Abstract
Ovarian cancer is a component of the autosomal-dominant hereditary breast-ovarian cancer syndrome and may be due to a mutation in either the BRCA1 or BRCA2 genes. Two mutations in BRCA1 (185delAG and 5382insC) and one mutation in BRCA2 (6174delT) are common in the Ashkenazi Jewish population. One of these three mutations is present in ∼2% of the Jewish population. Each mutation is associated with an increased risk of ovarian cancer, and it is expected that a significant proportion of Jewish women with ovarian cancer will carry one of these mutations. To estimate the proportion of ovarian cancers attributable to founding mutations in BRCA1 and BRCA2 in the Jewish population and the familial cancer risks associated with each, we interviewed 213 Jewish women with ovarian cancer at 11 medical centers in North America and Israel and offered these women genetic testing for the three founder mutations. To establish the presence of nonfounder mutations in this population, we also completed the protein-truncation test on exon 11 of BRCA1 and exons 10 and 11 of BRCA2. We obtained a detailed family history on all women we studied who had cancer and on a control population of 386 Ashkenazi Jewish women without ovarian or breast cancer. A founder mutation was present in 41.3% of the women we studied. The cumulative incidence of ovarian cancer to age 75 years was found to be 6.3% for female first-degree relatives of the patients with ovarian cancer, compared with 2.0% for the female relatives of healthy controls (relative risk 3.2; 95% CI 1.5–6.8; P=.002). The relative risk to age 75 years for breast cancer among the female first-degree relatives was 2.0 (95% CI 1.4–3.0; P=.0001). Only one nonfounder mutation was identified (in this instance, in a woman of mixed ancestry), and the three founding mutations accounted for most of the observed excess risk of ovarian and breast cancer in relatives.
Introduction
Ovarian cancer is among the most common forms of hereditary cancer in adults and is the leading cause of death caused by gynecological malignancy in North America. Both reproductive and genetic factors have been implicated in ovarian-cancer etiology (Risch 1998), and a family history of ovarian cancer is among the strongest risk factors for the disease (Amos and Struewing 1993). The familial breast-ovarian cancer syndrome accounts for ∼5% of all ovarian cancer cases in Canada (Narod et al. 1994). The majority of families with this syndrome carry a mutation in BRCA1 (MIM 113705) or BRCA2 (MIM 600185) (Narod et al. 1995a, 1995b). The lifetime risk of ovarian cancer conferred by a BRCA1 mutation has been estimated to be 60% (Ford et al. 1994; Easton et al. 1995), and the risk for carriers of a BRCA2 mutation has been estimated to be 27% (Ford et al. 1998). These risks greatly exceed the population risk of 1.4% by age 70 years. However, these cumulative risk estimates are made on the basis of studies of families with multiple instances of early-onset breast or ovarian cancer; a risk estimate made on the basis of a sample of (mostly) unaffected Jewish individuals was much lower (Struewing et al. 1997). The risks conferred by BRCA1 or BRCA2 mutations, ascertained through women with cancer but unselected for family history, may also be different. The cancer risks in carriers may also vary by specific mutation and among ethnic groups.
In North America, the incidence of ovarian cancer is higher among Ashkenazi Jewish women than among Sephardic Jewish women or non-Jewish women. The rate of ovarian cancer among Israeli Jews born in Europe or North America is among the highest reported and greatly exceeds the rate for Israeli non-Jews (Parkin et al. 1997). We hypothesize that the excess risk of ovarian cancer observed in the Jewish population might be predominantly due to a high prevalence of mutations in the ovarian cancer–susceptibility genes BRCA1 and BRCA2. Three common mutations are reported in this population: two in BRCA1 (185delAG and 5382insC) (Struewing et al. 1995a; Roa et al. 1996) and one in BRCA2 (6174delT; Oddoux et al. 1996). The combined frequency of these three mutations in the Ashkenazi population is ∼2% (reviewed in Tonin et al. 1996).
It is important to measure directly the proportion of ovarian cancers in Jewish women that are attributable to mutations for the purposes of genetic counseling, screening, and prevention. We interviewed a total of 213 Ashkenazi Jewish women with ovarian cancer, unselected for age or family history, from 11 hospitals in North America and Israel, and we evaluated 208 of these women for the presence of a founding mutation in BRCA1 or BRCA2.
Materials and Methods
Data Collection
Study population
A hospital-based study of Jewish women with epithelial ovarian cancer was conducted among 11 centers in North America and Israel. Two hundred Jewish women with ovarian cancer were recruited from the Departments of Gynecology and Oncology of the collaborating hospitals. In some hospitals, religious affiliation was recorded on the medical record. In other hospitals, the patient lists were reviewed for patients who were known to be of Jewish ancestry by the treating physician or who were likely to be Jewish by surname. The treating physicians were then asked to write letters to the selected patients and request their permission for a member of the study team to contact them. The letter described the study goals and offered the patient an opportunity to participate. The patient was then contacted by a member of the study team and was interviewed by telephone or in person for her family history and risk-factor profile. Patients confirmed that they were Jewish by birth (i.e., they were not adopted and had not converted). The study protocol also included the collection of a blood sample for genetic testing. Patients who wished to participate in the genetic testing protocol were given pretest counseling by a member of the study team or by the genetic counselor affiliated with the host institution. Patients were offered the option of receiving the results of the genetic tests, and most indicated their willingness to receive the results. We were able to obtain a copy of the pathology report from 85% of these patients. Pathology reports were reviewed for tumor stage (Fédération Internationale de Gynécologie et d'Obstetrique classification), for grade, and for histologic subtype.
