Abstract
Costello syndrome is characterized by mental retardation, loose skin, coarse face, skeletal deformations, cardiomyopathy, and predisposition to numerous malignancies. The genetic origin of Costello syndrome has not yet been defined. Using immunohistochemistry and metabolic labeling with [3H]-valine, we have established that cultured skin fibroblasts obtained from patients with Costello syndrome did not assemble elastic fibers, despite an adequate synthesis of tropoelastin and normal deposition of the microfibrillar scaffold. We found that impaired production of elastic fibers by these fibroblasts is associated with a functional deficiency of the 67-kD elastin-binding protein (EBP), which is normally required to chaperone tropoelastin through the secretory pathways and to its extracellular assembly. Metabolic pulse labeling of the 67-kD EBP with radioactive serine and further chase of this tracer indicated that both normal fibroblasts and fibroblasts from patients with Costello syndrome initially synthesized comparable amounts of this protein; however, the fibroblasts from Costello syndrome patients quickly lost it into the conditioned media. Because the normal association between EBP and tropoelastin can be disrupted on contact with galactosugar-bearing moieties, and the fibroblasts from patients with Costello syndrome revealed an unusual accumulation of chondroitin sulfate–bearing proteoglycans (CD44 and biglycan), we postulate that a chondroitin sulfate may be responsible for shedding EBP from Costello cells and in turn for their impaired elastogenesis. This was further supported by the fact that exposure to chondroitinase ABC, an enzyme capable of chondroitin sulfate degradation, restored normal production of elastic fibers by fibroblasts from patients with Costello syndrome. We also present evidence that loss of EBP from fibroblasts of Costello syndrome patients is associated with an unusually high rate of cellular proliferation.
Introduction
In 1977, Costello described two unrelated children with poor postnatal growth, mental retardation, curly hair, coarse facies, loose skin, and nasal papillomata, suggesting the existence of a previously undescribed syndrome of uncertain genetic etiology (Costello 1977). The delineation of Costello syndrome (MIM 218040) as a separate entity was further substantiated by the description of >30 other patients (for current review, see van Eeghen et al. 1999). Numerous diagnoses—including cutis laxa (Patton and Baraitser 1993; Davies and Hughes 1994a, 1994b; Vila Torres et al. 1994), Donohue and Leprechaun syndromes, Berardinelli-type lipodystrophy (Zampino et al. 1993), Noonan or cardiofaciocutaneous syndromes (Borochowitz et al. 1992; Teebi and Shaabani 1993; Wieczorek et al. 1997), and ectodermal dysplasias (van Eeghen et al. 1999)—have been initially considered in patients showing the clinical features of Costello syndrome.
The natural history of Costello syndrome is characterized by two distinct phases. The first phase is often marked by polyhydramnios and increased birth weight and is followed by the second phase, which includes failure to thrive, severe short stature, mental retardation, and a distinctive appearance with craniofacial and dermatologic findings resembling those observed in lysosomal storage disorders (fig. 1). Affected individuals may have a silent or clinically significant hypertrophic cardiomyopathy, cardiac valve malformations, and dysrhythmia (Fukao et al. 1996; Siwik et al. 1998; Tomita et al. 1998). Other manifestations of Costello syndrome include soft skin with excess wrinkling over the dorsum of the hands and deep creases on the palms and soles, hyperextensibility of digits, generalized hyperpigmentation, pigmented nevi, vascular birthmarks, acanthosis nigricans, and papillomata and other tumors that develop at later ages (Say et al. 1993; Costello 1996; Kerr et al. 1998; Philip and Sigaudy 1998; Suri and Garrett 1998; Feingold 1999; Franceschini et al. 1999). The genetic basis of Costello syndrome is unknown; however, the occurrence of Costello syndrome in two families with affected siblings (Zampino et al. 1993) and two consanguineous matings (Borochowitz et al. 1992) suggested autosomal recessive inheritance or gonadal mosaicism. In contrast, a review of 20 reported families affected by Costello syndrome (Lurie 1994) indicated that advanced paternal age (⩾38 years) and paternal-maternal age difference (⩾7.36 years) is a factor implicating sporadic autosomal dominant mutations in this disease. There has been only one report of a chromosomal translocation t(1,22) (q25, q11) in a girl with Costello syndrome (Czeizel and Timar 1995), and a single report of neurofibromatosis type 2 mapped to 22q12.2 (Suri and Garrett 1998) in another patient with Costello syndrome.
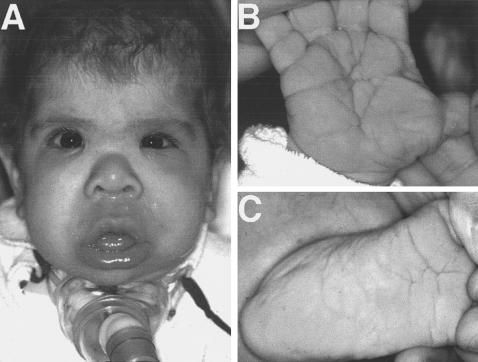
Figure 1.
Photographs depicting one of our patients with Costello syndrome at 6 mo of age, whose fibroblasts were used in the presented studies. A, sparse and curly hair, “coarse” face, depressed nasal bridge, bulbous and upturned nose, low-set ears, full cheeks, large tongue, pouting lower lip, strabismus. B, deep palmar creases. C, plantar creases.
There have only been a few reports defining the biochemical basis of this condition. Qualitative and quantitative histological analyses showed impaired elastin deposition in the skin of children with Costello syndrome (Vila Torres et al. 1994). In fact, an abnormal size, shape, and arrangement of elastic fibers in patients with Costello syndrome resembled those described in cutis laxa. Histopathologic studies of autopsy cases revealed that impaired deposition of elastin also occurs in the tongue, pharynx, larynx, and upper esophagus of patients with Costello syndrome, and that elastic fibers of the bronchi, alveoli, aorta, and coronary arteries of these children are thinner than in normal patient tissue specimens (Mori et al. 1996). Despite the fact that some patients with Costello syndrome resemble those with Williams syndrome, genetic analysis of the cells of these patients did not reveal any deletion of the elastin gene and showed normal expression of elastin mRNA (Mori et al. 1996). This suggested that the observed disruption of elastic fibers in tissues of patients with Costello syndrome may be due to some posttranslational modifications of tropoelastin, to problems arising from its inadequate secretion and extracellular deposition, or to both.
Insoluble elastin is composed of covalently cross-linked polypeptide chains of tropoelastin, which constitutes the major component of the extracellular elastic fibers that provide tissues with the property of elastic recoil. This elastin polymer is assembled along a scaffold of 12-nm microfibrils composed of heterogeneous glycoproteins such as fibrillin type 1, fibrillin type 2, and microfibril-associated glycoprotein (MAGP) (Mecham and Hauser 1991; Rosenbloom et al. 1993; Christiano and Uitto 1994; Vrhowski and Weiss 1998; Debelle and Tamburro 1999). It has been shown that a highly hydrophobic and nonglycosylated 68–72-kD tropoelastin (produced by fibroblasts, smooth muscle cells, and chondrocytes) is chaperoned through the intracellular secretory pathways by the 67-kD elastin-binding protein (EBP) (Hinek et al. 1988, 1996; Hinek 1994, 1996; Hinek and Rabinovitch 1994). The 67-kD EBP is an enzymatically inactive spliced variant of β-galactosidase (S-GAL) (Hinek et al. 1993; Privitera et al. 1998) that binds to the repeating hydrophobic domains on elastin, but may also bind to moieties containing β-galactosugars through a separate “lectin-like” binding domain (Mecham et al. 1991). Binding of β-galactosugar-bearing moieties to the lectin domain of EBP causes conformational changes of the entire 67-kD protein, such that it loses its affinity for elastin and separates from the cell surface. This property of the EBP seems to be particularly important in the process of elastic-fiber assembly, because tropoelastin delivered to the cell surface has to be released from its EBP chaperone. We have proposed that an orderly release of tropoelastin occurs on interaction of EBP with galactosugars, presumably protruding from the microfibrillar glycoproteins that provide the scaffold for growing elastic fibers (Hinek et al. 1991; Hinek 1994, 1996). We have shown, however, that a high pericellular concentration of free galactosugar-bearing moieties, such as chondroitin sulfate or dermatan sulfate glycosaminoglycans, may lead to a premature release of tropoelastin from EBP and consequent disruption of elastic fiber production (Hinek et al. 1988, 1991, 1992, 1993; Hinek 1996).
