Abstract
Wolfram syndrome, which is sometimes referred to as “DIDMOAD” (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness), is an autosomal recessive neurodegenerative disorder for which only insulin-dependent diabetes mellitus and optic atrophy are necessary to make the diagnosis. Researchers have mapped Wolfram syndrome to chromosome 4p16.1, and, recently, a gene encoding a putative transmembrane protein has been cloned and mutations have been identified in patients. To pursue the possibility of locus heterogeneity, 16 patients from four different families were recruited. These patients, who have the Wolfram syndrome phenotype, also have additional features that have not previously been reported. There is an absence of diabetes insipidus in all affected family members. In addition, several patients have profound upper gastrointestinal ulceration and bleeding. With the use of three microsatellite markers (D4S432, D4S3023, and D4S2366) reported to be linked to the chromosome 4p16.1 locus, we significantly excluded linkage in three of the four families. The two affected individuals in one family showed homozygosity for all three markers from the region of linkage on chromosome 4p16.1. For the other three families, genetic heterogeneity for Wolfram syndrome was verified by demonstration of linkage to chromosome 4q22-24. In conclusion, we report the unique clinical findings and linkage-analysis results of 16 patients with Wolfram syndrome and provide further evidence for the genetic heterogeneity of this disorder. We also provide data on a new locus that plays a role in the etiology of insulin-dependent diabetes mellitus.
Introduction
Wolfram syndrome, which is also known as “DIDMOAD” (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness [MIM 222300]) and which was first described by Wolfram and Wagner (1938), is associated with high morbidity and mortality. Only insulin-dependent diabetes mellitus (IDDM) and progressive optic-nerve atrophy (OA) are necessary to make the diagnosis, but associated findings include sensorineural hearing loss (SNHL), ataxia, peripheral neuropathy, urinary-tract atony, and psychiatric illnesses (Rose et al. 1966; Cremers et al. 1977; Swift et al. 1990; Salih and Tuvemo 1991). Typically, but not invariably, IDDM is detected before OA is detected, whereas diabetes insipidus (DI) and SNHL may or may not develop. Wolfram syndrome is an autosomal recessive disorder with probable locus heterogeneity, based on reported clinical heterogeneity (Rose et al. 1966; Page et al. 1976; Barrett and Bundey 1997). The literature contains a wealth of familial and individual reports as well as several comprehensive clinical reviews (Bretz et al. 1970; Blasi et al. 1986; Kinsley et al. 1995; Barrett and Bundey 1997).
Wolfram syndrome has previously been shown to be linked to markers at chromosome 4p16.1 (Polymeropoulos et al. 1994; Barrientos et al. 1996a, 1996b; Collier et al. 1996). Recently, mutations in a gene known as “wolframin” were identified in patients with Wolfram syndrome (Inoue et al. 1998; Strom et al. 1998). This gene, which encodes a novel protein containing predicted transmembrane domains, is expressed ubiquitously, with high expression in the pancreatic islets. However, evidence of locus heterogeneity exists, in that there was no linkage to this region in one small nuclear family in which OA presented one decade prior to diabetes mellitus (DM), which is an atypical Wolfram syndrome phenotype (Collier et al. 1996).
In this study, we present clinical data on 16 affected individuals with a clinical presentation that fits the diagnosis of Wolfram syndrome. These individuals are from four consanguineous Jordanian families. The genetic heterogeneity for Wolfram syndrome was verified by mapping of another locus to chromosome 4q22-24, by use of a DNA-pooling linkage strategy, and Neuromedin K receptor 3 (NKR3), a candidate gene that maps in the critical region, was screened for mutations in patients with Wolfram syndrome.
Patients and Methods
Patient Evaluation
Nineteen individuals affected with Wolfram syndrome were identified from seven families that were seen in the clinical service provided by the National Center for Diabetes, Endocrine, and Genetic Diseases in Amman, Jordan. Of these seven families, four families with several affected siblings (n=16) were recruited to participate in the study after informed consent was obtained from each individual or from a legal guardian. All recruited family members were examined thoroughly, and detailed family history and medical history were obtained. All available family members underwent a detailed ophthalmologic examination, and audiography was performed. Most of those who showed signs of the disease also underwent intravenous urography and magnetic-resonance imaging of the pituitary gland. A water-deprivation test was done for most family members. Any family member who showed at least two of the four cardinal signs of Wolfram syndrome was considered to be affected.
