Abstract
Cerebro-oculo-facio-skeletal (COFS) syndrome is a rapidly progressive neurological disorder leading to brain atrophy with calcification, cataracts, microcornea, optic atrophy, progressive joint contractures, and growth failure. Cockayne syndrome (CS) is a recessively inherited neurodegenerative disorder characterized by low-to-normal birth weight; growth failure; brain dysmyelination with calcium deposits; cutaneous photosensitivity; pigmentary retinopathy, cataracts, or both; and sensorineural hearing loss. CS cells are hypersensitive to UV radiation because of impaired nucleotide excision repair of UV radiation–induced damage in actively transcribed DNA. The abnormalities in CS are associated with mutations in the CSA or CSB genes. In this report, we present evidence that two probands related to the Manitoba Aboriginal population group within which COFS syndrome was originally reported have cellular phenotypes indistinguishable from those in CS cells. The identical mutation was detected in the CSB gene from both children with COFS syndrome and in both parents of one of the patients. This mutation was also detected in three other patients with COFS syndrome from the Manitoba Aboriginal population group. These results suggest that CS and COFS syndrome share a common pathogenesis.
Introduction
Although initially reported in 1971 by Lowry et al., cerebro-oculo-facio-skeletal (COFS) syndrome (MIM 214150) was explicitly delineated by Pena and Shokeir in 1974 as an autosomal recessive progressive brain and eye disorder leading to cerebral atrophy, hypoplasia of the corpus callosum, hypotonia, severe mental retardation, cataracts, microcornea, optic atrophy, progressive joint contractures, and postnatal growth deficiency (Pena and Shokeir 1974). Subsequent reports suggested a spectrum ranging from severe perinatal lethal forms to milder forms that allowed affected individuals to survive childhood (Preus and Fraser 1974; Lurie et al. 1976; Surana et al. 1978; Grizzard et al. 1980; Linna et al. 1982; Casteels et al. 1991; Gershoni-Baruch et al. 1991; Scott-Emuakpor et al. 1977). Several authors have since commented on the clinical similarity between COFS syndrome and Neu-Laxova syndrome (MIM 256520) (Winter et al. 1981; Silengo et al. 1984). Other entities appropriately considered in the differential diagnosis include Martsolf syndrome (MIM 212720) (Martsolf et al. 1978; Sánchez et al. 1985; Hennekam et al. 1988; Strisciuglio et al. 1988; Harbord et al. 1989); cataract, hypertrichosis, mental retardation (CAHMR) syndrome (MIM 211770) (Temtamy and Sinbawy 1991); and Warburg micro (MICRO) syndrome (MIM 600118) (Warburg et al. 1993). COFS syndrome has not been associated with any specific biochemical, chromosomal, or molecular defect, despite intensive investigations and recurrence in a number of consanguineous families.
In this article, we report studies of three patients with the typical COFS syndrome phenotype. Two patients (patients 1 and 2) were dizygotic twins from a family related to the Saulteaux tribe of the Ebb and Flow band in Manitoba, Canada (fig. 1). The third proband (patient 3) is also from the Saulteaux tribe. When first reported in 1974 by Pena and Shokeir, members of this tribe resided on an Indian reservation with a fluctuating population of ∼500 residents. French immigrants were thought to have married into the tribe in the late 19th century and are presumed to have introduced the culpable gene into a geographically restricted population where consanguineous marriages were common (Pena et al. 1978).
Figure 1.
Patients 1 and 2 at age 5 years. They demonstrate classic COFS syndrome facial features, including microcephaly, microphthalmia with deep-set eyes, prominent nasal root, and large ear pinnae.
In the present study, phenotypic characterization of fibroblast cell lines, derived from patients 1 and 3, has revealed features indistinguishable from those observed in classical Cockayne syndrome (CS) (MIM 216400). Additionally, sequencing of cDNA from patients 1 and 3 shows the identical homozygous dinucleotide deletion mutation in the CS group B (CSB) gene, with no mutations in the CSA open-reading frame (ORF). The same mutation is present in one of the CSB alleles in both parents of patient 1. This mutation was also found in DNA obtained from archival tissues from three other tribe members diagnosed with COFS syndrome, the neuropathological findings in whom have been reported elsewhere (Del Bigio et al. 1997). Finally, transfection of fibroblasts derived from patient 1 with normal CSB cDNA complemented the phenotype of hypersensitivity to killing by UV radiation. These results indicate that COFS syndrome is due to mutations in the CSB gene and that COFS syndrome should be considered a distinctive clinical variant of CS.