Because disruption of elastic-fiber production may arise either from low production of tropoelastin and microfibrillar proteins (e.g., in Williams and Marfan syndromes, respectively) or from their inadequate secretion and extracellular assembly (Hurler syndrome), the present study was aimed at assessing the major steps of elastogenesis in fibroblasts derived from six children with Costello syndrome and from three age-matched normal children.
Our data indicate that fibroblasts from patients with Costello syndrome produce normal levels of the soluble tropoelastin and properly deposit an extracellular microfibrillar scaffold but are unable to assemble elastic fibers, because of a secondary deficiency in EBP. We suggest that abnormal accumulation of chondroitin sulfate–bearing moieties by fibroblasts from patients with Costello syndrome may induce shedding of EBP from the cell surface and prevent normal recycling of this reusable tropoelastin chaperone. We also found an increased rate of proliferation of fibroblasts from patients with Costello syndrome, which could be influenced by loss of EBP.
Material and Methods
Material
All chemical grade reagents and the monoclonal antibody to chondroitin sulfate A were from Sigma. Alpha-minimum essential medium (MEM), FCS, and other cell-culture products were obtained from Gibco Life Technologies. Insoluble elastin and the polyclonal antibody to tropoelastin were purchased from Elastin Products Co., Inc. Peanut agglutinin was purchased from EY Laboratories. Microfibrillar proteins were detected with a polyclonal antibody to MAGP and to human fibrillin 1 from Elastin Products Co., Inc. Monoclonal antibody to fibronectin (mAB1940) was from Chemicon and polyclonal antifibronectin antibody was obtained from ICN. Polyclonal antibody to human collagen type I and to human biglycan (LF 39 and 112, respectively) were a generous gift from L. W. Fisher of The National Institutes of Health. Antibody-recognizing CD44 (Hermes-1) was obtained from Endogen. Fluorescein-conjugated antibodies, goat anti-rabbit (GAR-FITC) and goat anti-mouse (GAM-FITC), were purchased from Sigma. Horseradish peroxidase-conjugated goat anti-rabbit antibody (GAR-HRP) used for western blotting was from Bio-Rad. The chemiluminescence detection kit and radiolabeled reagents, [3H]-valine, [3H]-serine, [35S]-methionine, and [3H]-thymidine were purchased from Amersham Canada Ltd.
Fibroblast Cultures
Six children ranging in age from 1 mo to 16 years (7669, 9951, 10595, 12195, 12196, and 12368) were given a diagnosis of Costello syndrome on the basis of the presence of cardinal clinical features previously ascribed to this phenotype (Costello 1996; Johnson et al. 1998; van Eeghen et al. 1999). These features include characteristic coarse facies and thick lips, mental retardation, postnatal growth retardation, sparse and curly hair, deep palmar and plantar creases, loose skin of the hands and feet, hypertrophic cardiomyopathy and arrhythmia, and papillomata and other tumors. Biopsies of forearm skin from three patients with Costello syndrome (7669, 9951, and 10595) and from three normal children of matching ages (4212, 3858, and 4184) were used as the primary source of fibroblasts tested in immunohistochemical, biochemical, and cell proliferation studies. Moreover, fibroblasts obtained from three other patients with Costello syndrome (12195, 12196, and 12368) were analyzed by immunohistochemistry alone. Fibroblasts were originally isolated by collagenase digestion of the skin fragments and then passaged 2–6 times by trypsinization and maintained in alpha-MEM supplemented with 20 mM Hepes, 1% antibiotics/antimycotics, and 10% fetal bovine serum (FBS).
Immunostaining
Subconfluent, 48-h cultures of normal and Costello fibroblasts, fixed in cold 100% methanol at −20°C for 30 min, were incubated with 10 μg/ml of polyclonal antibody raised to the elastin/laminin-binding domain of the alternatively spliced variant of β-galactosidase (anti-S-GAL) (Hinek et al. 1993) and with the BCZ monoclonal antibody, which recognizes a different epitope on the EBP (Mecham et al. 1988). Parallel cultures fixed for 10 min in 4% paraformaldehyde, with or without detergent (0.2% Triton X-100) that permeabilizes cell membranes, were treated with 10 μg/ml of monoclonal antibody to chondroitin sulfate or with 10 μg/ml of antibodies that recognize the chondroitin sulfate–bearing proteoglycans biglycan and CD44.
In addition, the histochemical detection of galactosugar-bearing glycoconjugates was also performed by staining nonpermeabilized and permeabilized fibroblasts with 0.1 mg/ml of fluorescein-conjugated peanut agglutinin (FITC-PNA), which selectively binds to Galβ1-3GalNac domains (Zhou et al. 1999).
Ten-day-old cultures, containing abundant extracellular matrix and fixed in cold 100% methanol, were incubated with 20 μg/ml of polyclonal antibody to tropoelastin (Prosser et al. 1991), 2 μg/ml of monoclonal antibody to fibronectin (Vartio et al. 1987), and 10 μg/ml of polyclonal antibody to collagen type I. The parallel cultures scheduled for immunohistochemical assessment of microfibrillar components were fixed in 0.5% paraformaldehyde for 15 min, were blocked in PBS containing 0.1 M ammonium chloride, were treated with specific 20 μg/ml of polyclonal antibody to fibrillin or additionally pretreated for 10 min with PBS containing 50 mM DTT, were alkylated with 100 mM iodoacetamide for 15 min, were washed in PBS, and then were immunostained with specific polyclonal antibody to MAGP at the same concentration. All cultures were incubated with appropriate fluorescein-conjugated secondary antibodies (GAR-FITC or GAM-FITC) for an additional hour. Nuclei were counterstained with propidium iodide.
Morphometric analysis of 10-d-old cultures immunostained with antibodies recognizing extracellular matrix components was performed by an Olympus AH-3 microscope attached to a CCD camera (Optronix) and a computerized video analysis system (Image-Pro Plus software 3.0 for Macintosh [Media Cybernetics]).
Tropoelastin and Insoluble Elastin Assays
Fibroblasts from normal patients and patients with Costello syndrome were grown to confluency in 100-mm dishes in quadruplicates. [3H]-valine (20 μCi) was added to each dish along, with fresh media. Cultures were then incubated for 72 h, and the soluble and insoluble elastin was assessed separately in each culture. First, conditioned media was collected and immunoprecipitated with a polyclonal antibody to tropoelastin; then, soluble proteins present in the intracellular compartments were extracted with 0.1 M acetic acid; and then, intracellular tropoelastin was immunoprecipitated from extracts and was assessed quantitatively by scintillation counting, as described elsewhere (Hinek and Rabinovitch 1994). The remaining cultures containing cell remnants and deposited insoluble extracellular matrix were scraped and boiled in 0.5 ml of 0.1 N NaOH for 45 min to solubilize all matrix components except elastin. The resulting pellets containing the insoluble elastin were then solubilized by boiling in 200 μl of 5.7 N HCl for 1 h, and the aliquots were mixed with scintillation fluid and were counted.