An additional 13 study subjects were identified through the Ontario Cancer Registry as part of a provincewide study of genetic factors in ovarian cancer. These patients were all incident cases of ovarian cancer diagnosed in the province between 1995 and 1996 and who indicated on the questionnaire that they were of Jewish ancestry.
A total of 465 potential study subjects were identified. Of these, 80 subjects were dead, and it was not possible to locate 98 women. A further 33 patients were excluded because review of the pathology report indicated a diagnosis other than invasive epithelial ovarian cancer. Of the total of 254 women who were approached to participate, 213 agreed to complete the family history questionnaire, and 208 agreed to provide a blood sample for genetic testing. Forty-nine subjects refused to participate; 16 were ill, 8 were concerned about the insurance implications of genetic testing, 3 did not speak English, 2 were part of other genetic studies, and 2 were concerned that participating would be stressful. For 18 patients, the reason of refusal was not specified.
Epidemiological information and demographic information were collected by questionnaire, and a detailed family history was taken by interview. The questionnaire inquired about the patient's ethnic origin and the birthplace of her parents and grandparents. Three-generation pedigrees were drawn to include all women with breast and ovarian cancer. The current age (or the age of death) of all first-degree relatives was recorded on the pedigree. The ages at diagnosis and the sites of cancer were obtained for affected relatives. The diagnosis of cancer in the proband was confirmed by review of pathology reports, but in general, it was not possible to confirm cancer in relatives.
Control population
Control women were used in order to evaluate the importance of family history for ovarian cancer in Jewish women. The control population comprised Jewish women with no history of breast or ovarian cancer, and they were selected from two sources. First, staff members of several of the collaborating hospitals were approached to participate. These controls included 126 paid and unpaid employees of the Toronto Hospital, the Montreal Jewish General Hospital, the Cedars-Sinai Hospital, the Columbia Presbyterian Medical Center, the Albert Einstein Medical Center, the Central Emek Hospital, and the Yale University Medical Center. A second group of 260 control women was obtained by sending a mailed invitation to women on the membership lists of a Toronto synagogue and a Jewish women's group. Control women were aware that they were participating in a study of breast and ovarian cancer, but they were unaware that family history was the principal factor under study. The control women provided a detailed family history, but they did not supply a blood sample for genetic testing.
Mutation Analysis
High-molecular-weight DNA was extracted from whole blood. Red blood cells were lysed by absorbing to ammonium ions in red-blood-cell lysis buffer. The leukocytes were isolated and stored at −70°C. Leukocytes were then digested by adding buffer (NaCl and EDTA), 100 μl of 20 mg/ml proteinase K, 4 μl of 1 U/ml ribonuclease A, and 250 μl of 20% SDS to this solution. DNA was then extracted by the standard phenylchloroform procedures. Exons 2 and 20 in the BRCA1 gene and exon 11 in the BRCA2 gene were amplified by standard PCR amplification protocols. Exon 20 of BRCA1 was evaluated for 5382insC mutations by SSCP analysis, and exon 2 of BRCA1 was evaluated for 185delAG mutations by heteroduplex analysis.
The protein-truncation test was used to screen for truncating mutations in exon 11 of BRCA1 and exons 10 and 11 of BRCA2. Truncating mutations in these exons represent ∼70% of the mutations found to date in families with deleterious mutations in these genes. A protein-truncation test of exon 11 is also used to identify the abnormal band corresponding to the BRCA2 6174delT mutation.
The aberrant bands generated by each of these techniques were sequenced by annealing 5 μl exon 2 (delAG) PCR product, 1 μl exon 20 (insC), and 7 μl exon 11 (delT) PCR products to template and primers and sequenced by a standard protocol as outlined in Amersham sequencing kits US70170 and US79750. All samples were tested for all three founder mutations, and each mutant was confirmed by direct sequencing.
Statistical Analysis
Each case of ovarian cancer was classified as familial or nonfamilial on the basis of the presence of one case of ovarian cancer (other than the proband) or two cases of early-onset breast cancer (defined as <50 years of age at diagnosis) in the first- and second-degree relatives of the proband. The proportion of individuals with and without mutations was calculated by age at onset; by histologic type, grade, and stage; and for familial and nonfamilial instances.
We compared the cancer risks among the relatives of the women with ovarian cancer and the relatives of the Jewish control women without cancer. To do this, the cumulative incidence of breast and ovarian cancer was calculated for all first-degree relatives of the affected women and control women. Each relative was considered to be at risk for cancer from birth until either they developed cancer or until death. The cumulative cancer risks were calculated by means of an actuarial survival method, and the significance was assessed with the log-rank test. The relative risk (RR) for cancer was then estimated by comparison of the incidence rates for the relatives of the ovarian-cancer patients with the relatives of controls by use of a Cox proportional-hazards model. Risks were calculated for the entire patient population and then separately for the subgroups of women with BRCA1 or BRCA2 mutations. Because we recorded cancer histories on all first-degree relatives, we were able to estimate the penetrance of breast and ovarian cancer for each of the three mutations by making use of the kin-cohort method of Wacholder et al. (1998). The method is based on the assumption that half of the first-degree relatives of the mutation carriers are also expected to be carriers and that relatives are also at risk of carrying a different mutation, consistent with the population estimates.