Genotyping and Linkage Analysis
DNA was extracted from leukocytes in venous blood, by use of standard procedures. Four microsatellite DNA markers (D4S432, D4S3023, D4S2366, and D4S2639) known to be linked to the Wolfram syndrome locus on chromosome 4p16.1 were used to test for linkage in the four families (Polymeropoulos et al. 1994; Collier et al. 1996).
Homozygosity mapping was done with the use of a DNA-pooling strategy (Sheffield et al. 1995). Equimolar amounts of samples from families WS-3 and WS-4 were independently pooled into affected and unaffected pools from each family. Genotyping was performed by means of standard protocols (Lidral et al. 1997).
A 20-cM genomewide scan was performed, with an initial focus on those markers from the Weber mapping panel, version 8.0 (Yuan et al. 1997), that are on gene-rich chromosomes (Antonarakis 1994; Inglehearn 1997). Additional markers for fine mapping of the critical region were chosen from maps from the Center for Medical Genetics, Marshfield Medical Research Foundation (Broman et al. 1998), the Cooperative Human Linkage Center (CHLC) (Murray et al. 1994), and GeneMap'98 (Deloukas et al. 1998).
Two-point LOD score analysis was performed with the use of the LINKAGE (Lathrop et al. 1984) and GENEHUNTER (Kruglyak et al. 1996) programs, and multipoint analysis was performed with the use of GENEHUNTER, under the assumption of autosomal recessive inheritance with 95% penetrance and a disease-gene frequency of .005. The reference genetic map and allele frequencies used for the analysis were generated from CHLC and CEPH data (Broman et al. 1998). The order and distances designated for the chromosome 4p16 markers were D4S432-.014-D4S3023-.0469-DS2366-.205-D4S2639, and those designated for the chromosome 4q markers were D4S3243-.051-D4S2361-.114-D4S1647-.020-D4S1591-.021-D4S3256-.036-D4S1531-.001-D4S1564-.014-D4S3240-.001-D4S2623-.024-D4S2945-.128-D4S2394. Recalculation of the LOD scores, by use of equal allele frequencies, resulted in minimal changes and in no change in significance (data not shown).
Mutation Screen
The NKR3 gene for was screened for mutations with the use of primers designed to amplify the five exons, including splice sites and splice-branch sites, (Buell et al. 1992; primer sequences available on request). Sequences from one affected individual each from families WS-2–WS-4, along with sequences from a control individual, were sequenced with the use of the Big Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems) and were analyzed on an ABI 310 capillary DNA analyzer (PE Biosystems). Templates were prepared by means of PCR amplification and were purified with the use of Qiaquick PCR columns (Qiagen).
Results
Clinical Features
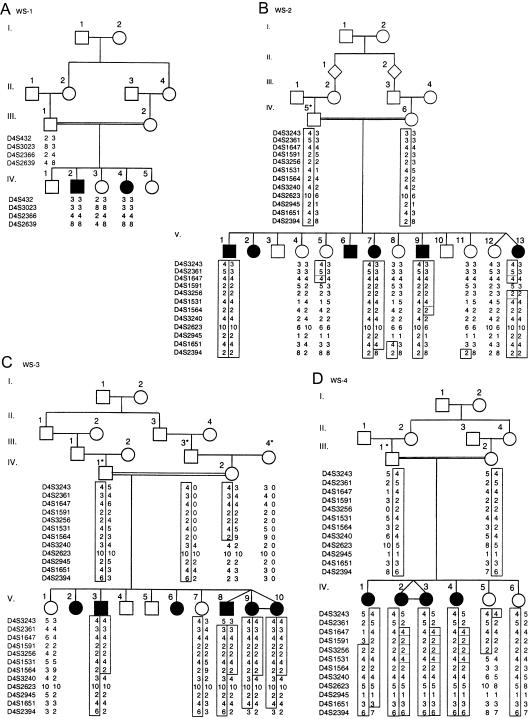
Family WS-1.—The two affected individuals in family WS-1 are a 17-year-old boy and a 10-year-old girl (table 1). Both presented with DM at the age of ∼5 years; shortly thereafter, a visual deficit occurred in both, and a hearing deficit occurred in the boy. The visual deficit was attributed to progressive OA, whereas the hearing loss was caused by high-frequency SNHL. The boy has urinary-tract dilatation with impaired kidney function, and both the boy and the girl had a normal response (urine osmolarity doubled at the end of 3 h) to the water-deprivation test. They both have soft, dysmorphic features, probably as a result of decreased facial subcutaneous fat. Recently, after a severe chest infection, the boy died of respiratory failure of central origin. The parents in family WS-1 are first cousins (fig. 1).