The integrity of tropoelastin produced by fibroblasts from normal and Costello syndrome patients was also assessed after confluent cultures (initially plated 5 × 106cells/dish) were pulsed with 15 μCi/ml [14C]-valine in valine-free medium for 2 h. The intracellular tropoelastin was then immunoprecipitated with specific antitropoelastin antibody (Prosser et al. 1991) from the cell layer extracts produced with 1% Nonidet P-40 in 50 mM TBS pH 8 containing proteinase inhibitors (2 mM benzamidine, 2 mM EACA, 2 mM PMSF, 1 mM EDTA, 2 mM leupeptin, and 1 mg/ml Trasylol) and analyzed by SDS-PAGE followed by autoradiography and by western immunoblotting.
Isolation of EBP
To compare patterns of the EBP expression by fibroblasts from normal children and those with Costello syndrome, we performed pulse-chase experiments. Fibroblasts were initially plated 1×106cells/dish to form a subconfluent culture, incubated in triplicate in serum-free Medium 199 for 6 h, and then pulsed with 15 μCi/ml [14C]-serine in serine-free medium for 1 h. The cultures were then rinsed well and chased in fresh Medium 199 for 5, 15, 30, and 45 min. At the end of each chase period, the cell layers and the media were processed separately. To isolate the EBP, we used the standard elastin-affinity chromatography technique as described elsewhere (Hinek et al. 1993, Hinek and Wilson 2000). The elastin-bound proteins released from elastin slurries were resolved by 7.5%–12% SDS-PAGE followed by autoradiography. The identity of EBP was additionally confirmed by immunoblotting with affinity-purified anti-S-Gal antibody recognizing 67-kD EBP, followed by GAR-HRP conjugated secondary antibody and amplification with the ECL chemiluminescence detection system. The media from each culture was also mixed with a protease-inhibitor cocktail and then subjected to immunoprecipitation with 2 μg/ml of anti-S-Gal antibody (Privitera et al. 1998).
Assessment of Fibroblast Proliferation
Fibroblasts from normal and Costello syndrome children were suspended in alpha-MEM containing 10% FBS and were initially plated in six-well dishes at a density of 50,000 cells per well. The medium was changed 24 h later, and parallel cultures were maintained for the next 48 h in normal medium containing 10% FBS in the presence or absence of insoluble elastin (1 mg/well). Cell proliferation in 72 h–cultures were first assessed with an inverted microscope with Nomarski optic, and then cells were trypsinized and were counted in a hemocytometer. Parallel sextuplicate cultures incubated with the earlier-mentioned agents were also exposed to [3H]-thymidine (2 μCi/well) for the last 24 h. These cultures were then washed in PBS and treated with cold trichloroacetic acid twice for 10 min at 4°C. For 30 min, 0.5 ml of 0.3 N NaOH was added to all dishes, and 200-μl aliquots of each culture were then mixed with scintillation fluid and counted. In all biochemical studies, means and standard deviations were calculated, and statistical analyses were done by ANOVA.
Results
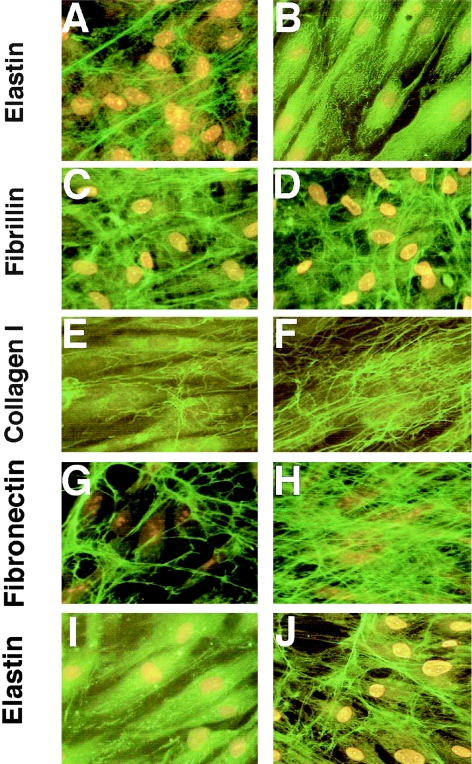
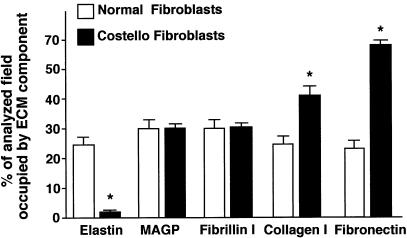
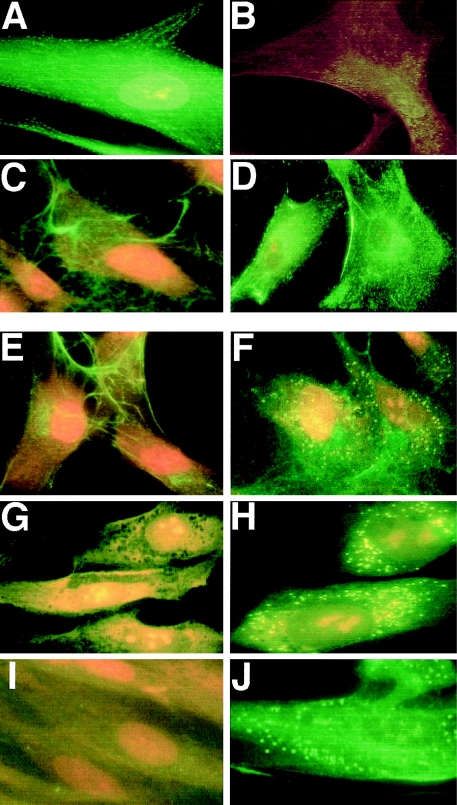
Comparison of 10-d-old fibroblast cultures immunostained with antitropoelastin antibody revealed that—in contrast to normal fibroblasts, which produced a dense network of elastic fibers (fig. 2A)—the fibroblasts from patients with Costello syndrome showed only intracellular accumulation of the immunodetectable material (fig. 2B). The lack of elastic fibers in cultures of fibroblasts from Costello syndrome patients contrasted with the presence of normally assembled microfibrillar scaffolds containing the immunodetectable fibrillin type I (fig. 2C, 2D). Moreover, the extracellular matrix produced by Costello fibroblasts was consistently found to have higher-than-normal expression of collagen type I (fig. 2F) and fibronectin (fig. 2H). Quantitative analysis of immunostaining with matrix component–specific antibodies revealed that this abnormal pattern of extracellular matrix distribution was consistent in cultures of fibroblasts derived from all six children with Costello syndrome (fig. 3).
Figure 2.
Representative photomicrographs of 10-d-old cultures immunostained with antitropoelastin antibody indicate that normal fibroblasts (A) produced long, branching elastic fibers, whereas fibroblasts from patient with Costello syndrome (B) did not deposit any extracellular elastin. Deposition of fibrillin I by Costello fibroblasts (C) does not differ from normal fibroblasts (D). Costello fibroblasts, however, deposit more collagen type 1 (F) and fibronectin (H) than do normal fibroblasts (E and G, respectively). Whereas Costello fibroblasts cultured for 10 d in normal medium did not display any extracellular elastic fibers (I), treatment with chondroitinase ABC (J) restored their ability to deposit elastic fibers in the extracellular matrix.
Figure 3.
Morphometric analysis of extracellular matrix (ECM) components immunostained with specific antibodies in 10-d-old cultures of normal and Costello fibroblasts. Fibroblasts from patients with Costello syndrome deposit only negligible amounts of immunodetectable extracellular elastin. Amounts of fibronectin and collagen type I produced by Costello fibroblasts significantly exceed those present in cultures of normal fibroblasts, whereas deposition of fibrillin I and MAGP by Costello fibroblasts does not differ from normal fibroblasts. In each analyzed group, 50 low-power fields (´20) from three separate cultures (per independent patients) were analyzed and the area occupied by the particular immunodetectable component quantified. The abundance of each component was then expressed as a percentage of the entire analyzed field (mean ± SD), and results from cultures of Costello fibroblasts were statistically compared with those in cultures of normal skin fibroblasts (P<.001).
