adults, assisted in refinement of the certainty of PCD-status assignment. Hence, the PCD phenotype was qualitative and was based on a composite of dyslexia-related components that other groups have analyzed separately and have found to show various strengths of linkage to the 6p21.3 region. It has been shown that partitioning a quantitative trait into a qualitative phenotype may be less powerful for detection of linkage than using the quantitative data directly (Wijsman and Amos 1997). Furthermore, it is possible that use of a composite measure of dyslexia may be less powerful than analysis of single measures of the component skills involved in reading ability. It was therefore questioned (Fisher et al. 1999) whether the absence of linkage between PCD and chromosome 6p in our families was a true nonreplication or whether our inability to detect linkage was the result of the qualitative and composite nature of the PCD phenotype that we used. To better understand the relationship between the PCD phenotype and the quantitative reading measures on which it was based, Pearson correlation analysis and correlation-ratio analysis were performed with the use of all members of the 79 families. The PCD phenotype was analyzed with the use of three categories (category 1 denoted unaffected; category 2, uncertain; and category 3, affected), whereas the quantitative reading-component traits were continuous variables in which a higher test score corresponded to increased skill. Thus, the correlations between PCD and the quantitative measures were negative, because as the PCD status increased from unaffected to affected, the skill in the reading-component tests decreased. The magnitudes of the square root of correlation ratios (data not shown), which are more appropriate for categorical variables, were similar to the Pearson correlation coefficients. As shown in table 3, the correlation coefficients (r) between PCD and the phonological-coding and spelling measures were substantial at−0.73 and −0.77, respectively. The strength of these correlations indicates that the qualitative PCD phenotype is an accurate indicator of reading disability, thereby lending credibility to our previous report of absence of linkage between dyslexia and 6p23-p21.3. Although the high correlation between PCD and phonological coding was anticipated, since the PCD diagnosis was primarily based on the results of the phonological-coding tests, the high correlation to spelling was somewhat unexpected, given that spelling is thought to be comprised of orthographic as well as phonological components and given that it was only used to assist in diagnosis of PCD. However, the correlation between the phonological-coding and spelling traits was also substantial (r=.75), indicating that spelling ability significantly involves phonological skills. The correlation of PCD to phonological awareness and RAN speed was moderate (r =−0.46 and r=-0.50, respectively; table 3). The phonological awareness and RAN-speed measures were also not highly correlated with any of the other reading-component measures (range, r = 0.34–0.52). The low RAN-speed correlations are consistent with the fact that the RAN test uses a purely orthographic task. The reason for the modest correlations between phonological awareness and the other phonologically based measures (PCD, phonological coding, and spelling) may be that the higher-level phonological skills are only partially dependent on the phonological-awareness skill. Pearson-correlation analyses and correlation-ratio analyses utilizing both the all-ages sib-pair sample and the sample of sib pairs <18 years of age yielded results similar to those obtained for all members of the 79 families (data not shown).
Table 3.
Pearson Correlation Coefficient Matrix of PCD, Phonological Awareness, Phonological Coding, Spelling, and RAN Speed, by Use of All Members of the 79 Families[Note]
| PCD | Phonological Awareness | Phonological Coding | Spelling | |
| Phonological awareness | −.50 | |||
| Phonological coding | −.73 | .52 | ||
| Spelling | −.77 | .49 | .75 | |
| RAN Speed | −.45 | .34 | .43 | .46 |
Note.—All correlations are significant at P<10-4.
Quantitative-trait sib-pair linkage analyses did not detect significant evidence for a locus influencing reading disability in the 6p23–p21.3 region, supporting our previous linkage results obtained with the use of the qualitative PCD phenotype. Table 4 shows P values for two-point simple linear regression of the squared sib-pair trait difference on the estimated proportion of IBD alleles at each marker. In both the all-ages sib-pair sample and the sample of sib pairs <18 years of age, none of the regressions were significant at the 5% level, for any of the quantitative traits, with use of any of the markers tested. Although P values (.10 in the all-ages sib-pair sample and .07 in the sample of sib pairs < 18 years of age) were nearly significant at the 5% level, for linkage between the spelling trait and TNFB, the results with D6S105 (located 2 cM telomeric to TNFB) (Cardon et al. 1994) provided no supportive evidence for linkage to spelling ability. Multipoint sib-pair linkage analyses also did not find significant evidence for linkage between any of the quantitative traits and the 6p region (data not shown). The strongest evidence for linkage was found by use of multipoint nonparametric methods, which report a Z score that is asymptotically normally distributed, thereby allowing determination of significance levels. Analyses of the phonological-awareness measure found maximum Z scores of 0.5 (with weighting of sib pairs; P=.31) and 0.75 (with no weighting of sib pairs; P=.23) in the region of D6S105 and TNFB, with use of the all-ages sib-pair sample. By using the sample of sib pairs <18 years of age, we found that the maximum Z score was .9 (with both weighting schemes; P=.18) in this region. Multipoint nonparametric analyses of the spelling measure detected a maximum Z score of .8 at D6S299 (with both weighting schemes; P=.21), in the sample of sib pairs <18 years of age; however, the maximum Z score obtained with the use of the all-ages sib-pair sample was near zero. The phonological-coding measure had maximum Z scores <.2 across the 6p region, whereas the RAN-speed measure had negative Z scores over the region.