Results
A total of 213 cases of epithelial ovarian cancer were collected from 11 different medical centers. These represent the majority of living cases of ovarian cancer in Jewish women under active follow-up at the departments of gynecology and oncology of the 11 participating hospitals. Our study attempted to recruit all prevalent cases, and the median time since diagnosis was 2.2 years (range, 0–25.6 years). The average age at diagnosis of ovarian cancer was 57.6 years (range 19–88 years), and the average age of the women at the time of the interview was 61.2 years (range 21–90 years).
A total of 86 founder mutations was found among the 208 patients (41.3%), including 57 in BRCA1 (43 in 185delAG and 14 in 5382insC) and 29 in BRCA2 (6174delT). The frequency of mutations varied by age at onset (table 1); a BRCA1 mutation was found in 41.1% of women diagnosed between the ages of 30 and 60 years and in 11.8% of those diagnosed when they were older than age 60 (P<.0001 for difference). A BRCA2 mutation was found in 9.8% of women diagnosed between the ages of 30 and 60 and in 19.4% of women diagnosed after age 60 (P=.07). Women with BRCA1 mutations were diagnosed with ovarian cancer at a younger age, on average, than women for whom no mutation was detected (50.8 years and 59.2 years, respectively; P<.0001). In contrast, women with BRCA2 mutations were older when they were diagnosed than were women for whom no mutation was detected, but the difference was not significant (62.1 years vs. 59.2 years; P=.27). BRCA2 mutations were more numerous than BRCA1 mutations in women diagnosed with ovarian cancer after age 60 years (18 women vs. 11 women, respectively).
Table 1.
Frequency of Mutations in Cases of Ovarian Cancer by Age at Diagnosis
|
No. (%) Positive for Mutation in |
||||
| Age Group (years) | Total No. of Patients | BRCA1 | BRCA2 | Either |
| 19–29 | 3 | 0 (.0) | 0 (.0) | 0 (.0) |
| 30–39 | 15 | 7 (46.7) | 1 (6.7) | 8 (53.3) |
| 40–49 | 54 | 24 (44.4) | 2 (3.7) | 26 (48.1) |
| 50–59 | 43 | 15 (34.9) | 8 (18.6) | 23 (53.5) |
| 60–69 | 49 | 9 (18.4) | 10 (20.4) | 19 (38.8) |
| 70–90 | 44 | 2 (4.5) | 8 (18.2) | 10 (22.7) |
| Total | 208 | 57 (27.4) | 29 (13.9) | 86 (41.3) |
The majority of mutations (75%) were found in women with papillary serous tumors (table 2). Endometrioid and Mullerian tumors were relatively uncommon, but these were associated with a high mutation frequency. In contrast, mucinous and clear-cell tumors were underrepresented in the women who carried the mutation. The great majority (92%) of women who carried the mutation presented with grade III tumors (table 3), and 52% of the women with grade III tumors were positive for mutation. There was no difference in the stage distribution between the mutation-positive and mutation-negative tumors (table 3).
Table 2.
Frequency of Mutations in Cases of Ovarian Cancer by Histology[Note]
|
No. (%) Positive for Mutation in |
||||
| Histology | Total No. of Patients | BRCA1 | BRCA2 | Either |
| Serous | 105 | 33 (31.4) | 18 (17.1) | 51 (48.6) |
| Mullerian or mixed | 21 | 6 (28.6) | 3 (14.3) | 9 (42.9) |
| Endometrioid | 12 | 3 (25.0) | 3 (25.0) | 6 (50.0) |
| Mucinous | 7 | 1 (14.3) | 0 (.0) | 1 (14.3) |
| Clear cell | 7 | 1 (14.3) | 0 (.0) | 1 (14.3) |
| Transitional cell | 1 | 0 (.0) | 0 (.0) | 0 (.0) |
| Brenner | 1 | 0 (.0) | 0 (.0) | 0 (.0) |
| Total | 154 | 44 (28.6) | 24 (15.6) | 68 (44.2) |
Note.—Eighteen carriers and 36 noncarriers had missing histology information.
Table 3.
Frequency of Mutations in Cases of Ovarian Cancer by Stage and Grade[Note]
|
No. (%) Positive for Mutation in |
||||
| Stage or Grade | Total No. of Patients | BRCA1 | BRCA2 | Either |
| Stage: | ||||
| I | 30 | 6 (20.0) | 2 (6.7) | 8 (26.7) |
| II | 15 | 7 (46.7) | 2 (13.3) | 9 (60.0) |
| III | 120 | 32 (26.7) | 23 (19.2) | 55 (45.8) |
| IV | 11 | 5 (45.5) | 0 (.0) | 5 (45.5) |
| Total | 176 | 50 (28.4)a | 27 (15.3)b | 77 (43.8)c |
| Grade: | ||||
| I | 13 | 1 (7.7) | 0 (.0) | 1 (7.7) |
| II | 22 | 1 (4.5) | 4 (18.2) | 5 (22.7) |
| III | 128 | 47 (36.7) | 19 (14.8) | 66 (51.6) |
| Total | 136 | 49 (30.1)d | 23 (14.1)e | 72 (44.2)f |
Note.—Nine carriers and 21 noncarriers had missing stage information, whereas 14 carriers and 28 noncarriers had missing grade information.
Overall P=.40.
Overall P=.34.
Overall P=.15.
Overall P=.001.
Overall P=.31.
Overall P=.0002.