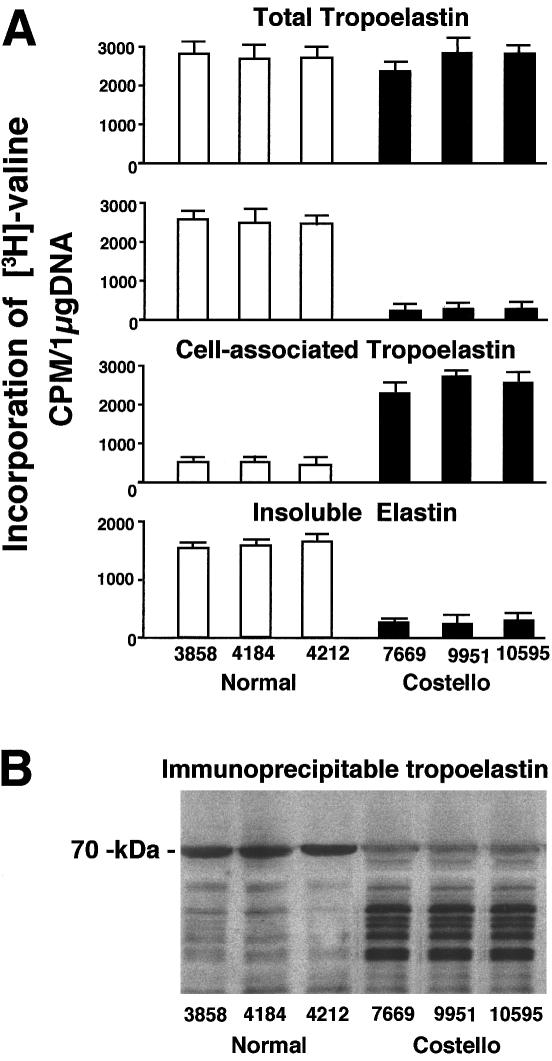
Quantitative assessment of [3H]-valine incorporation into immunoprecipitatable soluble tropoelastin extracted jointly from the cell layers and from the conditioned media showed that fibroblasts from patients with Costello syndrome and fibroblasts from normal children synthesized comparable amounts of tropoelastin (fig. 4A). The separate immunoprecipitation of metabolically labeled tropoelastin from the cell-layer extracts and from the conditioned media clearly indicated that fibroblasts from patients with Costello syndrome were characterized by an impaired secretion of newly synthesized tropoelastin. In contrast to cultures of normal fibroblasts, in which cellular extracts contained only a small portion of total [3H]-valine-labeled tropoelastin, the cell-layer fractions of fibroblasts from patients with Costello syndrome retained the majority of the metabolically labeled tropoelastin. Consistently, fibroblasts from patients with Costello syndrome incorporated much less radioactive valine into the NaOH-insoluble elastin than did their counterparts taken from normal children. Moreover, SDS-PAGE and autoradiography of metabolically radiolabeled proteins immunoprecipitated with antitropoelastin antibody indicated that the bulk of soluble tropoelastin extracted from the fibroblasts of patients with Costello syndrome was partially degraded. Whereas cell extracts from normal children contained significant amounts of radiolabeled 70-kD tropoelastin, the cell-layer extracts of fibroblasts from patients with Costello syndrome showed 3–5 times lower levels of the intact 70-kD tropoelastin (as measured by densitometry) and showed numerous species of lower molecular weight, representing tropoelastin degradation products (fig. 4B).
Figure 4.
A, Quantitative analysis of [3H]-valine-labeled immunoprecipitatable tropoelastin indicates that both normal (3858, 4184, and 4212) and Costello fibroblasts (7669, 9951, and 10595) synthesize comparable amounts of total metabolically labeled tropoelastin. In contrast to normal fibroblasts, Costello fibroblasts retain the majority of their metabolically labeled tropoelastin intracellularly. Consistently, Costello fibroblasts incorporate much less [3H]-valine into extracellular insoluble elastin than do normal fibroblasts. B, Representative autoradiographs of metabolically radiolabeled proteins immunoprecipitated with antitropoelastin antibody from the cell-layer extracts. In contrast to normal fibroblasts (3858, 4184, and 4212) that contain a significant amount of radiolabeled 70-kD tropoelastin, the Costello fibroblasts (7669, 9951, and 10595) show much lower levels of intact 70-kD tropoelastin and show numerous lower molecular weight species that likely represent tropoelastin degradation products.
Immunostaining with anti-S-GAL antibody (which recognizes the elastin-binding domain of EBP) and BCZ antibody (which recognizes a different epitope on EBP) consistently indicated that the levels of this protein were greatly diminished in fibroblasts from patients with Costello syndrome. As depicted in figure 5A, EBP (detected with anti-S-GAL antibody) was particularly well expressed on the surface of normal fibroblasts. In contrast, the fibroblasts from patients with Costello syndrome showed only negligible cell surface immunostaining (fig. 5B).
Figure 5.
A–D, Representative photomicrographs of 48-h-old cultures of lightly fixed and nonpermeabilized fibroblasts. Immunostaining with anti-S-Gal antibody, which recognizes EBP, indicates that normal fibroblasts (A) show strong cell-surface expression of this protein, whereas Costello fibroblasts (B) show greatly diminished levels of the EBP. Immunostaining with monoclonal antibody recognizing chondroitin sulfate A indicates that, in contrast to cultures of normal fibroblasts (C), which show immunodetectable epitope only in the extracellular matrix, Costello fibroblasts show strong cell surface–associated expression of chondroitin sulfate (D). E–J, Representative photomicrographs of 48-h-old cultures of permeabilized fibroblasts immunostained with antibodies to chondroitin sulfate A epitope (E, F) to CD44 (G, H) and to biglycan (I, J). In contrast to normal fibroblasts (E, G, I), which do not show any intracellular immunostaining with anti-CD44 and antibiglycan antibodies, and only perinuclear localization of the chondroitin sulfate epitope, Costello fibroblasts (F, H, J) show strong and overlapping intracellular (lysosomal) localization of all three epitopes.
Another striking difference between normal fibroblasts and fibroblasts from patients with Costello syndrome was their expression of the epitope detected with the monoclonal antibody raised to chondroitin sulfate A. Immunostaining of nonpermeabilized cells showed that, in contrast to normal fibroblasts that deposited chondroitin sulfate extracellularly (fig. 5C), the fibroblasts from patients with Costello syndrome were spotted with large amounts of immunogenic materials, likely representing accumulations of chondroitin sulfate–containing proteoglycans on their surface (fig. 5D). Immunostaining of permeabilized cultures indicated that, in addition to extracellular matrix, the normal fibroblasts showed the presence of chondroitin sulfate epitopes only in perinuclear regions (fig. 5E). Permeabilized fibroblasts from patients with Costello syndrome revealed accumulation of the chondroitin sulfate–containing material in multiple cytoplasmic vesicles (fig. 5F). Immunostaining of parallel cultures with antibody to the α subunit of hexosaminidase (data not shown) indicated that most of these chondroitin sulfate–positive vesicles could be identified as lysosomes. Interestingly, histochemistry with peanut agglutinin, which selectively binds to Galβ1-3GalNac domains, showed identical patterns of cell surface and intracellular localization as anti–chondroitin sulfate antibodies (data not shown). Additional immunochemical studies aimed at the positive identification of the chondroitin sulfate–bearing moieties accumulating in fibroblasts from patients with Costello syndrome indicated that their intracellular vesicles (lysosomes) were also immunoreactive to antibodies recognizing the protein components of the chondroitin sulfate–bearing proteoglycans, CD44 (fig. 5H) and biglycan (fig. 5J). In contrast to fibroblasts from patients with Costello syndrome, normal fibroblasts did not reveal any storage of CD44 (fig. 5G) or biglycan (fig. 5I). Immunostaining of nonpermeabilized cells with these antibodies also showed a cell-surface aggregation of such moieties on the cell surfaces of fibroblasts from patients with Costello syndrome, but not on normal fibroblasts (data not shown).