Table 1.
Diagnosis and Age at Diagnosis in Patients with Wolfram Syndrome
|
Diagnosis and Age at Diagnosis in Affected Individuals in Each Familya |
||||||||||||||||
| Family WS-1 |
Family WS-2 |
Family WS-3 |
Family WS-4 |
|||||||||||||
| Characteristics | IV.2 | IV.4 | V.1 | V.2 | V.6 | V.7 | V.9 | V.13 | V.3 | V.8 | V.9 | V.10 | IV.1 | IV.2 | IV.3 | IV.4 |
| Age (years) | 17 | 10 | 38 | 35 | 25 | 23 | 20 | 15 | 28 | 14 | 14 | 14 | 14 | 12 | 12 | 6 |
| Sex | M | F | M | F | M | F | M | F | M | M | F | F | F | F | F | F |
| DM: | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Age at diagnosis (years) | 5 | 5 | 15 | 15 | 12 | 14 | 15 | 12 | 10 | 10 | 10 | 10 | 13 | NA | NA | NA |
| OA: | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Age at diagnosis (years) | 7 | 7 | 20 | 22 | 18 | 18 | 19 | 14 | 20 | 12 | 12 | 12 | 13 | 11 | 11 | 5 |
| SNHL: | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Age at diagnosis (years) | 10 | NA | 25 | 25 | 18 | 18 | NA | 14 | 24 | 12 | 12 | 12 | 13 | 11 | 11 | 5 |
| DI | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Urinary-tract dilatation: | Yes | No | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | No | Yes | No | No | No |
| Age at diagnosis (years) | 14 | NA | 35 | 34 | 24 | NA | NA | NA | 24 | 12 | 12 | NA | 13 | NA | NA | NA |
| Peptic ulcer: | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No |
| Age at diagnosis (years) | NA | NA | 20 | 20 | 17 | 20 | 18 | 14 | 26 | 13 | 13 | 13 | NA | 11 | NA | NA |
| Diabetic retin | No | No | Yes | No | Yes | No | No | No | Yes | No | No | No | No | No | No | No |
| Status | Dead | Dead | Alive | Alive | Alive | Dead | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
M = male; F = female; NA = not available.
Figure 1.
Pedigrees of families with Wolfram syndrome (DIDMOAD). A, Family WS-1 shows homozygosity for markers at the chromosome 4p16 WFS1 locus. B–D, Families WS-2–WS-4 show haplotypes for markers at chromosome 4q22-24. Recombinations in individuals V.9 and V.13 from family WS-2, individual IV.2 from family WS-3, and individual IV.1 from family WS-4 define the critical region. For individuals denoted by an asterisk, genotypes were inferred from the genotypes of existing family members, and maximum-likelihood haplotypes were deduced by use of GENEHUNTER. Blackened squares denote haplotypes and recombinants, and broken lines indicate alternative crossover events that could also explain the data.
Family WS-2.—Family WS-2 includes six affected individuals in one sibship (table 1; fig. 1). All affected individuals had DM develop in the early part of their second decade; this was soon followed by both visual and hearing deficits, except in the case of the 20-year-old man (individual V.9), who still has normal hearing. All affected individuals have documented OA and high-frequency SNHL, and all have severe complicated peptic-ulcer disease. They also all had a urine volume of <3 liters in 24 h. Individual V.6 had a normal response to the water-deprivation test. The parents in this family are distantly related (they are at least second cousins), and they come from a very inbred family.
Family WS-3.—Family WS-3 includes naturally conceived triplets that are affected with Wolfram syndrome (table 1; fig. 1). Two additional members of this family were suspected of having Wolfram syndrome, but they were unavailable for study, since individual V.2 is living abroad and since individual V.6 had died of a cause unrelated to the disorder. The other four affected individuals all have DM that manifested when each individual was ∼10 years old. They all have documented OA and high-frequency SNHL. Three of the individuals have urinary-tract dilatation, and one individual (V.3), who was 28 years old at the time of the present study, has end-stage renal failure. The triplets had a normal response to the water-deprivation test. The parents are second cousins.