Table 4.
P Values for Simple Linear-Regression Analysis, by Use of SIBPAL, of Both the All-Ages Sib-Pair Sample and the Sample of Sib Pairs <18 Years of Age
|
Phonological Awareness |
Phonological Coding |
Spelling |
RAN Speed |
||||||
| Marker | Distance (cM)a | All Ages | Age <18 Years | All Ages | Age <18 Years | All Ages | Age <18 Years | All Ages | Age <18 Years |
| F13A1 | 18 | .27 | .20 | .86 | .40 | .65 | .25 | .54 | .16 |
| D6S89 | 12 | .55 | .72 | .13 | .37 | .66 | .68 | .99 | .96 |
| D6S299 | 4 | .62 | .79 | .26 | .29 | .50 | .24 | .82 | .93 |
| D6S105 | 2 | .70 | .75 | .93 | .56 | .63 | .18 | 1.00 | .97 |
| TNFB | 4 | .78 | .87 | .44 | .58 | .10 | .07 | .65 | .78 |
| D6S291 | 3 | .59 | .63 | .33 | .81 | .33 | .50 | .45 | .77 |
| GLP1R | .65 | .79 | .35 | .65 | .24 | .49 | .50 | .70 | |
Between marker and that on line below.
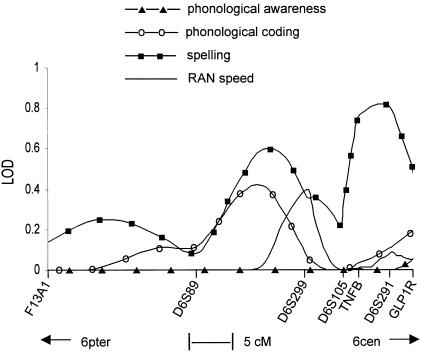
Variance-components analyses also failed to find significant evidence for a locus in the 6p region influencing any of the reading measures. Under the assumption of no dominance variance for the QTL or unlinked polygenes, the LOD scores across the region, for each of the four quantitative measures, were essentially zero. However, under the assumption of dominance variance for the QTL, weak evidence was found for a locus affecting the spelling, phonological-coding, and RAN-speed components of reading. As shown in figure 1, analysis of the spelling figure found a peak maximum LOD score of 0.82 in the region of TNFB and D6S291, and a lesser maximum LOD score of 0.60 occurred between D6S89 and D6S299. Analyses of the phonological-coding measure identified a peak maximum LOD score of 0.42 between D6S89 and D6S299, whereas analyses of RAN speed found a peak maximum LOD score of 0.40 at D6S299. Results of the phonological-awareness measure showed a maximum LOD <0.1 across the 6p region. Although the results of analyses with the use of the phonological-coding and spelling measures were not significant, they were consistent with the results of two-point sib-pair linkage analyses, where P values were lower for these same markers with the respective quantitative measure (table 4).
Figure 1 .
LOD-score curves from GENEHUNTER variance-components analysis of phonological awareness, phonological coding, spelling, and RAN speed. Analysis was performed under the assumption of dominance variance for the QTL.
The results of these sib-pair and variance-components linkage analyses, in which quantitative measures of reading disability are used, support our previous findings of no evidence for linkage to chromosome 6p23-p21.3, by use of a qualitative PCD phenotype. Although these quantitative results provide weak evidence for a locus affecting reading in this region, they are far from being statistically significant. The reason for the lack of significant linkage to chromosome 6p in our families, when four other studies have found significant linkage, remains unclear. We analyzed the same markers that showed significant linkage in the other studies, and we used accredited psychometric tests that, in some cases, were identical to those used in other studies (e.g., Grigorenko et al. [1997] used the same phonological-awareness test). Our phenotypic measures had sufficient variability to allow for detection of linkage, and the large number of families and sib pairs in our study conferred high power to detect linkage. In addition, we used the same quantitative-trait sib-pair linkage method and variance-components method that were used elsewhere (Fisher et al. 1999). Therefore, we propose that the most likely explanation for our inability to detect linkage to chromosome 6p is that the studies with positive linkage results were enriched for subtypes of dyslexia that were not well represented in our sample, as a result of either chance or varying ascertainment criteria. Our ascertainment scheme specified that at least two siblings meet the criterion of having PCD; thus, we may have selected a larger proportion of highly familial major-gene forms of dyslexia than would have been seen with the use of ascertainment schemes based on presence of a single dyslexic proband with no specific requirement for a dyslexic sibling (e.g., Cardon et al. 1994; Gayán et al. 1999). We are currently performing a genomewide linkage screen of our families, to detect such major loci, and we have identified a locus on chromosome 6q13-q16.2 that is involved in susceptibility to PCD (Petryshen et al. 1999).