Material and Methods
Biological Material
Fibroblast cultures from patients 1 and 3 were prepared from skin biopsies. These cells were the source of mRNA. Genomic DNA from both parents of patient 1 was obtained from blood. DNA was obtained from paraffin-embedded cerebral cortex samples from three deceased subjects with COFS syndrome (Del Bigio et al. 1997) as well as from four age-matched controls, as described elsewhere (Stein and Raoult 1992). Wild-type primary and immortalized fibroblast cell lines GM1604 and Tel1604, respectively, were provided by Lisa McDaniel, University of Texas Southwestern Medical Center. The CS-B cell line CS2PB was obtained from the Camden Cell Repository. Fibroblast cell lines were immortalized by infection with a retroviral vector containing human telomerase cDNA. Immortalized COFS syndrome cell lines are designated TelCOFS03MA (patient 1) and TelCOFS02MA (patient 3). Transfection of immortalized cell lines was done with Fugene (Roche Molecular Biochemicals), according to the manufacturer's directions. Cell lines were maintained in Eagle minimum essential medium, supplemented with 15%–20% fetal bovine serum, 2× nonessential amino acids, 2 mM L-glutamine, and antibiotic/antimycotic (Gibco). Selection for cointegration of pSV2neo and pSLME6(+), a plasmid vector containing the CSB cDNA under control of the SV40 late promoter (Troelstra et al. 1992), was with 200 mg/ml G418 (Gibco).
UV Radiation Sensitivity
Six-well cluster dishes were inoculated with 20,000 cells per well in 2 ml of Eagle minimum essential medium/20% irradiated, hybridoma-tested fetal bovine serum. The next day, dishes were rinsed with PBS and irradiated with UVC light, after which they were reincubated in culture medium. Trypsin was added to wells containing confluent cells, and cells were counted with a particle counter so that we could generate quantitative survival curves.
Complementation of UV Radiation Sensitivity
After transfection with either pSV2neo or cotransfection with pSV2neo and pSLME6(+), cells were selected with G418. G418-resistant clones were pooled, and cells (3×105) were plated in 10-cm dishes in duplicate. The next day, cells were washed twice with PBS and irradiated with UVC radiation. After 5 d, surviving cells were rinsed in PBS, fixed with 1% glutaraldehyde in PBS for 15 min, rinsed with PBS, and stained with crystal violet for 30 min. The dye was removed, and dishes were washed to remove excess dye. After drying the dishes, dye was extracted with 70% ethanol for ⩾30 min. Spectrophotometric readings (575 nm) of dye concentration were used to quantitate survival. Experiments were performed in triplicate.
Unscheduled DNA Synthesis and Recovery of RNA Synthesis after UV Irradiation
Analysis of unscheduled DNA synthesis (DNA repair synthesis) was performed as described by De Vries et al. (1995). Recovery of RNA synthesis in UV-irradiated cells was measured as described by Troelstra et al. (1992).
Mutational Analysis
Total RNA was prepared from fibroblast cell lines by the TRIZOL reagent (Gibco BRL), according to manufacturer's instructions. cDNA was synthesized from total RNA by the Superscript preamplification system (Gibco BRL), followed by PCR with the Expand High Fidelity PCR system (Boehringer-Mannheim). Sequencing of amplified fragments was by standard automated methods with an ABI PRISM 310 (PE Biosystems). Amplification and sequencing of the CSA ORF was done as described by Henning et al. (1995). CSB cDNA sequencing was performed with the following primers: forward, 31–53, 5′-TCTCTGTTTCCTTGTGGGCGCTC-3′; 828–848, 5′-CCCAGATCCCTCAGAAACAGG-3′; 2450–2472, 5′-GGAGAGATGCAGATTTTCTCCGG-3′; and 3236–3255, 5′-CGCAAGAAGTTCCCTGCTTC-3′; and reverse, 999–980, 5′-GGGGCTGGAGGCGTGACTGG-3′; 2827–2805, 5′-CACCCGCGTGGTCAGAAGAAACA-3′; 3683–3663, 5′-GTCTCAGATGTTTCTCCAGGG-3′; and 4529–4507, 5′-CTTCACCACCAGAAGTTCTATGG-3′.