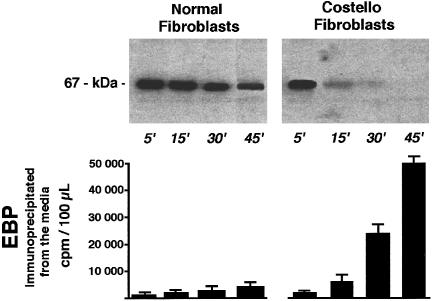
The metabolic pulse-chase labeling of EBP followed by the chase indicated that both types of fibroblasts initially synthesized similar amounts of this protein. In contrast to normal fibroblasts, which showed only a small decrease in labeled EBP during the chase period, the amounts of EBP extractable from fibroblasts of patients with Costello syndrome steadily decreased during the 45-min chase. The autoradiography did not detect any degradation products of EBP in the cell extracts from patients with Costello syndrome (fig. 6), and this metabolically labeled protein could be immunoprecipitated in increased amounts from the respective conditioned media during the chase, suggesting extensive shedding of EBP molecules from the cell surface of fibroblasts from patients with Costello syndrome.
Figure 6.
Representative autoradiographs (upper panel) showing levels of the 67-kD EBP during the chase after metabolic pulse labeling with radioactive serine. Results indicate that both normal and Costello fibroblasts initially synthesize comparable amounts of EBP, isolated by elastin affinity columns. In contrast to normal fibroblasts, which retain the majority of the labeled EBP, Costello fibroblasts steadily lose this newly produced protein, which can be immunoprecipitated in increased amounts from the respective conditioned media during the chase (lower panel) .
Because the results mentioned earlier strongly indicated that impaired elastic-fiber assembly in cultures of Costello fibroblasts coincides with accumulation of the immunodetectable chondroitin sulfate–bearing moieties, which induce shedding of EBP (Hinek et al. 1988, 1991), we decided to test whether enzymatic removal of these galactosugar-bearing moieties from the cell surface would improve elastogenesis. Indeed, treatment of Costello syndrome–fibroblast cultures with an enzyme that degrades chondroitin sulfates (chondroitinase ABC, 0.1 U/ml/day, for 10 d) led to restoration of normal elastic-fiber assembly (fig. 2I, J).
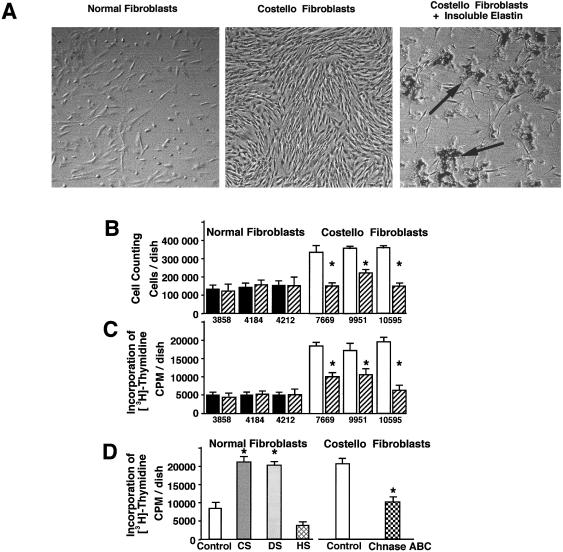
We have established, in addition to the results mentioned earlier, that the growth rate of cultured fibroblasts from patients with Costello syndrome was higher than that of normal fibroblasts. The striking difference in the cell density between normal and Costello fibroblasts was clearly noticeable in 3-d-old cultures (fig. 7A). Whereas normal fibroblasts initially plated at concentrations of 50,000 cells/well grew to an average 140,000 cells/well in the 3 d, the fibroblasts from patients with Costello syndrome plated at the same initial density reached 320,000 cells/well (fig. 7B). Similar results indicating an increased rate of proliferation in cultures of fibroblasts from patients with Costello syndrome were obtained when incorporation of [3H]-thymidine was assessed (fig. 7C). Interestingly, addition of exogenous insoluble elastin to cultures of fibroblasts derived from patients with Costello syndrome significantly decreased their proliferation as assessed by inverted microscopy, direct cell counting, and incorporation of [3H]-thymidine (fig. 7A, B, C ). Exogenous insoluble elastin not only caused inhibition of cell growth but also induced changes in the cell shape. Although some Costello cells that did not maintain direct contact with clusters of insoluble elastin remained elongated, cells attached to elastin clusters were spread and resembled normal fibroblasts. It should also be mentioned that virtually all fibroblasts maintaining direct contact with insoluble elastin did not display bromodeoxyuridine incorporation or positive staining with Ki-67 antibody (data not shown). Treatment with insoluble elastin did not affect the growth rate of normal fibroblasts.
Figure 7.
A, Representative phase-contrast micrographs of 3-d-old cultures illustrating that the growth rate of fibroblasts from patients with Costello syndrome (middle panel) were considerably higher than those taken from normal children (left panel). Addition of insoluble elastin to cultures of Costello fibroblasts substantially reduced their cell density (right panel). Arrows indicate clusters of insoluble elastin attached to the cultured fibroblast. B, Cell counting and C, incorporation of [3H]-thymidine also show the increased growth rate of Costello fibroblasts (blackened bars) as compared with normal fibroblasts (unblackened bars) in 3-d-old cultures. These assays also illustrate that high proliferation of Costello fibroblasts was significantly reduced in cultures treated with exogenous insoluble elastin (striated bars). Treatment with insoluble elastin did not change the proliferative rate of normal fibroblasts. D, Parallel cultures of normal fibroblasts treated with chondroitin sulfate (CS) or dermatan sulfate (DS), but not heparan sulfate (HS) (all in concentration 400 μg/ml), significantly increased the rate of their [3H]-thymidine incorporation. At the same time, addition of chondroitinase ABC (0.2 U/d) (Chnase ABC) significantly decreased incorporation of [3H]-thymidine in cultures of fibroblasts taken from patients with Costello syndrome. In all experiments, cells were plated with the same initial density of 50,000 cells/well. Values of mean ± SD from three different experiments were statistically compared with untreated controls within the same cell type (P<.002).
Discussion
Although the basic genetic defect in Costello syndrome remains obscure, the cardinal phenotypic features observed in patients with Costello syndrome indicate that pathology of mesenchyme-derived tissues may constitute fundamental problems in this clinical entity. Findings of defective elastic fibers in tissue samples from patients with Costello syndrome (Vila Torres et al. 1994; Mori et al. 1996) reinforced this suggestion and focused our studies on the pathomechanism of impaired elastogenesis in Costello syndrome.