Family WS-4.—Family WS-4 includes two affected siblings and an affected monozygotic twin pair born to parents who are first cousins (table 1; fig. 1). From a previous marriage to a woman to whom he was not related, the father has a daughter, who was age 16 years at the time of the present study (data not shown). The daughter presented with normal ophthalmologic findings, normal fasting blood glucose levels, and no other symptoms. The affected children include a girl (individual IV.1), who is currently 14 years old and who presented with DM at age 13 years. Although she had no visual or hearing deficits, she has documented OA and high-frequency SNHL. In addition, she has urinary-tract dilatation with normal kidney function. The twins (individuals IV.2 and IV.3), who are currently 12 years old, have documented and asymptomatic OA and high-frequency SNHL . One of them had a high fasting blood glucose level that later normalized. A girl (individual IV.4), who is currently 6 years old, has documented and asymptomatic OA and high-frequency SNHL. All affected individuals in this family produced a urine volume of <3 liters per 24 h with high osmolarity. The sisters (individuals IV.5 and IV.6), who are 5 and 4 years old, have optic nerves that appeared to be normal on ophthalmologic examination.
Excluding Linkage to the Chromosome 4p16 Locus
Linkage to the previously described Wolfram syndrome locus on chromosome 4p16 was explored by genotyping of the markers D4S432, D4S3023, DS2366, and D4S2639 in members of all four families. Visual inspection revealed allelic heterozygosity for these markers in affected individuals from families WS-2–WS-4 (data not shown), whereas, affected individuals from family WS-1 were found to be homozygous for markers D4S432, D4S3023, D4S2366, and D4S2639 (fig. 1). For family WS-1, the results of two-point linkage analysis showed a maximum LOD score of 1.28 (table 1), and multipoint linkage analysis indicated a maximum LOD score of 1.87 near marker D4S3023 (data not shown). Linkage to this region was excluded for families WS-2–WS-4, each of which had a LOD score <2.0 (table 2).
Table 2.
Two-Point LOD Scores between Wolfram Syndrome and Chromosome 4p16 Markers
|
LOD Score for |
||||
| Marker | Family WS-1 | Family WS-2 | Family WS-3 | Family WS-4 |
| D4S432 | 1.28 | −2.49 | −2.57 | −3.95 |
| D4S3023 | .88 | −2.54 | −2.11 | −2.76 |
| D4S2366 | 1.27 | −2.67 | −2.90 | −2.50 |
| D4S2639 | 1.27 | −2.08 | −.63 | NAa |
NA = Not available.
Linkage Mapping with the Use of Pooled DNA Samples
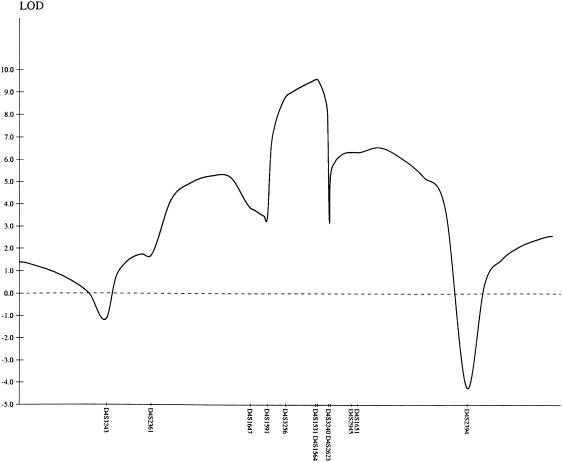
Given the exclusion of linkage in three of the four families, a 20-cM genomewide scan for additional loci was performed with the use of a DNA-pooling strategy for families WS-3 and WS-4. Approximately 30% of the genome had been screened—initially, with the use of markers from gene-rich chromosomes (Antonarakis 1994; Inglehearn 1997)—before homozygosity was identified with the marker D4S1647 (data not shown). The results of linkage analysis of additional markers indicated a maximum two-point LOD score of 6.84 for marker D4S1564, at recombination fraction (θ) = 0 (table 3), and a multipoint maximum LOD score of 9.55 near markers D4S1531 and D4S1564 (fig. 2). Recombinations in individuals V.9 and V.13 in family WS-2, individual IV.2 in family WS-3, and individual IV.1 in family WS-4 defined the critical region as a 7.1-cM region between D4S1591 and D4S3240 (fig. 1). Haplotype analysis revealed a common haplotype for markers D4S3256, D4S1531, and D4S1564 in members of all three families, suggesting that they may have a common ancestor.
Table 3.