CSB cDNA from patients was amplified in four parts with primers 31–53/999–980, 828–848/2827–2805, 2450–2472/3683–3663, and 3236–3255/4529–4507. Sequencing was done with these primers plus additional ones (sequence not shown) necessary to cover the entire coding region.
Genomic DNA from the parents was digested with XbaI and subjected to agarose gel electrophoresis. A fragment ∼4 kb in size was shown by Southern hybridization to contain the relevant sequence. A gel slice containing 3–5 kb DNA fragments was isolated. The fragments were harvested from the gel slice with QiaQuick (Qiagen). This DNA was used in a PCR reaction with the following primers: 3641–3670 forward, 5′-CATAGTGTGGCAGAAGAAGAGACCCTGGAG-3′; and 3830–3800 reverse, 5′-CATAATCGTCATTGCTCTGTTCCTTGGCCTC-3′. PCR fragments were either sequenced directly or cloned into pGEM-T-Easy (Promega), and several clones were sequenced. For the paraffin-embedded samples, the procedures we followed were as described for total genomic DNA, except that it was unnecessary to submit samples to XbaI digestion.
Results
Case Reports
Patients 1 and 2 were dizygotic male-female twins born small for their gestational age. They had microcephaly, deep-set eyes, bilateral microphthalmia and cataracts, overhanging upper lips, and prominent noses (fig. 1), and the boy had undescended testes. Their parents were from the same Manitoba tribe in which COFS syndrome was originally reported, and family history revealed three normal siblings and no known consanguinity. The twins manifested failure to thrive, recurrent pneumonias, axial hypotonia, appendicular hypertonia, hyperreflexia, and progressive contractures. Both were diagnosed with typical features of COFS syndrome (fig. 1). Despite high-calorie feeding via gastrostomy tubes, the twins showed minimal weight gain and no developmental progress. The girl died at age 5 years; she suffered from dehydration, metabolic acidosis, and seizures. Before her death, cranial computed tomography scans revealed enlargement of the ventricles and subarachnoid spaces, with calcifications in the periventricular frontal white matter and basal ganglia. Autopsy revealed severe neurodegeneration, with markedly reduced brain weight, patchy demyelination in the cerebrum and hindbrain, neuronal loss and gliosis in the cerebral cortex, and pericapillary and parenchymal mineralization in the cortex and basal ganglia (case 7 in the study by Del Bigio et al. [1997]).
Teeth erupted at age 5 years in the surviving boy, but they became carious, even though he was fed by gastrostomy tube. At age 2 years his hearing was normal, but there was profound hearing loss by age 6 years, when he developed insulin resistance. At age 7 years he had further neurological deterioration, and computed tomography showed brain atrophy with basal ganglia calcifications similar to that observed in his sister. Electroencephalogram indicated that he had no seizure activity. This boy never manifested cutaneous photosensitivity or actinic keratoses, but at age 10 years he had ocular photosensitivity with consistently constricted pupils. Cranial magnetic resonance imaging scan revealed severe atrophy of brain stem, cerebellum, and upper cervical spinal cord, with diffuse cerebral atrophy. Areas of increased signal intensity on T2 weighting in the periventricular cerebral white matter were interpreted as demyelination. The patient died at age 11 years.
Patient 3 was a girl first seen by geneticists at age 2 years, when the diagnosis of COFS syndrome was made. She had three younger siblings, all normal. She was the daughter of Aboriginal parents from the same population as patients 1 and 2, although consanguinity was denied. She presented at birth with growth deficiency, microcephaly, and bilateral microphthalmia with cataracts. She failed to thrive and manifested profound developmental deficiency, recurrent pneumonia, and seizures. She had small, deep-set eyes, a prominent nasal root and tip, an overhanging upper lip, and mild micrognathia. Appendicular tone was increased with decreased axial tone, and she developed progressive contractures. Eruption of teeth was delayed until age 4 years, and she was inattentive to visual stimuli. She had no freckling, actinic keratoses, or photosensitivity, and she died at age 6 years.