Patients with one relative with ovarian cancer or with two relatives with early-onset breast cancer were classified as familial. In total, 53 of 199 women (27%) for whom complete family histories on second-degree relatives were available satisfied this definition of familial cancer. An additional 27 patients (14%) had a single relative with early-onset breast cancer. As expected, mutations were more among familial patients than among nonfamilial patients (table 4). Mutations were present in 78% of the women with two or more affected relatives (breast or ovarian cancer), in 58% of women with one affected relative, and in 28% of patients with no relative with ovarian cancer or early-onset breast cancer. A family history of one or more affected relatives was present in 61% of the patients with a mutation.
Table 4.
Frequency of Mutations in Women with Ovarian Cancer by Family History[Note]
|
No. (%) Positive for Mutation in |
||||
| Family History | Total No. of Patients | BRCA1 | BRCA2 | Either |
| None | 119 | 23 (19.3) | 10 (8.4) | 33 (27.7) |
| Breast cancer (no relatives with ovarian cancer): | ||||
| 1 instance of breast cancer under age 50 years | 27 | 12 (44.4) | 3 (11.1) | 15 (55.6) |
| ⩾2 instances of breast cancer under age 50 years | 8 | 6 (75.0) | 1 (12.5) | 7 (87.5) |
| Ovarian cancer (no relatives with breast cancer): | ||||
| ⩾1 instances of ovarian cancer | 30 | 9 (30.0) | 9 (30.0) | 18 (60.0) |
| Breast and ovarian cancer | ||||
| 1 instance of breast cancer under age 50 years | 9 | 5 (55.6) | 2 (22.2) | 7 (77.8) |
| ⩾2 instances of breast cancer under age 50 years | 6 | 2 (33.3) | 2 (33.3) | 4 (66.7) |
Note.—Nine women had missing information on second-degree relatives, so they are not included in this table.
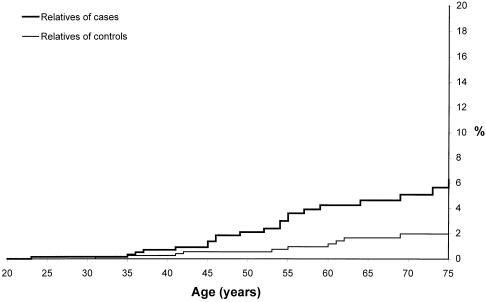
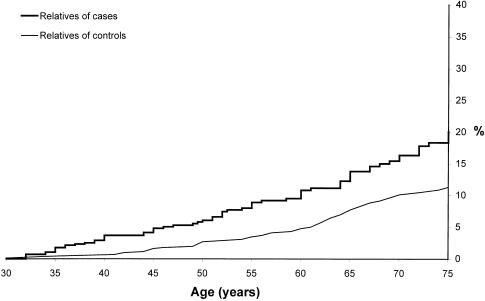
Among the first-degree relatives of women with ovarian cancer, the cumulative incidence of ovarian cancer was estimated to be 6.3% to age 75 years. This risk was significantly greater than the risk in relatives of the control women (RR = 3.2; P=.002) (fig. 1). The risk of breast cancer to age 75 years for first-degree relatives of the ovarian-cancer patients was also increased (RR = 2.0; P=.0001) (fig. 2). The cumulative incidence of breast cancer in the first-degree relatives of the ovarian-cancer patients reached 20.4% at age 75 years, compared with 11.3% for the relatives of the controls.
Figure 1.
Cumulative incidence of ovarian cancer to age 75 years in female first-degree relatives of women with cancer and control women. We observed 20 ovarian cancers among the 638 female relatives of women with ovarian cancer and 10 ovarian cancers among the 1,130 female relatives of control women (P=.0015).
Figure 2.
Cumulative incidence of breast cancer to age 75 years in female first-degree relatives of women with cancer and control women. We observed 63 breast cancers among the 638 female relatives of cases with ovarian cancer and 50 breast cancers among the 1,130 female relatives of control women (P<.0001).
The risks of cancer in the probands' relatives also depended on the age at onset of the index case. Among the female relatives of women diagnosed with ovarian cancer before the age of 55 years, the cumulative incidence of ovarian cancer to age 75 years was 11.6%, compared with 4.4% for relatives of women diagnosed after age 55 years (RR = 2.3; P=.05). This was not due to the effect of the mutation carriers; after excluding the carriers, the risk for relatives of early-onset ovarian cancer remained higher than the risk for relatives of women diagnosed after age 55 years (RR = 7.4; P=.02). Among relatives of early-onset ovarian cancer, the cumulative incidence to age 75 years for breast cancer was 25.7%, compared with 18.1% for the relatives of women diagnosed after age 55 years (RR = 2.0; P=.006). After excluding carriers, the RR for breast cancer was 2.93 (P=.02).
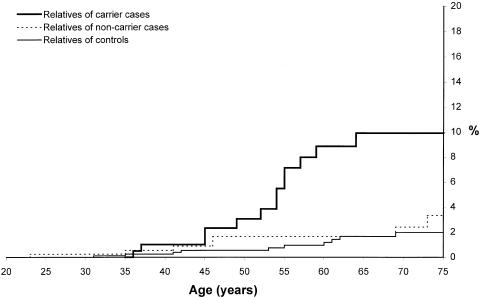
Among the subgroup of patients with BRCA1 or BRCA2 mutations, the familial risks were particularly high (figs. 3 and 4). The RR of ovarian cancer to age 75 years among the female relatives of BRCA1 mutation carriers compared with healthy control women was 5.4 (95% CI 2.4–12.3), and the RR for breast cancer was 3.2 (95% CI 2.1–4.9). There was no significant difference in risks for the two BRCA1 mutations. For the first-degree relatives of the 29 BRCA2 carriers, the RRs to age 75 years were 6.2 for ovarian cancer (95% CI 2.3–17.1) and 2.4 for breast cancer (95% CI 1.2–4.5). The cumulative incidence of ovarian cancer to age 75 years was slightly higher in the relatives of carriers of BRCA2 mutations than carriers of BRCA1 mutations (12.3% vs. 8.3% to age 75 years), but this difference was not statistically significant. The average age at onset of breast cancer in the relatives of the BRCA1 carriers was 48.7 years and was less than that of the BRCA2 carriers (57.5 years; P=.06). The cumulative incidence of breast cancer to age 55 years was 18.8% for first-degree relatives of BRCA1 carriers, compared with only 6.2% for first-degree relatives of BRCA2 carriers (P=.02).