Acknowledgments
We gratefully acknowledge the families who participated in this study, and we thank the following individuals for their assistance: Norma Schmill de French, Martha Hughes, and Rose Tobias, for technical assistance; Rita Humphreys and Andrea Maben, for psychological assessment; and Susan Crawford, for data management. This research was supported by a grant from the Alberta Mental Health Research Fund (to B.J.K. and L.L.F.), by the Alberta Children’s Hospital Foundation (support to B.J.K.), by the Network of Centres of Excellence Programme of the Canadian Federal Government (support to L.L.F.), and by scholarships from the Natural Sciences and Engineering Research Council of the Canadian Federal Government and the Alberta Heritage Foundation for Medical Research (support to T.L.P.). The SAGE program package used in this study was supported by U.S. Public Health Service Resource Grant 1 P41 RR03655 from the National Center for Research Resources. L.L.F. is an Alberta Heritage Medical Scientist.
References
- Bowers PG, Swanson LB (1991) Naming speed deficits in reading disability: multiple measures of a singular process. J Exp Child Psychol 51:195–219 [DOI] [PubMed]
- Cardon LR, Smith SD, Fulker DW, Kimberling WJ, Pennington BF, DeFries JC (1994) Quantitative trait locus for reading disability on chromosome 6. Science 266:276–279 [DOI] [PubMed]
- Denckla MB, Rudel RG (1974) Rapid “automatized” naming of pictured objects, colors, letters and numbers by normal children. Cortex 10:186–202 [DOI] [PubMed]
- ——— (1976) Rapid “automatized” naming (R.A.N.): dyslexia differentiated from other learning disabilities. Neuropsychologia 14:471–479 [DOI] [PubMed]
- Felton RH, Naylor CE, Wood FB (1990) Neuropsychological profile of adult dyslexics. Brain Lang 39:485–497 [DOI] [PubMed]
- Field LL, Kaplan BJ (1998) Absence of linkage of phonological coding dyslexia to chromosome 6p23-p21.3 in a large family data set. Am J Hum Genet 63:1448–1456 (erratum: 64:334) [DOI] [PMC free article] [PubMed]
- Fisher SE, Marlow AJ, Lamb J, Maestrini E, Williams DF, Richardson AJ, Weeks DE, et al (1999) A quantitative-trait locus on chromosome 6p influences different aspects of developmental dyslexia. Am J Hum Genet 64:146–156 [DOI] [PMC free article] [PubMed]
- Gayán J, Smith SD, Cherny SS, Cardon LR, Fulker DW, Brower AM, Olson RK, et al (1999) Quantitative-trait locus for specific language and reading deficits on chromosome 6p. Am J Hum Genet 64:157–164 [DOI] [PMC free article] [PubMed]
- Grigorenko EL, Wood FB, Meyer MS, Hart LA, Speed WC, Shuster A, Pauls DL (1997) Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. Am J Hum Genet 60:27–39 [PMC free article] [PubMed]
- Jastak S, Wilkinson GS (1984) The wide range achievement test–revised. Jastak Associates, Wilmington, DE [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed]
- Kruglyak L, Lander ES (1995) Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet 57:439–454 [PMC free article] [PubMed]
- Petryshen TL, Kaplan BJ, Field LL (1999) Evidence for a susceptibility locus for phonological coding dyslexia on chromosome 6q13-q16.2. Am J Hum Genet Suppl 65:A32 [Google Scholar]
- Rosner J, Simon DP (1971) The auditory analysis test: an initial report. J Learn Dis 4:382–392 [Google Scholar]
- SAGE (Statistical Analysis for Genetic Epidemiology), release 3.1. (1997) Department of Epidemiology and Biostatistics, Rammelkamp Center for Education and Research, MetroHealth Campus, Case Western Reserve University, Cleveland [Google Scholar]
- SAS, version 6, 4th ed. (1990) SAS Institute, Cary, NC [Google Scholar]
- Wijsman EM, Amos CI (1997) Genetic analysis of simulated oligogenic traits in nuclear and extended pedigrees: summary of GAW10 contributions. Genet Epidemiol 14:719–735 [DOI] [PubMed]
- Wolf M, Bally H, Morris R (1986) Automaticity, retrieval processes, and reading: a longitudinal study in average and impaired readers. Child Dev 57:988–1000 [DOI] [PubMed]
- Woodcock RW, Johnson MB (1989) Woodcock-Johnson psychoeducational battery–revised. DLM Teaching Resources, Allen, TX [Google Scholar]