Phenotypic Characterization of COFS Syndrome Cells
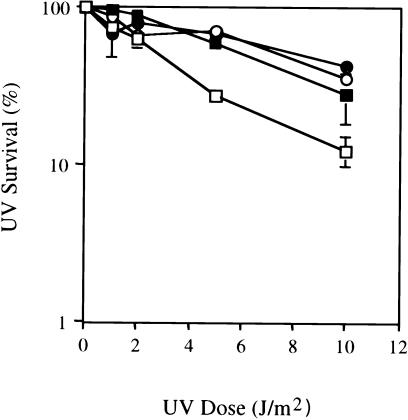
Primary fibroblasts were established from patients 1 and 3, and sublines were immortalized by infection with the human telomerase (hTERT) gene, a recently developed strategy that results in immortalization with no other discernible alterations in the phenotype of diploid primary fibroblasts (Bodnar et al. 1998). Phenotypic characterization of cells was carried out independently in two laboratories (those of D.B.B. and E.C.F.). Both the primary (fig. 2) and the immortalized cell lines (data not shown) were distinctly more sensitive to killing by UV radiation than were cells from an unaffected control individual. Additionally, both immortalized (fig. 3) and mortal (data not shown) cell lines revealed normal levels of global nucleotide excision repair (NER), as determined by repair synthesis in living cells exposed to UV radiation. Finally, measurement of repair synthesis of plasmid DNA treated with the DNA-damaging agent acetylaminofluorene and incubated with cell-free extracts showed normal levels of NER in extracts of one of the immortalized cell lines (data not shown). Collectively, these results indicate that sensitivity to killing after exposure to UV radiation is not the result of defective global NER, in contrast to what is observed in the NER-defective hereditary disease xeroderma pigmentosum (Cleaver and Kraemer 1989). Base excision repair of oxidative damage in vitro was also normal in these two cell lines (data not shown).
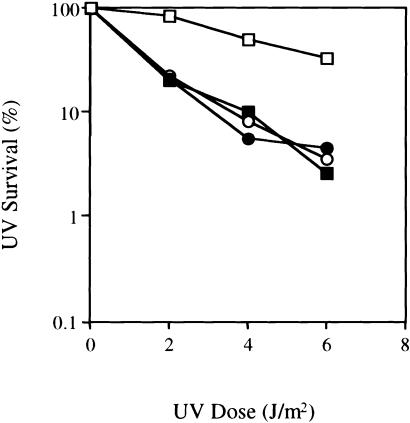
Figure 2.
Survival of COFS cell lines after exposure to UV radiation. Wild-type fibroblast cell line GM1604 (unblackened squares), COFS syndrome patient 1 (blackened circles), COFS syndrome patient 3 (unblackened circles), and cells from a CS group B patient (blackened squares). Survival values are the mean of triplicate experiments.
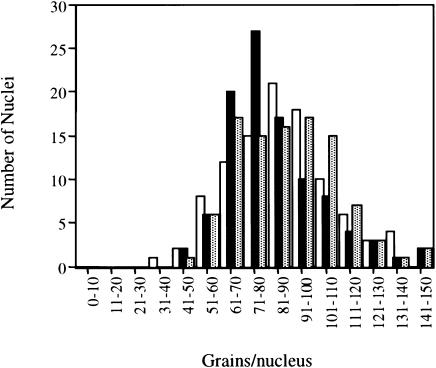
Figure 3.
COFS cell lines are proficient in unscheduled DNA synthesis. Quantitation of grains/nucleus in 100 non-S phase cells of a wild-type (Tel1604) cell line (blackened bars), COFS cell line TelCOFS03MA (unblackened bars), and COFS cell line TelCOFS02MA (gray bars). Grain counts are expressed as histograms and show that the COFS cell lines are proficient in unscheduled DNA synthesis.
Moderately increased sensitivity to UV radiation is a characteristic phenotype of cells from patients with CS. CS cells manifest normal global NER but are defective in NER of actively transcribed genes or transcription-coupled NER (van Hoffen et al. 1993). The latter phenotype manifests with a delay in the rate of recovery of RNA synthesis after exposure to UV radiation. Primary fibroblasts from COFS syndrome patients 1 and 3 (data not shown), as well as cell lines immortalized by transfection with the telomerase gene, manifested reduced kinetics of recovery of RNA synthesis comparable to that observed in cells of people with CS (fig. 4).