Figure 3.
Cumulative incidence of ovarian cancer to age 75 years in female first-degree relatives of carriers, noncarriers, and controls. We observed 13 ovarian cancers among the 253 female relatives of carrier women with ovarian cancer, 7 ovarian cancers among the 368 female relatives of noncarrier cases, and 10 ovarian cancers among the 1,130 female relatives of control women (P<.0001).
Figure 4.
Cumulative incidence of breast cancer to age 75 years in female first-degree relatives of carriers, noncarriers, and controls. We observed 36 breast cancers among the 253 female relatives of carrier women with ovarian cancer, 27 breast cancers among the 368 female relatives of noncarrier women, and 50 ovarian cancers among the 1,130 female relatives of control women (P<.0001).
The excess risks of ovarian or breast cancer in the relatives of the women, compared with that of the controls, were mostly, but not entirely, attributable to the presence of one of three founding mutations (figs. 3 and 4). Among the female relatives of women who tested negative for all three mutations, the cumulative risk of ovarian cancer to age 75 years was 4%, compared with a risk of 2% in the first-degree relatives of the healthy controls (RR = 1.9; P = .20; fig. 3). Similarly, the cumulative risk of breast cancer in the relatives of the mutation-negative women was 16%, compared with 11% for the relatives of the healthy controls (RR = 1.4; P = .15; fig. 4).
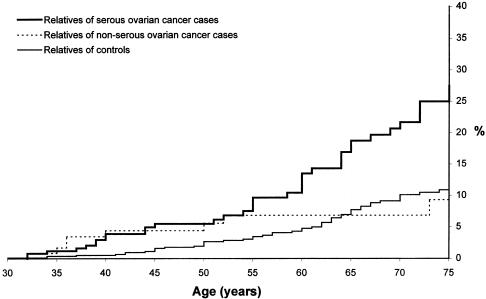
The majority of the mutation-positive tumors was of serous papillary histology. The cancer risks for relatives of women with serous papillary tumors were elevated, but those for relatives of women with other tumor types were not (fig. 5). The RR of breast cancer among first-degree relatives of serous papillary tumors was 2.7 (P<.001) and was 1.2 for first-degree relatives of women with other tumor types (P=.6). Among the noncarrier population with serous tumors, the RR for breast cancer was 1.73 (P = .08).
Figure 5.
Cumulative incidence of breast cancer to age 75 years in female first-degree relatives of women with serous and nonserous ovarian cancer and control women. We observed 37 breast cancers among the 305 female relatives of women with serous ovarian cancer, 9 breast cancers among the 141 female relatives of women with nonserous ovarian cancer, and 50 ovarian cancers among the 1,130 female relatives of control women (P<.0001).
The cumulative incidence of breast and of ovarian cancer was compared for the mothers and sisters of the ovarian cancer patients. In the entire group of women we studied, the risk of breast cancer was greater for sisters (29% to age 75 years) than it was for mothers (17% to age 75 years; P=.04). There was no difference in the risk of ovarian cancer between mothers and sisters. Among the subgroup of mutation-positive women, the risk of breast cancer in the sisters (38%) moderately exceeded that in the mothers (25%), but the difference was not significant (P=.26). The risk of ovarian cancer in the carriers' sisters (14%) was similar to that of the mothers (14%).
The lifetime risk of ovarian cancer in BRCA1 and BRCA2 carriers can be estimated from our data in two ways. First, RRs can be constructed by comparing the frequency of mutations in women with the reported frequencies of mutations in healthy Jewish control women. Second, we can use the data on the observed cases of ovarian cancer in the relatives of the carriers to construct penetrance estimates, according to the kin-cohort method described by Wacholder et al. (1998).
Among the 191 women diagnosed with ovarian cancer before age 75 years, we identified 42 carriers of the 185delAG mutation, 14 carriers of the 5382insC mutation, and 26 carriers of the 6174delT mutation. The frequencies of these mutations in North American Jews are reported to be 7.7 per 1,000 for the 185delAG mutation, 3.8 per 1,000 for the 5382insC mutation, and 11.1 per 1,000 for the 6174delT mutation (Struewing et al. 1997). Therefore, the odds ratios for ovarian cancer to age 75 years are 36.6 for the 185delAG mutation, 20.8 for the 5382insC mutation, and 14.2 for the 6174delT mutation. Assuming baseline population risks for ovarian cancer of 1.4% to age 75 years (SEER Database, white women, 1988–1992; see Parkin et al. 1997) these risks are roughly equivalent to cumulative incidences to age 75 years of 51.2%, 29.1%, and 19.9% for the 185delAG, 5382insC, and 6174delT mutations, respectively.