Two-Point LOD Scores between Wolfram Syndrome and Chromosome 4q Markers in Families WS-2, WS-3, and WS-4
|
LOD Score at θ = |
|||||||
| Locus | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D4S3243 | −1.48 | −1.22 | −.43 | .07 | .32 | .21 | .04 |
| D4S2361 | 1.10 | 1.31 | 1.61 | 1.61 | 1.25 | .72 | .20 |
| D4S1647 | −.67 | .00 | .42 | .48 | .37 | .22 | .08 |
| D4S1591 | −2.10 | −1.41 | −.56 | −.09 | .20 | .18 | .80 |
| D4S3256 | 3.38 | 3.30 | 2.97 | 2.54 | 1.72 | .96 | .34 |
| D4S1531 | 6.10 | 5.96 | 5.41 | 4.71 | 3.30 | 1.92 | .68 |
| D4S1564 | 6.84 | 6.70 | 6.11 | 5.35 | 3.82 | 2.31 | .92 |
| D4S3240 | 2.53 | 3.47 | 3.82 | 3.59 | 2.68 | 1.57 | .53 |
| D4S2623 | 2.21 | 3.10 | 3.32 | 3.08 | 2.29 | 1.40 | .53 |
| D4S2945 | 1.70 | 2.59 | 2.84 | 2.61 | 1.88 | 1.09 | .39 |
| D4S1651 | 2.85 | 3.74 | 3.90 | 3.56 | 2.58 | 1.51 | .55 |
| D4S2394 | −4.77 | −2.08 | .19 | .90 | 1.07 | .77 | .35 |
Figure 2.
Multipoint LOD plot for markers in the chromosome 4q22-24 region of families WS-2–WS-4
Mutation Screen of the NKR3 Gene
The NKR3 gene, which has been mapped near D4S411 and D4S3256 on GeneMap'98, was screened for mutations, since it is expressed in the brain and kidney and since it is involved in the regulation of neurotransmission, inflammation, and pain sensation (Buell et al. 1992). Sequencing of the five exons of the NKR3 gene did not indicate any coding or splicing mutations in affected individuals from families WS-2–WS-4.
Discussion
In the present study, the clinical symptoms of the affected members of four families demonstrate phenotypic heterogeneity, since DI was not diagnosed in any individual, although it was suspected in a few. The method (urine volume) used for diagnosis of DI is not the most accurate, but several patients underwent the water-deprivation test. Even if milder forms of DI exist, it is definitely less common than has been reported in previous series (Bretz et al. 1970). In family WS-1, onset of DM and OA occurred at an earlier age than it did in families WS-2–WS-4. Also, in family WS-1, the morbidity of the disease is more severe, resulting in early mortality. Patients from families WS-2 and WS-3 seem to have an unusual peptic-ulcer disease that has not previously been reported in patients with Wolfram syndrome. This finding is currently undergoing further study. DM was diagnosed in all affected individuals, with the exception of the three younger siblings from family WS-4, who presented with OA and SNHL (table 1). This is not the typical sequence of clinical symptoms; however, OA has been the initial presentation in other clinical series (Barrett and Bundey 1997).
Homozygosity mapping by means of a DNA-pooling strategy and subsequent linkage analysis successfully located the presence of another locus for Wolfram syndrome, WFS2, to a 7.1-cM region between D4S1591 and D4S3240. Previously, the NKR3 gene had been mapped to this interval. However, mutation analysis of the five coding exons did not reveal any mutations, thus excluding NKR3 as a gene for Wolfram syndrome. Within the WFS2 critical region, 22 genes have been mapped, in addition to multiple expressed sequence tags, which will be the basis for positional-cloning efforts.
The pathology of Wolfram syndrome has been speculated to be a result of nuclear mutations that cause deletions in the mitochondrial genome, and a semidominant model of inheritance, in which heterozygous carriers are at greater risk for these deletions and may manifest some of the clinical features of Wolfram syndrome, has been proposed (Barrientos et al. 1996a, 1996b). A high frequency of mitochondrial deletions has been found in the CNS tissue of one patient (Barrientos et al. 1996b). However, studies of lymphocytes have been variable, ranging from no deletions (Barret et al. 1997) to a low frequency of deletions (Rotig et al. 1993; Barrientos et al. 1996a, 1996b). In addition, neither the tRNALeu(3243) mutation associated with mitochondrial DM nor the mitochondrial deletions have been found in other studies of patients with Wolfram syndrome (Hofmann et al. 1997; Strom et al. 1998; Hardy et al. 1999), thereby leading researchers to question the role of mitochondrial genome anomalies in the etiology of Wolfram syndrome. It has been suggested that the wolframin gene may be a mitochondrial membrane protein and that mutations may affect mitochondrial function, although not through effects on the mitochondrial genome (Strom et al. 1998). Subcellular localization of Wolframin and identification of the WFS2 gene will help discern the role of the mitochondria in the pathophysiology of Wolfram syndrome.