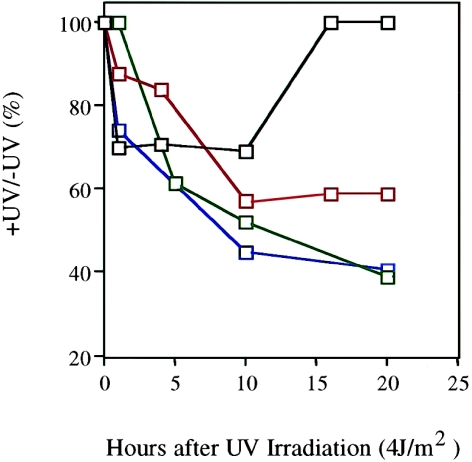
Figure 4.
Recovery of RNA synthesis in a wild-type cell line (Tel1604; blackened squares) and COFS cell lines TelCOFS02MA (green squares) and TelCOFS03MA (red squares). Data for a CSB mutant cell line (CS2PB; blue squares) are shown for comparison. Unirradiated or UV-irradiated (with 4 J/m2) cells were pulse-labeled with [3H] at different times after irradiation (see Material and Methods section for details). Data points represent the mean of triplicate plates. The SEM falls within the size of the symbols.
Mutational Analysis of CSA and CSB cDNA
The findings described above prompted examination of the nucleotide sequence of the CSA and CSB ORFs by use of cDNA from fibroblasts of patients 1 and 3. No mutations were detected in the CSA ORF (data not shown). In contrast, we detected homozygous deletion of two nucleotides at position 3794 of the CSB ORF in both patients analyzed with COFS syndrome (fig. 5B). This deletion generates the nonsense codon TGA at amino acid position 1240 and is expected to result in a truncated polypeptide missing the C-terminal 254 amino acids (fig. 5B). The identical mutation was observed in one CSB allele in the parents of patient 1 (fig. 5C). Examination of genomic DNA extracted from formalin-fixed, paraffin-embedded cerebral cortex archival material from three confirmed cases of COFS syndrome (Del Bigio et al. 1997), as well as from four age-matched controls, revealed the identical mutation in all three samples from the COFS syndrome patients but not in the age-matched controls (data not shown).
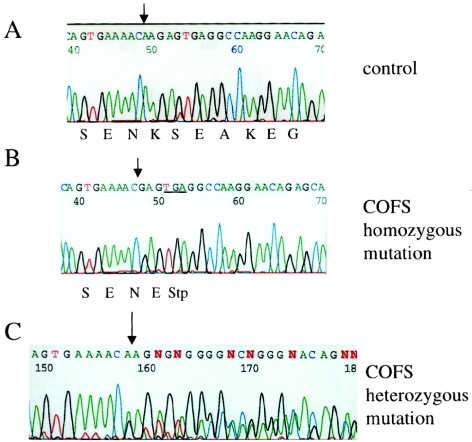
Figure 5.
Sequence chromatograms showing the mutation found in patients with COFS syndrome. Arrows mark the position where the mutation is localized. A, Wild-type nucleotide and predicted amino acid sequence. B, Sequence of one patient with COFS syndrome; the anticipated stop codon is underlined. C, Sequence of the father of the patient depicted in B. Note the frameshift shown by the overlap of both wild-type and mutant alleles in the carrier (arrow).
Complementation of UV Radiation Sensitivity of COFS Cells
To confirm that the phenotype of COFS cells is due to a defect in the CSB gene, we introduced wild-type CSB cDNA into immortalized COFS cells from patient 1 and examined UV radiation sensitivity. Cotransfection of a plasmid vector, pSLME6(+), from the study by Troelstra et al. (1992) containing the CSB cDNA under control of the SV40 late promoter together with plasmid pSV2neo (expressing the gene for resistance to G418), into wild-type and COFS cells yielded G418-resistant clones. Cells were also transfected with pSV2neo alone as a control. COFS cells transfected with plasmid pSV2neo alone were abnormally sensitive to killing by UV radiation (fig. 6). This sensitivity was significantly corrected by cotransfection with pSV2neo and the vector containing the CSB gene (fig. 6). On the basis of all the results presented above, we conclude that the mutation in the CSB gene observed in COFS cells is the direct cause of the cellular phenotypes.
Figure 6.
Complementation of UV radiation sensitivity in immortalized COFS cells (TelCOFS03MA). COFS and wild-type cell lines were transfected with either pSV2neo alone or cotransfected with pSV2neo and the expression vector pSLME6 carrying the CSB gene. G418-resistant clones were pooled and examined for UV radiation sensitivity. Repair-proficient line Tel1604 transfected with pSV2neo (unblackened circles) or with pSV2neo plus pSLME6 (blackened circles). COFS cells (TelCOFS03MA) transfected with pSV2neo (unblackened squares) or with pSV2neo plus pSLME6 (blackened squares). Vertical bars represent the SEM of three experiments.