There were a total of 13 ovarian cancers observed before age 75 years among the 253 female first-degree relatives of the carriers. The relatives of the control population have a risk of 2.0% to age 75 years of ovarian cancer (this risk is slightly greater than the lifetime risk of 1.4% for women in the general population in North America). The penetrances to age 75 years are estimated to be 10.1%, 21.0%, and 26.6% for the 185delAG, 5382insC, and 6174delT mutations, respectively, on the basis of the method of Wacholder et al. (1998). Because these estimates were made on the basis of a total of 13 observed ovarian cancers, the confidence limits are wide.
Data on the first-degree relatives of the carrier women and the noncarrier women were also used to construct estimates of breast-cancer penetrance. The estimated penetrance of the BRCA1 mutations for breast cancer was 31.3% to age 55 years, 45.9% to age 65 years, and 43.8% to age 75 years. The penetrance of the 185delAG mutation (44.2% to age 75 years) was similar to that of the 5382insC mutation (39.3% to age 75 years). The estimated penetrance of breast cancer to age 75 years for carriers of the 6174delT BRCA2 mutation was only slightly lower (36.8% to age 75 years). However, the difference was striking for breast cancer risk before age 55 years (31.3% for BRCA1 mutations vs. 6.1% for BRCA2; P=.02).
A history of cancer other than ovarian cancer was reported by 42 of 213 women (19.7%), including 29.1% of the mutation-positive women and 12.3% of the mutation-negative women (P=.003 for the difference). Overall, 25 of 42 women with multiple primary cancer (59.5%) carried a BRCA1 or BRCA2 mutation, including 18 of 25 women with a past history of breast cancer. The other cancers in carriers included colorectal cancer (two cases), skin cancer (three cases), head and neck cancer (one case), and lung cancer (one case).
We assessed the risks of cancers at other sites in the relatives of the women we studied by comparing the cancer histories of the 1,222 first-degree relatives of the women with ovarian cancer with the cancer histories of the 2,213 first-degree relatives of the control women. Because the average age of the relatives of the women with cancer was greater than that of the relatives of the control women (53.8 years and 48.2 years, respectively; P<.0001), a survival analysis was performed, taking into account the at-risk period for each relative (see Methods). The risks of cancer of all sites were compared for the relatives of the women and control women (table 5).
Table 5.
Cumulative Incidence of Cancer in First-Degree Relatives of Jewish Ovarian-Cancer Patients and Jewish Controls[Note]
|
Cumulative Incidence of Cancer (%) to Age |
||||||||||||
| 55 Yearsa |
65 Yearsa |
75 Yearsa |
||||||||||
| Site | Control | Case | Pb | RRc | Control | Case | Pb | RRc | Control | Case | Pb | RRc |
| Breast | 3.5 | 9.1 | .0001 | 2.87 | 7.7 | 14.2 | .0003 | 2.12 | 11.3 | 20.4 | .0001 | 2.04 |
| Ovary | 1.0 | 3.9 | .002 | 3.96 | 1.7 | 5.0 | .003 | 3.21 | 2.0 | 6.3 | .002 | 3.20 |
| Prostate | .0 | .6 | .081 | ∞ | .3 | .6 | .339 | 3.05 | 2.6 | 7.5 | .007 | 3.50 |
| Uterus | .3 | .6 | .644 | 1.58 | .3 | 1.5 | .153 | 3.22 | .6 | 2.2 | .163 | 2.67 |
| Colon | .4 | .8 | .290 | 1.88 | 1.6 | 2.2 | .310 | 1.48 | 3.5 | 5.3 | .152 | 1.51 |
| Pancreas | .0 | .4 | .031 | ∞ | .1 | 1.5 | .002 | 12.66 | 1.0 | 2.0 | .027 | 3.15 |
| Lung | .1 | .1 | .756 | 1.55 | .3 | 1.0 | .167 | 2.64 | 2.4 | 3.4 | .319 | 1.46 |
| Head and neck | .1 | .1 | .747 | 1.57 | .3 | .8 | .316 | 2.11 | .5 | .8 | .512 | 1.58 |
| Melanoma | .2 | .2 | .656 | 1.56 | .2 | .2 | .656 | 1.56 | .4 | .8 | .323 | 2.09 |
| Primary site unknown | .0 | .6 | .013 | ∞ | .3 | 1.0 | .036 | 4.73 | 1.5 | 1.4 | .542 | 1.37 |
| Any: | ||||||||||||
| Womend | 3.4 | 5.0 | .184 | 1.51 | 7.0 | 11.6 | .026 | 1.67 | 14.6 | 20.2 | .047 | 1.45 |
| Women | 7.4 | 16.4 | .0001 | 2.41 | 15.1 | 27.2 | .0001 | 2.06 | 25.6 | 40.4 | .0001 | 1.85 |
| Men | 2.7 | 4.3 | .234 | 1.49 | 7.4 | 12.0 | .056 | 1.56 | 21.5 | 31.3 | .005 | 1.60 |
| All | 5.1 | 10.6 | .0001 | 2.13 | 11.5 | 19.9 | .0001 | 1.88 | 23.6 | 35.9 | .0001 | 1.74 |
Note.—Cancers in women include cancers in 1,130 female relatives of control women and 638 female relatives of probands. Cancers in men include 1,083 male relatives of control women and 584 male relatives of probands.
Kaplan-Meier estimates.
Log-rank test.
Relative risks are obtained from a univariate Cox proportional hazard model. Baseline: index controls.
Women with no breast or ovarian cancer. Female relatives with breast cancer or ovarian cancer are considered to be at risk for other cancers.