Mapping of Wolfram syndrome loci may provide information regarding more common psychiatric and diabetic diseases. Given that the carrier frequency is estimated to be 1/350 (Barrett and Bundey 1997), carriers may contribute to a significant frequency of psychiatric illness and diabetes. The majority of patients with Wolfram syndrome have been shown to have had a history of psychiatric illness, including severe depression, psychosis, or organic brain syndrome (Swift et al. 1990). In addition, heterozygous carriers have a 26-fold increase in risk for psychiatric illness (Swift et al. 1998). Bipolar disorder, which is among a variety of psychiatric disorders that have been mapped to chromosome 4 (Kennedy et al. 1999), shows weak linkage to marker D4S1647, which maps to a location 5 cM proximal to the WFS2 region (Detara-Wadleigh et al. 1997). Also, weak linkage for schizophrenia exists at marker D4S2623 (Levinson et al. 1998), with a recent report showing suggestive linkage to D4S2917 (reviewed by Kennedy et al. 1999). D4S2917 is within the WFS2 critical region, and D4S2623 is very close to the distal critical region. Susceptibility loci for bipolar disorder have also been mapped to markers flanking the WFS1 locus on chromosome 4p16 (Blackwood et al. 1996; Ginns et al. 1998; reviewed by Kennedy et al. 1999), further supporting a possible role for Wolfram syndrome genes in the etiology of mental illness.
Heterozygous carriers of WFS1 also have an increased risk for DM (Ohata et al. 1998). A locus contributing to insulin activity has been mapped near fatty acid–binding protein 2 (FABP2) in Pima Indians (Prochazka et al. 1993) and Mexican Americans (Mitchell et al. 1995). FABP2 maps to markers flanking the distal aspect of the WFS2 critical region. This mapping is in the same region that shows suggestive linkage for type 2 DM in Mexican Americans (Duggirala et al. 1999). Weak linkage of IDDM to D4S1604, which maps 30 cM distal to the WFS2 critical region, has previously been reported (Hashimoto et al. 1994). Thus, cloning of the WFS2 gene may provide an understanding of diabetes and psychiatric disorders, in addition to providing a further understanding of the pathophysiology of Wolfram syndrome.
Acknowledgments
We are very grateful to the participation of the members of the families involved in this study. We offer appreciation for the essential support provided by the Jordan University of Science and Technology, Irbid, and the University of Jordan, Amman. In addition, the contributions of Karen Neal and Katy Nash and the consultations of Dr. Mary Marazita and Meg Cooper have been invaluable. This study was funded by the Higher Council for Science and Technology, Amman, Jordan, and by start-up funds from the Ohio State University College of Dentistry (to A.C.L.) and from NIH grant R03 DE12533-01 (A.C.L.).
Electronic-Database Information
The accession number and URLs for data in this article are as follows:
- Center for Medical Genetics, Marshfield Medical Research Foundation, http://www.marshmed.org/genetics/Map_Markers/maps/indexmap.html (for the reference genetic map and markers used to fine map the critical region)
- CEPH, http://cephb.fr/ (for markers used to fine map the critical region and for allele frequencies)
- CHLC, http://lpg.nci.nih.gov/CHLC/ (for allele frequencies, the reference genetic map, and markers used to fine map the critical region)
- GeneMap'98, http://www.ncbi.nlm.nih.gov/genemap98/ (for the identification of candidate genes in the critical region)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for DIDMOAD [MIM 222300])
References
- Antonarakis SE (1994) Genome linkage scanning: systematic or intelligent? Nat Genet 8:211–212 [DOI] [PubMed]
- Barrett TG, Bundey SE (1997) Wolfram (DIDMOAD) syndrome. J Med Genet 34:838–841 [DOI] [PMC free article] [PubMed]