Results of our immunocytochemical and metabolic studies indicated that Costello fibroblasts showed a normal rate of tropoelastin production but were unable to secrete tropoelastin or to assemble extracellular insoluble elastin. The coincident finding of a very low immunosignal for EBP in Costello cells initially suggested a deficiency in this recyclable tropoelastin chaperone (Hinek et al. 1988, 1996; Hinek and Rabinovitch 1994) as a factor for the disruption of elastic-fiber formation in Costello syndrome. The pulse-chase experiments indicated, however, that fibroblasts from both normal patients and patients with Costello syndrome initially produced equal amounts of the metabolically labeled 67-kD EBP, but only Costello fibroblasts quickly lost it into the conditioned media. We therefore propose that EBP was rapidly shed from the Costello fibroblast. Because immunocytochemistry detected accumulation of the chondroitin sulfate–bearing moieties in Costello fibroblasts, we additionally suggest that shedding of EBP may occur on contact of this protein with cell-surface chondroitin-sulfate moieties. This conclusion is based on our previous observations (1) that binding of galactosugar-containing moieties to the galactolectin domain of the EBP induces structural changes of this protein that make it unable to associate with tropoelastin and with the cell membranes (Hinek et al. 1988, 1993) and (2) that addition of exogenous galactosugar-bearing moieties (chondroitin sulfate or dermatan sulfate) to cultures of normal fibroblasts, chondroblasts, and smooth-muscle cells caused depletion of EBP and disruption of elastic-fiber production (Hinek et al. 1991, 1992). Decreased deposition of insoluble elastin by rat-lung fibroblasts cultured in the presence of galactosamine-containing chondroitin sulfate and dermatan sulfate has also been reported by McGowan et al. (1993). Despite the fact that extracts of fibroblasts from patients with Costello syndrome revealed the presence of degraded tropoelastin, cultures of these fibroblasts did not show any higher-than-normal activity of secretable elastolytic enzymes that could degrade exogenous insoluble elastin substrate (data not shown). Thus, impaired formation of extracellular fibers—but not their accelerated degradation—is likely responsible for the low net content of insoluble elastin in the tissues of Costello patients. In fact, increased elastolysis would not be expected because chondroitin sulfate can sequestrate and inactivate leukocytic elastase (Volpi 1997; Ying et al. 1997).
Our proposal that the impaired elastogenesis observed in cells from patients with Costello syndrome may be triggered by the accumulation of chondroitin sulfate–bearing moieties was reinforced by the fact that enzymatic degradation of these moieties by exogenous chondroitinase ABC led to restoration of normal elastic-fiber assembly. It should be also mentioned that results of our present study, indicating links between impaired elastogenesis in Costello syndrome and functional deficiency of EBP induced by chondroitin sulfate–bearing moieties, are consistent with our recent data of a very similar effect in Hurler disease in which an accumulation of dermatan sulfate (another galactosugar-bearing glycosaminoglycan) is associated with impaired elastogenesis (Hinek and Wilson 2000). Existence of phenotypic similarities between patients with Costello syndrome, with Hurler disease, and with other mucopolysaccharidoses (Belcher 1972; Schieken et al. 1975; Johnson et al. 1981; Haust 1987; Hopwood and Morris 1990; Nelson et al. 1990; Vinallonga et al. 1992; Neufeld and Muenzer 1997) may encourage future studies aimed at elucidation of the primary genetic defect in Costello syndrome. The objective would be to question whether Costello syndrome might belong to the group of metabolic-storage diseases (mucopolysaccharidoses) caused by a primary deficiency in enzymes responsible for degradation of chondroitin sulfate moieties. Our finding that permeabilized Costello fibroblasts display an apparent accumulation of the chondroitin sulfate-bearing proteoglycans (CD44 and biglycan), in multiple cytoplasmic vesicles that overlap with immunolocalization of the lysosomal enzyme hexosaminidase, further supports this claim. We suggest that Costello syndrome cells (similar to known mucopolysaccharidoses) are not capable of normal degradation of the chondroitin sulfate attached to several different compounds, and that this inability may result in a storage of undegraded proteoglycans (CD44, biglycan, and others) in lysosomes. We speculate that a lack of a proper enzymatic degradation of chondroitin sulfate components of these proteoglycans may obscure their proteinase-binding domains and consequently may prevent further degradation of their protein cores.
Elastin is often thought to be a component produced in the latest stages of fetal development and in the perinatal period (Cleary et al. 1967; Mecham and Hauser 1991; Rosenbloom et al. 1993; Pasquali-Ronchetti and Baccarani-Contri 1997). Recent studies with the developing chick embryo, by means of in situ hybridization techniques, revealed that tropoelastin mRNA is expressed early during development (Selmin et al. 1991; Holzenberger et al. 1993). Studies by Hurle et al. (1994) also showed the presence of elastic fibers during early morphogenesis of the limb skeleton, in vivo and in vitro, and suggested that the elastic-fiber scaffold plays an important role in coordinating the size and the spatial location of the cartilaginous skeletal elements within the limb buds. They also observed precise patterns of elastic-fiber arrangement present in the outflow tract and atrioventricular cushion tissue of the heart, the early developing lung, the notochord, and the somites. These observations pointed to previously unsuspected functions for elastic matrices during embryonic development and substantiated our hypothesis that impaired elastogenesis in the developing skeleton, heart, or skin may be play an important role in the overall pathophysiology of the Costello syndrome.
Our observation that fibroblasts from patients with Costello syndrome, which are unable to assemble normal elastic fibers, displayed an increased rate of proliferation, as compared with normal skin fibroblasts, indicates a potential pathophysiological link between the absence of insoluble elastin and increased cell proliferation. Further support for this link comes from a recent observation of Li et al. (1998), who showed that newborn transgenic mice lacking elastin protein die as a result of arterial blockage with abnormal intimal thickenings composed of proliferating smooth-muscle cells. The possibility that insoluble elastin may, in fact, negatively modulate mitogenic signals is further substantiated by the fact that administration of exogenous insoluble elastin to cultured Costello fibroblasts normalized their proliferation rate. Increased cellular proliferation associated with decreased insoluble elastin in extracellular matrix produced by Costello fibroblasts may result from several overlapping mechanisms. First, large hydrophobic particles of exogenous insoluble elastin may attract soluble tropoelastin-derived peptides present in the conditioned media and cause their precipitation (coacervation). Such a depletion of soluble fragments of tropoelastin, known to be stimulators of cell-cycle progression (Jung et al. 1998; Hinek et al. 1999) may therefore downregulate cellular proliferation. Second, establishment of physical contact between large particles of insoluble elastin and the cell surface may cause binding and trapping of EBP molecules that prevent their shedding. Aggregation of cell-surface EBP may, in turn, lead to masking of adjacent growth factor receptors that normally transduce mitogenic signals induced by serum-derived growth factors. Conversely, extensive chondroitin sulfate–induced shedding of EBP from the cell surface of Costello fibroblasts may lead to unmasking of adjacent cell-surface receptors important for mitogenic signal transduction. This suggestion is consistent with our previously reported results showing that chondroitin sulfate–dependent shedding of EBP from cell surfaces of arterial smooth muscle cells unmasks their adjacent interleukin type I receptors interacting with IL-1β and facilitates cellular response to this cytokine (Hinek et al. 1996), resulting in a net increase in fibronectin production (also noted in cultures of Costello fibroblasts) and stimulation of cell migration and proliferation. This proposed mechanism does not preclude the possibility that chondroitin sulfate–containing proteoglycans accumulating on the surface of fibroblasts from patients with Costello syndrome may also act as coreceptors for major growth factors (e.g., bFGF). In this regard, our finding of increased expression of CD44 in fibroblasts from patients with Costello syndrome may be of special interest. This cell-surface hyaluronate receptor containing a chondroitin sulfate moiety, which may act as a low-affinity fibroblast growth-factor receptor, has been widely implicated in the growth of numerous human tumors (Borland et al. 1998; Herrlich et al. 1998; Ponta et al. 1998; Sneath and Mangham 1998; Chiu et al. 1999; Humphrey et al. 1999) including rhabdomyosarcoma, which has been reported in patients with Costello syndrome (Kerr et al. 1998; Feingold 1999).