Overall, the relatives of the women with ovarian cancer experienced a 74% greater risk of any cancer to age 75 years compared with controls (P=.0001). The excess risk was present in men (RR = 1.6; P=.005) as well as in women (RR = 1.9; P=.001). The excess in men was largely attributable to cancers of the prostate and pancreas. Nonsignificant excesses of cancer of the colon, head and neck, and lung were also observed. Among the female first-degree relatives, the overall risk of cancer to age 75 years was increased 1.9 times (95% CI 1.4–2.4). Excluding cancer of the breast and ovary, the RR was 1.5 (95% CI 1.0–2.1). Sites of excess included the uterus, colon, pancreas, and lung; however, of these, only the excess of pancreatic cancer to age 65 years was statistically significant (P=.03).
The cancer risks were also compared for relatives of the mutation-positive women and mutation-negative women (table 6). The carrier relatives were at greater risk for breast, ovary, uterus, and prostate cancer. The endometrial cancer excess was limited to the relatives of the BRCA1 carriers. The RR of endometrial cancer to age 75 years in relatives of BRCA1 carriers compared with relatives of healthy controls was 9.4 (P=.0003). However, this risk was based on only four cases of endometrial cancer observed in the relatives of the BRCA1 carriers.
Table 6.
Cumulative Incidence of Cancer in First-Degree Relatives of Jewish Women with Ovarian Cancer: Carriers Versus Noncarriers[Note]
|
Cumulative Incidence of Cancer (%) to Age |
||||||||||||
| 55 Yearsa |
65 Yearsa |
75 Yearsa |
||||||||||
| Site | Noncarrier | Carrier | Pb | RRc | Noncarrier | Carrier | Pb | RRc | Noncarrier | Carrier | Pb | RRc |
| Breast | 6.3 | 13.9 | .006 | 2.41 | 8.1 | 24.1 | .0001 | 2.95 | 15.8 | 28.3 | .002 | 2.20 |
| Ovary | 1.9 | 7.2 | .031 | 3.07 | 1.9 | 10.0 | .004 | 4.00 | 4.0 | 10.0 | .018 | 2.89 |
| Prostate | .0 | 1.5 | .078 | ∞ | .0 | 1.5 | .078 | ∞ | 5.3 | 9.3 | .109 | 2.47 |
| Uterus | .0 | 1.6 | .078 | ∞ | .0 | 3.9 | .011 | ∞ | 1.1 | 3.9 | .053 | 6.53 |
| Colon | .7 | 1.1 | .593 | 1.54 | 2.3 | 2.2 | .978 | .99 | 5.3 | 5.7 | .895 | 1.06 |
| Pancreas | .5 | .3 | .830 | .77 | 1.5 | 1.4 | .945 | .95 | 1.5 | 2.9 | .448 | 1.61 |
| Lung | .0 | .3 | .211 | ∞ | 1.1 | .3 | .590 | .54 | 3.1 | 3.4 | .759 | 1.20 |
| Head and neck | .2 | .0 | .427 | .00 | 1.3 | .0 | .115 | .00 | 1.3 | .0 | .115 | .00 |
| Melanoma | .4 | .0 | .250 | .00 | .4 | .0 | .250 | .00 | .9 | .7 | .582 | .54 |
| Primary site unknown | .5 | .4 | .828 | .77 | .8 | .9 | .961 | 1.05 | 1.4 | .9 | .798 | .80 |
| Any: | ||||||||||||
| Womend | 4.0 | 6.3 | .483 | 1.38 | 10.4 | 13.3 | .464 | 1.26 | 19.6 | 21.1 | .625 | 1.15 |
| Women | 11.4 | 24.0 | .002 | 2.13 | 18.5 | 40.2 | .0001 | 2.31 | 35.4 | 48.6 | .001 | 1.76 |
| Men | 4.1 | 5.0 | .852 | 1.10 | 12.6 | 10.2 | .625 | .84 | 31.4 | 29.1 | .992 | .97 |
| All | 7.8 | 15.0 | .004 | 1.85 | 15.6 | 26.4 | .0008 | 1.77 | 33.4 | 39.3 | .011 | 1.44 |
Note.—Cancers in women include cancers in 368 female relatives of noncarriers and 253 female relatives of probands. Cancers in men include cancers in 349 male relatives of noncarriers and 223 male relatives of probands.
Kaplan-Meier estimates.
Log-Rank test.
Relative risks are obtained from a univariate Cox proportional hazard model. Baseline: noncarriers.
Women with no breast or ovarian cancer. Female relatives with breast cancer or ovarian cancer are considered to be at risk for other cancers.
The cumulative incidences of prostate cancer to age 75 years were 2.6% for relatives of healthy controls, 5.3% for relatives of noncarrier cases, 8.0% for relatives of BRCA1 carriers, and 12.0% for relatives of BRCA2 carriers. The prostate cancer risk was significantly increased for both the relatives of the BRCA1 carriers (P=.01) and BRCA2 carriers (P=.002) compared with healthy controls.
Overall, the relatives of the BRCA2 carriers were at greater risk for cancer of any type than the relatives of the BRCA1 carriers (46.3% vs. 34.9%; P=.35; table 7). The risk was significantly higher for cancer in men before age 65 years (21.4% vs. 4.4%; P=.01), attributable to an excess of prostate, pancreatic, and colon cancers observed in male relatives of the BRCA2 carriers at that age.
Table 7.