- Barret TG, Bundey SE, Fielder AR, Good PA (1997) Optic atrophy in Wolfram (DIDMOAD) syndrome. Eye 11:882–888 [DOI] [PubMed]
- Barrientos A, Casademont J, Saiz A, Cardellach F, Volpini V, Solans A, Tolosa E, et al (1996a) Autosomal recessive Wolfram syndrome associated with an 8.5-kb mtDNA single deletion. Am J Hum Genet 58:963–970 [PMC free article] [PubMed]
- Barrientos A, Volpini V, Casademont J, Genis D, Manzanares J-M, Ferrer I, Corral J, et al (1996b) A nuclear defect in the 4p16 region predisposes to multiple mitochondrial DNA deletions in families with Wolfram syndrome. J Clin Invest 97:1570–1576 [DOI] [PMC free article] [PubMed]
- Blackwood DH, He L, Morris SW, McLean A, Whitton C, Thomson M, Walker MT, et al (1996) A locus for bipolar affective disorder on chromosome 4p. Nat Genet 12:427–430 [DOI] [PubMed]
- Blasi C, Pierelli F, Rispoli E, Saponara M, Vingolo E, Andreani D (1986) Wolfram's syndrome: a clinical, diagnostic, and interpretative contribution. Diabetes Care 9:521–528 [DOI] [PubMed]
- Bretz GW, Baghdassarian A, Graber JD, Zacherle BJ, Norum RA, Blizzard RM (1970) Coexistence of diabetes mellitus and insipidus and optic atrophy in two male siblings: studies and review of the literature. Am J Med 48:398–403 [DOI] [PubMed]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869 [DOI] [PMC free article] [PubMed]
- Buell G, Schulz MF, Arkinstall SJ, Maury K, Missotten M, Adami N, Talabot F, et al (1992) Molecular characterisation, expression and localisation of human neurokinin-3 receptor. FEBS Lett 299:90–95 [DOI] [PubMed]
- Collier DA, Barrett TG, Curtis D, Macleod A, Arranz MJ, Maassen JA, Bundey S (1996) Linkage of Wolfram syndrome to chromosome 4p16.1 and evidence for heterogeneity. Am J Hum Genet 59:855–863 [PMC free article] [PubMed]
- Cremers CW, Wijdeveld PG, Pinckers AJ (1977) Juvenile diabetes mellitus, optic atrophy, hearing loss, diabetes insipidus, atonia of the urinary tract and bladder, and other abnormalities (Wolfram syndrome): a review of 88 cases from the literature with personal observations on 3 new patients. Acta Paediatr Scand Suppl 264:1–16 [DOI] [PubMed]
- Deloukas P, Schuler GD, Gyapay G, Beasley EM, Soderlund C, Rodriguez-Tome P, Hui L, et al (1998) A physical map of 30,000 human genes. Science 282:744–746 [DOI] [PubMed]
- Detera-Wadleigh SD, Badner JA, Yoshikawa T, Sanders AR, Goldin LR, Turner G, Rollins DY, et al (1997) Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 4, 7, 9, 18, 19, 20, and 21q. Am J Med Genet 74:254–262 [DOI] [PubMed]
- Duggirala R, Blangero J, Almasy L, Dyer TD, Williams KL, Leach RJ, O'Connell P, et al (1999) Linkage of type 2 diabetes mellitus and of age at onset to a genetic location on chromosome 10q in Mexican Americans. Am J Hum Genet 64:1127–1140 [DOI] [PMC free article] [PubMed]
- Ginns EI, St Jean P, Philibert RA, Galdzicka M, Damschroder-Williams P, Thiel B, Long RT, et al (1998) A genome-wide search for chromosomal loci linked to mental health wellness in relatives at high risk for bipolar affective disorder among the Old Order Amish. Proc Natl Acad Sci USA 95:15531–15536 [DOI] [PMC free article] [PubMed]
- Hardy C, Khanim F, Torres R, Scott-Brown M, Seller A, Poulton J, Collier D, et al (1999) Clinical and molecular genetic analysis of 19 Wolfram syndrome kindreds demonstrating a wide spectrum of mutations in WFS1. Am J Hum Genet 65:1279–1290 [DOI] [PMC free article] [PubMed]
- Hashimoto L, Habita C, Beressi JP, Delepine M, Besse C, Cambon-Thomsen A, Deschamps I, et al (1994) Genetic mapping of a susceptibility locus for insulin-dependent diabetes mellitus on chromosome 11q. Nature 371:161–164 [DOI] [PubMed]
- Hofmann S, Bezold R, Jaksch M, Kaufhold P, Obermaier-Kusser B, Gerbitz KD (1997) Analysis of the mitochondrial DNA from patients with Wolfram (DIDMOAD) syndrome. Mol Cell Biochem 174:209–213 [PubMed]
- Inglehearn CF (1997) Intelligent linkage analysis using gene density estimates. Nat Genet 16:15 [DOI] [PubMed]