Discussion
COFS syndrome is rare but well documented. The original reports by Pena and Shokeir (1974) and Pena et al. (1978) suggested an autosomal recessive mode of inheritance. Nine of the initial 10 cases reported by them were from two French-Indian families in a genetically isolated Manitoba Aboriginal population in which consanguineous marriages were common. The 10th case occurred in a French family from the same region, but the identity of the father was not established. Key features include congenital microcephaly with subsequent brain atrophy, reduced white matter, patchy gray matter, hypotonia, deep-set eyes with microphthalmia and cataracts, and camptodactyly with rocker-bottom feet. Movement is markedly decreased, leading to joint contractures, and life span is usually short (Pena and Shokeir 1974; Pena et al. 1978). Failure to thrive in these children is common because of feeding problems. Recurrent aspiration pneumonia led to death at age <30 mo in 8 of the initial 10 patients described (Pena and Shokeir 1974; Pena et al. 1978).
The initial report by Pena and Shokeir cited a study by Lowry et al. (1971) in which two siblings with microcephaly, microphthalmia, cataracts, kyphosis, limited joint movements, undescended testes, shallow acetabula, and coxa valga were described. Preus and Fraser (1974) reported a similar but more-severely affected child of Italian parents who were first cousins, living in Montreal. Subsequently, single patients with COFS syndrome whose parents were consanguineous were described (Lurie et al. 1976; Grizzard et al. 1980). Additionally, Scott-Emuakpor et al. (1977) reported four siblings with COFS syndrome whose parents were unrelated. These reports expanded the spectrum of severity in both directions and confirmed the autosomal recessive transmission of the disorder.
In a longitudinal follow-up study of some patients they originally studied, Pena et al. (1978) emphasized the progressive nature of COFS syndrome and suggested that it may represent a primary neurodegenerative disorder. They also commented on observations of patients reported earlier with anomalies such as cardiac defects due to defective atrioventricular septation (Lurie et al. 1976; Preus et al. 1977) and expressed doubt that these represented true COFS syndrome. However, a recent report (Casteels et al. 1991) described two unrelated infants with COFS syndrome. One was classically affected, but the other had associated heart defects.
The distinction between COFS syndrome and CS can pose a serious clinical problem. Linna et al. (1982) emphasized the diagnostic utility of foci of intracranial calcification in the lenticular nuclei and hemispheric white matter in affected siblings with COFS syndrome. However, progressive demyelination with brain calcification is also observed in the severe infantile form of CS (Nance and Berry 1992). In both conditions, intrauterine growth can be normal, but postnatal growth deficiency is striking and unrelenting. Hence, it has become increasingly more difficult to clinically distinguish between early-onset CS, classical COFS syndrome (with congenital cataracts, microphthalmia, and intracranial calcifications), and other progressive demyelinating diseases similar to early-onset CS (Linna et al. 1982; Patton et al. 1989; Talwar and Smith 1989; Nance and Berry 1992).
Studies have documented the location of mutations in the CSB gene in 16 CS patients (Mallery et al. 1998). The mutation reported in the present study is novel and is situated downstream of the most 3′ mutation reported. One might therefore anticipate that the COFS syndrome form of CSB protein would be less truncated and perhaps account for a less-severe phenotype than that shown by either classical CS type I patients or the more-severely affected type II form (Mallery et al. 1998; Colella et al. 1999). However, we have recently identified a mutation in another patient with COFS syndrome who is not a member of the aboriginal COFS syndrome kindred (C. Powell, L. Meira, and E. Friedberg, unpublished data). Molecular analysis of cells from this individual revealed a mutation in exon 10 of the CSB gene, 5′ to many of the mutations reported in CS. Collectively, these results suggest that the severity of the disease does not directly correlate with the site of mutation in the linear CSB gene.