In summary, we propose that chondroitin sulfate–dependent shedding of EBP from fibroblasts of patients with Costello syndrome eventually eliminates recycling of this tropoelastin chaperone and consequently disrupts tropoelastin secretion and extracellular assembly into elastic fibers. Thus, Costello syndrome should be added to the list of inherited diseases that are characterized by impaired elastogenesis. We also believe that lack of insoluble elastin coincident with high rates of cell proliferation may be relevant to the pathophysiological mechanisms responsible for the development of the cardinal phenotypic features of Costello syndrome, including skeletal, cardiovascular, and skin problems, as well as the development of benign and malignant tumors.
Acknowledgments
This work was supported by a grant (PG 13920) from the Medical Research Council of Canada and by Dr. A. Hinek's Career Investigator Award from the Heart and Stroke Foundation of Ontario. We thank Dr. Charles I. Scott Jr., Linda Nicholson, and Dr. Teresa Costa for obtaining patient samples and Cheryl Shuman and Cheryl Cytrynbaum for their clinical assistance with the patients. We also gratefully acknowledge the participation of the patients with Costello syndrome and their families.
Electronic-Database Information
The URL and accession number for data in this article is as follows:
- Online Mendelian Inheritance in Man (OMIM): http://www.ncbi.nlm.nih.gov/Omim (for Costello syndrome [MIM 218040])
References
- Belcher RW (1972) Ultrastructure of the skin in the genetic mucopolysaccharidoses. Arch Pathol 94:511–518 [PubMed]
- Borland G, Ross JA, Guy K (1998) Forms and functions of CD44. Immunology 93:139–148 [DOI] [PMC free article] [PubMed]
- Borochowitz Z, Pavone L, Mazor G, Rizzo R, Dar H (1992) New mental congenital anomalies: mental retardation syndrome (MR/MCA) with facio-cutaneous-skeletal involvement. Am J Med Genet 43:678–685 [DOI] [PubMed]
- Chiu RK, Droll A, Dougherty ST, Carpenito C, Cooper DL, Dougherty GJ (1999) Alternatively spliced CD44 isoforms containing exon v10 promote cellular adhesion through the recognition of chondroitin sulfate–modified CD44. Exp Cell Res 248:314–321 [DOI] [PubMed]
- Christiano AM, Uitto J (1994) Molecular pathology of elastic fibers. J Invest Dermatol Suppl 103:53S–57S [DOI] [PubMed]
- Cleary EG, Sandberg LB, Jackson DS (1967) The changes in chemical composition during development of the bovine nuchal ligament. J Cell Biol 33:469–479 [DOI] [PMC free article] [PubMed]
- Costello JM (1977) A new syndrome: mental subnormality and nasal papillomata. Aust Paediatr J 13:114–118 [DOI] [PubMed]
- ——— (1996) Costello syndrome: update on the original cases and commentary. Am J Med Genet 62:199–201 [DOI] [PubMed]
- Czeizel AE, Timar L (1995) Hungarian case with Costello syndrome and translocation t(1,22). Am J Med Genet 57:501–503 [DOI] [PubMed]
- Davies SJ, Hughes HE (1994a) Cutis laxa: a feature of Costello syndrome. J Med Genet 31:85 [DOI] [PMC free article] [PubMed]
- ——— (1994b) Costello syndrome: natural history and differential diagnosis of cutis laxa. J Med Genet 31:486–489 [DOI] [PMC free article] [PubMed]
- Debelle L , Tamburro AM (1999) Elastin: molecular description and function. Int J Biochem Cell Biol 31:261–272 [DOI] [PubMed]
- Feingold M (1999) Costello syndrome and rhabdomyosarcoma. J Med Genet 36:582–583 [PMC free article] [PubMed]
- Franceschini P, Licata D, Di Cara G, Guala A, Bianchi M, Ingrosso G, Franceschini (1999) Bladder carcinoma in Costello syndrome: report on a patient born to consanguineous parents and review. Am J Med Genet 86:174–179 [PubMed]
- Fukao T, Sakai S, Shimozawa N, Kuwahara T, Kano M, Goto E, Nakashima Y, et al (1996) Life-threatening cardiac involvement throughout life in a case of Costello syndrome. Clin Genet 50:244–247 [DOI] [PubMed]
- Haust MD (1987) Arterial involvement in genetic diseases. Am J Cardiovasc Pathol 1:231–285 [PubMed]
- Herrlich P, Sleeman J, Wainwright D, Konig H, Sherman L, Hilberg F, Ponta H (1998) How tumor cells make use of CD44. Cell Adhes Commun 6:141–147 [DOI] [PubMed]
- Hinek A (1994) Nature and multiple functions of the 67-kD elastin-/laminin-binding protein. Cell Adhes Commun 2:185–193 [DOI] [PubMed]
- ——— (1996) Biological roles of the non-integrin elastin/laminin receptor. J Biol Chem 377:471–480 [PubMed]
- Hinek A, Boyle J, Rabinovitch M (1992) Vascular smooth muscle cell detachment from elastin and migration through elastic laminae is promoted by chondroitin sulfate–induced “shedding” of the 67-kDa cell surface elastin binding protein. Exp Cell Res 203:344–353 [DOI] [PubMed]
- Hinek A, Jung S, Rutka JT (1999) Cell surface aggregation of elastin receptor molecules caused by suramin amplified signals leading to proliferation of human glioma cells. Acta Neuropathol (Berl) 97:399–407 [DOI] [PubMed]
- Hinek A, Mecham RP, Keeley F, Rabinovitch M (1991) Impaired elastin fiber assembly is related to reduced 67-kD elastin binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfate. J Clin Invest 88:2083–2094 [DOI] [PMC free article] [PubMed]
- Hinek A, Molossi S, Rabinovitch M (1996) Functional interplay between interleukin-1 receptor and elastin binding protein regulates fibronectin production in coronary artery smooth muscle cells. Exp Cell Res 225:122–131 [DOI] [PubMed]
- Hinek A, Rabinovitch M (1994) 67-kD elastin-binding protein is a protective “companion” of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol 126:563–574 [DOI] [PMC free article] [PubMed]
- Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J (1993) The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest 3:1198–1205 [DOI] [PMC free article] [PubMed]
- Hinek A, Wilson S (2000) A new insight into the mechanism of connective tissue disorders in Hurler syndrome: accumulation of dermatan sulfate causes inhibition of elastic fiber assembly and increased cell proliferation. Am J Pathol (in press) [Google Scholar]
- Hinek A, Wrenn DS, Mecham RP Barondes SH (1988) The elastin receptor: a galactoside-binding protein. Science 239:1539–1541 [DOI] [PubMed]
- Holzenberger M, Lievre CA, Robert L (1993) Tropoelastin gene expression in the developing vascular system of the chicken: an in situ hybridization study. Anat Embryol (Berl) 188:481–492 [DOI] [PubMed]
- Hopwood JJ, Morris CP (1990) The mucopolysaccharidoses: diagnosis, molecular genetics and treatment. Mol Biol Med 7:381–404 [PubMed]
- Humphrey G, Hazel DL, MacLennan K, Lewis I (1999) Expression of CD44 by rhabdomyosarcoma: a new prognostic marker? Br J Cancer 80:918–921 [DOI] [PMC free article] [PubMed]