- Inoue H, Tanizawa Y, Wasson J, Behn P, Kalidas K, Bernal-Mizrachi E, Mueckler M, et al (1998) A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20:143–148 [DOI] [PubMed]
- Kennedy JL, Basile VS, Macciardi FM (1999) Chromosome 4 workshop summary: Sixth World Congress on Psychiatric Genetics, Bonn, Germany, October 6–10, 1998. Am J Med Genet 88:224–228 [DOI] [PubMed]
- Kinsley BT, Swift M, Dumont RH, Swift RG (1995) Morbidity and mortality in the Wolfram syndrome. Diabetes Care 18:1566–1570 [DOI] [PubMed]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed]
- Levinson DF, Mahtani MM, Nancarrow DJ, Brown DM, Kruglyak L, Kirby A, Hayward NK, et al (1998) Genome scan of schizophrenia. Am J Psychiatr 155:741–750 [DOI] [PubMed]
- Lidral AC, Murray JC, Buetow KH, Basart AB, Schearer H, Shiang R, Naval A, et al (1997). Studies of the candidate genes TGFB2, MSX1, TGFA, and TGFB3 in the etiology of cleft lip and palate in the Philippines. Cleft Palate Craniofac J 34:1–6 [DOI] [PubMed]
- Mitchell BD, Kammerer CM, O'Connell P, Harrison CR, Manire M, Shipman P, Moyer MP, et al (1995) Evidence for linkage of postchallenge insulin levels with intestinal fatty acid-binding protein (FABP2) in Mexican-Americans. Diabetes 44:1046–1053 [DOI] [PubMed]
- Murray JC, Buetow KH, Weber JL Ludwigsen S, Scherpbier-Heddema T, Manion F, Quillen J, et al (1994) A comprehensive human linkage map with centimorgan density. Science 265:2049–2054 [DOI] [PubMed]
- Ohata T, Koizumi A, Kayo T, Shoji Y, Watanabe A, Monoh K, Higashi K, et al (1998) Evidence of an increased risk of hearing loss in heterozygous carriers in a Wolfram syndrome family. Hum Genet 103:470–474 [DOI] [PubMed]
- Page M, Asmal AC, Edwards CRW (1976) Recessive inheritance of diabetes: the syndrome of diabetes insipidus, diabetes mellitus, optic atrophy and deafness. Quart J Med 45:505–520 [PubMed]
- Polymeropoulos MH, Swift RG, Swift M (1994) Linkage of the gene for Wolfram syndrome to markers on the short arm of chromosome 4. Nat Genet 8:95–97 [DOI] [PubMed]
- Prochazka M, Lillioja S, Tait JF, Knowler WC, Mott DM, Spraul M, Bennett PH, et al (1993) Linkage of chromosomal markers on 4q with a putative gene determining maximal insulin action in Pima Indians. Diabetes 42:514–519 [DOI] [PubMed]
- Rose FC, Fraser GR, Friedmann AI, Kohner EM (1966) The association of juvenile diabetes mellitus and optic atrophy: clinical and genetical aspects. Quart J Med 35:385–405 [PubMed]
- Rotig A, Cormier V, Chatelain P, Francois R, Saudubray J-M, Rustin P, Munnich A (1993) Deletion of mitochondrial DNA in a case of early-onset diabetes mellitus, optic atrophy, and deafness (Wolfram syndrome, MIM 222300). J Clin Invest 91:1095–1098 [DOI] [PMC free article] [PubMed]
- Salih MAM, Tuvemo T (1991) Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD syndrome): a clinical study in two Sudanese families. Acta Paediatr Scand 80:567–572 [DOI] [PubMed]
- Sheffield VC, Nishimura DY, Stone EM (1995) Novel approaches to linkage mapping. Curr Opin Genet Dev 5:335–341 [DOI] [PubMed]
- Strom TM, Hortnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, Gerbitz KD, et al (1998) Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet 7:2021–2028 [DOI] [PubMed]
- Swift RG, Polymeropoulos MH, Torres R, Swift M (1998) Predisposition of Wolfram syndrome heterozygotes to psychiatric illness. Mol Psychiatry 3:86–91 [DOI] [PubMed]
- Swift RG, Sadler DB, Swift M (1990) Psychiatric findings in Wolfram syndrome homozygotes. Lancet 336:667–669 [DOI] [PubMed]
- Wolfram DJ, Wagener HP (1938) Diabetes mellitus and simple optic atrophy among siblings: report of four cases. Mayo Clin Proc 13:715–718 [Google Scholar]
- Yuan B, Valske D, Weber JL, Beck J, Sheffield VC (1997) Improved set for short-tandem-repeat polymorphisms for screening the human genome. Am J Hum Genet 60:459–460 [PMC free article] [PubMed]