We did not note clinical photosensitivity in the patients with COFS syndrome described in this study. Nonetheless, cells from these individuals are abnormally sensitive to killing by UV radiation, and this phenotype can be corrected by transfection with wild-type CSB cDNA. Additionally, the cells manifested other phenotypes documented earlier in CS, including delayed recovery of RNA synthesis after exposure to UV radiation (Nance and Berry 1992). Finally, and most conclusively, both cases of COFS syndrome revealed identical mutations in the CSB gene. On the basis of these findings, we suggest that COFS syndrome no longer constitutes a distinct genetic entity but represents an allelic, clinically severe form of CS.
Acknowledgments
We thank our laboratory colleagues for valuable discussions and for critical review of the manuscript, and Lisa McDaniel for providing cell lines GM1604 and Tel1604. We acknowledge support by SHARE's Child Disability Center and the Steven Spielberg Pediatric Research Center; University of California–Los Angeles Intercampus Training Program grant GM08243 from the National Institutes of Health; grants P01 HD22657-06 (J.M.G.) and CA44247 (E.C.F.) from the U.S. Department of Health and Human Services; and the American Registry of Pathology, Armed Forces Institute of Pathology.
Electronic-Database Information
The URL for data in this article is as follows:
References
- Bodnar AG, Ouelette M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, et al (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352 [DOI] [PubMed]
- Casteels I, Wijnants A, Casaer P, Eggermont E, Misotten L, Fryns JP (1991) Cerebro-oculo-facio-skeletal (COFS) syndrome. The variability of presenting symptoms as a manifestation of two subtypes? Genet Counseling 2:43–46 [PubMed] [Google Scholar]
- Cleaver JE, Kraemer KH (1989) Xeroderma pigmentosum. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited disease, vol 2. McGraw-Hill, New York, pp 2949–2971 [Google Scholar]
- Colella S, Nardo T, Mallery D, Borrone C, Ricci R, Ruffa G, Lehmann AR, et al (1999) Alterations in the CSB gene in three Italian patients with the severe form of Cockayne syndrome (CS) but without clinical photosensitivity. Hum Mol Genet 8:935–941 [DOI] [PubMed]
- Del Bigio MR, Greenberg CR, Rorke LB, Schnur R, McDonald-McGinn DM, Zachai EH (1997) Neuropathological findings in eight children with cerebro-oculo-facio-skeletal (COFS) syndrome. J Neuropathol Exp Neurol 56:1147–1157 [DOI] [PubMed]
- De Vries A, van Oostrom CThM, Hofhuis FMA, Dortant PM, Berg RJW, de Gruijl FR, Wester PW, et al (1995) Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene XPA. Nature 377:169–173 [DOI] [PubMed]
- Gershoni-Baruch R, Ludatscher RM, Lichtig C, Sujov P, Machoul I (1991) Cerebro-oculo-facio-skeletal syndrome: further delineation. Am J Med Genet 41:74–77 [DOI] [PubMed]
- Grizzard WS, O'Donnell JJ, Carey JC (1980) The cerebro-oculo-facio-skeletal syndrome. Am J Ophthalmol 89:293–298 [DOI] [PubMed]
- Harbord MG, Baraitser M, Wilson J (1989) Microcephaly, mental retardation, cataracts, and hypogonadism in sibs: Martsolf's syndrome. J Med Genet 26:397–406 [DOI] [PMC free article] [PubMed]
- Hennekam RCM, van de Meeberg AG, van Doorne JM, Dijkstra PF, Bijlsma JB (1988) Martsolf syndrome in a brother and sister: clinical features and pattern of inheritance. Eur J Pediatr 147:539–543 [DOI] [PubMed]
- Henning KA, Li L, Iyer N, McDaniel LD, Reagan MS, Legerski R, Schultz RA, et al (1995) The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 82:555–564 [DOI] [PubMed]
- Linna S-L, Finni K, Simila S, Kouvalainen K, Laitinen J (1982) Intracranial calcifications in cerebro-oculo-facio-skeletal (COFS) syndrome. Pediatr Radiol 12:28–30 [DOI] [PubMed]