- Hurle JM, Corson G, Daniels K, Reiter RS, Sakai LY, Solursh M (1994) Elastin exhibits a distinctive temporal and spatial pattern of distribution in the developing chick limb in association with the establishment of the cartilaginous skeleton. J Cell Sci 107:2623–2634 [DOI] [PubMed]
- Johnson GL, Vine DL, Cottrill CM, Noonan JA (1981) Echocardiographic mitral valve deformity in the mucopolysaccharidoses. Pediatrics 67:401–406 [PubMed]
- Johnson JP, Golabi M, Norton ME, Rosenblatt RM, Feldman GM, Yang SP, Hall BD, et al (1998) Costello syndrome: phenotype, natural history, differential diagnosis, and possible cause. J Pediatr 133:441–448 [DOI] [PubMed]
- Jung S, Rutka JT, Hinek A (1998) Tropoelastin and elastin degradation products induce proliferation of human astrocytoma cell lines. J Neuropathol Exp Neurol 57:439–448 [DOI] [PubMed]
- Kerr B, Eden OB, Dandamudi R, Shannon N, Quarrell O, Emmerson A, Ladusans E, et al (1998) Costello syndrome: two cases with embryonal rhabdomyosarcoma. J Med Genet 35:1036–1039 [DOI] [PMC free article] [PubMed]
- Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, et al (1998) Elastin is an essential determinant of arterial morphogenesis. Nature 393:276–280 [DOI] [PubMed]
- Lurie IW (1994) Genetics of the Costello syndrome. Am J Med Genet 52:358–359 [DOI] [PubMed]
- McGowan SE, Liu R, Harvey CS (1993) Effects of heparin and other glycosaminoglycans on elastin production by cultured neonatal rat lung fibroblasts. Arch Biochem Biophys 302:322–331 [DOI] [PubMed]
- Mecham RP, Hauser J (1991) The elastic fiber in cell biology of extracellular matrix. In: Hay E (ed) Cell biology of extracellular matrix, 2nd ed. Plenum Press, New York, pp 79–109 [Google Scholar]
- Mecham RP, Hinek A, Cleary EG, Kucich U, Rosenbloom J (1988) Development of immunoreagents to ciliary zonules that react with protein components of elastic fibers. Biochem Biophys Res Commun 151:822–826 [DOI] [PubMed]
- Mecham RP, Whitehouse L, Hay M, Hinek A, Sheetz MP (1991) Ligand affinity of the 67 kDa elastin/laminin binding protein is modulated by the protein's lectin domain: visualization of elastin/laminin-receptor complexes with gold-tagged ligands. J Cell Biol 113:187–194 [DOI] [PMC free article] [PubMed]
- Mori M, Yamagata T, Mori Y, Nokubi M, Saito K, Fukushima Y, Momoi MY (1996) Elastic fiber degeneration in Costello syndrome. Am J Med Genet 61:304–309 [DOI] [PubMed]
- Nelson J, Shields MD, Mulholland HC (1990) Cardiovascular studies in the mucopolysaccharidoses. J Med Genet 27:94–100 [DOI] [PMC free article] [PubMed]
- Neufeld E, Muenzer J (1997) The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited diseases. McGraw-Hill, New York, pp 2465–2494 [Google Scholar]
- Pasquali-Ronchetti I, Baccarani-Contri M (1997) Elastic fiber during development and aging. Microsc Res Tech 38:428–435 [DOI] [PubMed]
- Patton MA, Baraitser M (1993) Cutis laxa and the Costello syndrome. J Med Genet 30:622 [DOI] [PMC free article] [PubMed]
- Philip N, Sigaudy S (1998) Costello syndrome. J Med Genet 35:238–240 [DOI] [PMC free article] [PubMed]
- Ponta H, Wainwright D, Herrlich P (1998) The CD44 protein family. Int J Biochem Cell Biol 30:299–305 [DOI] [PubMed]
- Privitera S, Prody CA, Callahan JW, Hinek A (1998) The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem 273:6319–6326 [DOI] [PubMed]
- Prosser IW, Whitehouse LA, Parks WC, Stahle-Backdahl M, Hinek A, Park PW, Mecham RP (1991) Polyclonal antibodies to tropoelastin and specific detection and measurement of tropoelastin in vitro. Connect Tissue Res 265:265–279 [DOI] [PubMed]
- Rosenbloom J, Abrams WR, Mecham RP (1993) Extracellular matrix: the elastic fiber. FASEB J 7:1208–1218 [PubMed]
- Say B, Gucsavas M, Morgan H, York C (1993) The Costello syndrome. Am J Med Genet 47:163–165 [DOI] [PubMed]
- Schieken RM, Kerber RE, Ionasescu VV, Zellweger H (1975) Cardiac manifestations of the mucopolysaccharidoses. Circulation 52:700–705 [DOI] [PubMed]
- Selmin O, Volpin D, Bressan GM (1991) Changes of cellular expression of mRNA for tropoelastin in the intraembryonic arterial vessels of developing chick revealed by in situ hybridization. Matrix 11:347–358 [DOI] [PubMed]
- Siwik ES, Zahka KG, Wiesner GL, Limwongse C (1998) Cardiac disease in Costello syndrome. Pediatrics 101: 706–709 [DOI] [PubMed]
- Sneath RJ, Mangham DC (1998) The normal structure and function of CD44 and its role in neoplasia. Mol Pathol 51:191–200 [DOI] [PMC free article] [PubMed]
- Suri M, Garrett C (1998) Costello syndrome with acoustic neuroma and cataract: is the Costello locus linked to neurofibromatosis type 2 on 22q? Clin Dysmorphol 7:149–151 [DOI] [PubMed]
- Teebi AS, Shaabani IS (1993) Further delineation of Costello syndrome. Am J Med Genet 47:166–168 [DOI] [PubMed]
- Tomita H, Fuse S, Ikeda K, Matsuda K, Chiba S (1998) An infant with Costello syndrome complicated with fatal hypertrophic obstructive cardiomyopathy. Acta Paediatr Jpn 40:608–611 [DOI] [PubMed]
- van Eeghen AM, van Gelderen I, Hennekam RC (1999) Costello syndrome: report and review. Am J Med Genet 82:187–193 [DOI] [PubMed]
- Vartio T, Laitinen L, Narvanen O, Cutolo M, Thornell LE, Zardi L, Virtanen I (1987) Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci 88:419–430 [DOI] [PubMed]
- Vila Torres J, Marfa MP, Gonzalez Ense–at MA, Lloreta Trull J (1994) Pathology of the elastic tissue of the skin in Costello syndrome. Anal Quant Cytol Histol 16:421–429 [PubMed]
- Vinallonga X, Sanz N, Balaguer A, Miro L, Ortega JJ, Casaldaliga J (1992) Hypertrophic cardiomyopathy in mucopolysaccharidoses: regression after bone marrow transplantation. Pediatr Cardiol 13:107–109 [DOI] [PubMed]
- Volpi N (1997) Inhibition of human leukocyte elastase activity by chondroitin sulfates. Chem Biol Interact 105:157–167 [DOI] [PubMed]
- Vrhovski B, Weiss AS (1998) Biochemistry of tropelastin. Eur J Biochem 258:1–18 [DOI] [PubMed]
- Wieczorek D, Majewski F, Gillessen-Kaesbach G (1997) Cardio-facio-cutaneous (CFC) syndrome—a distinct entity? Report of three patients demonstrating the diagnostic difficulties in delineation of CFC syndrome. Clin Genet 52:37–46 [DOI] [PubMed]
- Ying QL, Kemme M, Saunders D, Simon SR (1997) Glycosaminoglycans regulate elastase inhibition by oxidized secretory leukoprotease inhibitor. Am J Physiol 272:533–541 [DOI] [PubMed]
- Zampino G, Mastrioacovo P, Ricci R, Zollino M, Sergni G, Martini-Neri ME, Neri G (1993) Costello syndrome: further clinical delineation, natural history, genetic definition and nosology. Am J Med Genet 47:176–183 [DOI] [PubMed]
- Zhou CJ, Kawabuchi M, He JW, Kuraoka A, Hirata K, Wang S, Nada O (1999) Changes in the distribution of peanut agglutinin (PNA) binding molecules during muscle reinnervation following nerve crush injury. Arch Histol Cytol 62:261–272 [DOI] [PubMed]