- Lowry RBR, McLean R, McLean DM, Tischler B (1971) Cataracts, microcephaly, kyphosis and limited joint movement in two siblings: a new syndrome. J Pediatr 79:282–284 [DOI] [PubMed]
- Lurie IW, Cherstvoy ED, Lazjuk GI, Nedzved MK, Usoev SS (1976) Further evidence for the autosomal-recessive inheritance of the COFS syndrome. Clin Genet 10:343–346 [DOI] [PubMed]
- Mallery DL, Tanganelli B, Colella S, Steingrimsdottir H, van Gool AJ, Troelstra C, Stefanini M, et al (1998) Molecular analysis of mutations in the CSB (ERCC6) gene in patients with Cockayne syndrome. Am J Hum Genet 62:77–85 [DOI] [PMC free article] [PubMed]
- Martsolf JT, Hunter AGW, Haworth JC (1978) Severe mental retardation, cataracts, short stature, and primary hypogonadism in two brothers. Am J Med Genet 1:291–299 [DOI] [PubMed]
- Nance MA, Berry SA (1992) Cockayne syndrome: review of 140 cases. Am J Med Genet 42:68–84 [DOI] [PubMed]
- Patton MA, Gianelli F, Francis AJ, Baraitser M, Harding B, Williams AJ (1989) Early onset Cockayne's syndrome: case reports with neuropathological and fibroblast studies. J Med Genet 26:154–159 [DOI] [PMC free article] [PubMed]
- Pena SDJ, Evans J, Hunter AGW (1978) COFS syndrome revisited. Birth Defects Original Article Series, vol 14(6B):205–213 [PubMed] [Google Scholar]
- Pena SDJ, Shokeir MHK (1974) Autosomal recessive cerebro-oculo-facio-skeletal (COFS) syndrome. Clin Genet 5:285–293 [DOI] [PubMed]
- Preus M, Fraser FC (1974) The cerebro-oculo-facio-skeletal syndrome. Clin Genet 5:294–297 [DOI] [PubMed]
- Preus M, Kaplan P, Kirkham TH (1977) Renal anomalies and oligohydramnios in the cerebro-oculofacio-skeletal syndrome. Am J Dis Child 131:62–64 [DOI] [PubMed]
- Sánchez JM, Barreiro C, Freilij H (1985) Two brothers with Martsolf's syndrome. J Med Genet 22:308–310 [DOI] [PMC free article] [PubMed]
- Scott-Emuakpor AB, Heffelfinger J, Higgins JV (1977) A syndrome of microcephaly and cataracts in four siblings: a new genetic syndrome? Am J Dis Child 131:167–169 [DOI] [PubMed]
- Silengo MC, Davi G, Bianco R, Biagioli M, Franceschini P, Cavallo M, Bussi G (1984) The NEU-COFS (cerebro-oculo-facio-skeletal) syndrome: report of a case. Clin Genet 25:201–204 [DOI] [PubMed]
- Stein A, Raoult D (1992) A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res 20:5237–5238 [DOI] [PMC free article] [PubMed]
- Strisciuglio P, Costabile M, Esposito M, Di Maio S (1988) Martsolf's syndrome in a non-Jewish boy. J Med Genet 25:267–269 [DOI] [PMC free article] [PubMed]
- Surana RB, Fraga JR, Sinkford SM (1978) The cerebro-oculo-facio-skeletal syndrome. Clin Genet 13:486–488 [DOI] [PubMed]
- Talwar D, Smith SA (1989) CAMFAK syndrome: a demyelinating inherited disease similar to Cockayne syndrome. Am J Med Genet 34:194–198 [DOI] [PubMed]
- Temtamy SA, Sinbawy AHH (1991) Cataract, hypertrichosis, and mental retardation (CAHMR): a new autosomal recessive syndrome. Am J Med Genet 41:432–433 [DOI] [PubMed]
- Troelstra C, van Gool A, de Wit J, Vermeulen W, Bootsma D, Hoeijmakers JHJ (1992) ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne's syndrome and preferential repair of active genes. Cell 71:939–953 [DOI] [PubMed]
- van Hoffen A, Natarajan AT, Mayne LV, van Zeeland AA, Mullenders LHF (1993) Deficient repair of the transcribed strand of active genes in Cockayne's syndrome. Nucleic Acids Res 21:5890–5895 [DOI] [PMC free article] [PubMed]
- Warburg M, Sjö O, Fledelius HC, Pedersen SA (1993) Autosomal recessive microcephaly, microcornea, congenital cataract, mental retardation, optic atrophy, and hypogenitalism: MICRO syndrome. Am J Dis Child 147:1309–1312 [DOI] [PubMed]
- Winter RM, Donnai D, Crawford MD (1981) Syndrome of microcephaly, microphthalmia, cataracts and joint contractures. J Med Genet 18:129–131 [DOI] [PMC free article] [PubMed]