Cumulative Incidence of Cancer in First-Degree Relatives of Jewish Women with Breast Cancer: Women Positive for BRCA1 Versus Women Positive for BRCA2[Note]
|
Cumulative Incidence of Cancer (%) to Age |
||||||||||||
| 55 Yearsa |
65 Yearsa |
75 Yearsa |
||||||||||
| Site | BRCA1 | BRCA2 | Pb | RRc | BRCA1 | BRCA2 | Pb | RRc | BRCA1 | BRCA2 | Pb | RRc |
| Breast | 18.8 | 6.2 | .022 | .30 | 27.0 | 19.7 | .095 | .53 | 29.8 | 26.3 | .174 | .62 |
| Ovary | 6.8 | 7.7 | .955 | .97 | 8.3 | 12.3 | .700 | 1.24 | 8.3 | 12.3 | .700 | 1.24 |
| Prostate | 1.0 | 2.7 | .618 | 2.00 | 1.0 | 2.7 | .618 | 2.00 | 8.0 | 12.0 | .626 | 1.45 |
| Uterus | 2.8 | .0 | .233 | .00 | 6.6 | .0 | .092 | .00 | 6.6 | .0 | .092 | .00 |
| Colon | 1.0 | 1.2 | .914 | .88 | 1.0 | 4.4 | .275 | 2.61 | 5.3 | 6.4 | .699 | 1.30 |
| Pancreas | .4 | .0 | .450 | .00 | .4 | 2.9 | .289 | 3.39 | 1.5 | 5.0 | .308 | 2.46 |
| Lung | .5 | .0 | .450 | .00 | .5 | .0 | .450 | .00 | 4.6 | 1.8 | .398 | .40 |
| Head and neck | .0 | .0 | … | … | .0 | .0 | … | … | .0 | .0 | … | … |
| Melanoma | .0 | .0 | … | … | .0 | .0 | … | … | .0 | 1.8 | .182 | ∞ |
| Primary site unknown | .0 | 1.1 | .188 | ∞ | .0 | 2.5 | .066 | ∞ | .0 | 2.5 | .066 | ∞ |
| Any: | ||||||||||||
| Womend | 8.3 | 3.4 | .261 | .42 | 15.1 | 10.6 | .421 | .65 | 21.8 | 19.7 | .591 | .79 |
| Women | 28.8 | 16.6 | .041 | .48 | 41.2 | 38.8 | .249 | .73 | 46.6 | 51.0 | .508 | .85 |
| Men | 3.0 | 8.9 | .167 | 2.76 | 4.4 | 21.4 | .012 | 4.16 | 23.7 | 39.5 | .041 | 2.22 |
| All | 15.8 | 13.4 | .356 | .75 | 23.3 | 31.6 | .605 | 1.14 | 34.9 | 46.3 | .353 | 1.22 |
Note.—Cancers in women include cancers in 162 female relatives of BRCA1 carriers and 91 female relatives of BRCA2 carriers. Cancers in men included cancers in 149 male relatives of BRCA1 carriers and 74 male relatives of BRCA2 carriers.
Kaplan-Meier estimates.
Log-rank test.
Relative risks are obtained from a univariate Cox proportional hazard model. Baseline: positive for BRCA1.
Women with no breast or ovarian cancer. Female relatives with breast cancer or ovarian cancer are considered to be at risk for other cancers.
We studied prevalent cases; if the survival of BRCA1 or BRCA2 mutation carriers is better than that of noncarriers, then we expect to see the prevalence of mutations increasing with time since diagnosis. However, because BRCA1-positive cases also occur at a younger age than nonhereditary cases and because BRCA2-positive women are older, it is necessary to control for age at diagnosis in this comparison. To do so, we matched each woman with a mutation-positive ovarian cancer with a mutation-negative woman diagnosed at the same age and compared the mutant frequencies by time elapsed since diagnosis (table 8). The overall mutation frequency in these subsamples are 50% (by definition), but the frequency of BRCA1 mutations increased with time since diagnosis. Although the trend did not reach statistical significance, this observation supports the hypothesis that the survival of women with BRCA1-associated ovarian cancer is better than expected. No similar trend was observed for BRCA2 mutations, suggesting that the survival benefit is limited to BRCA1 carriers.
Table 8.
Frequency of Mutations in Women with Ovarian Cancer by Time since Diagnosis
| Years since Diagnosis | No. of BRCA1 Controls | No. of Patients Positive for Mutations in BRCA1 (%) | No. of BRCA2 Controls | No. of Patients Positive for Mutations in BRCA2 (%) |
| .0–.9 | 16 | 13 (44.8) | 10 | 5 (33.3) |
| 1.0–3.9 | 23 | 22 (48.9) | 13 | 17 (56.7) |
| 4.0–7.9 | 11 | 12 (52.2) | 2 | 3 (60.0) |
| 8.0–26.0 | 7 | 10 (58.8) | 4 | 4 (50.0) |
| Total | 57 | 57 (50.0) | 29 | 29 (50.0) |
| P value for trend | .35 | .40 |
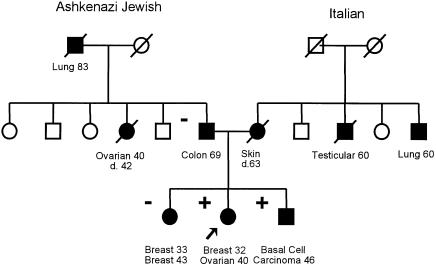
A founder mutation was not identified for 122 of 208 women. To estimate the frequency of nonfounder mutations in Jewish ovarian-cancer cases, we performed protein-truncation test analysis on the large exons of BRCA1 and BRCA2 (which comprise the majority of the coding region of the two genes) for these 122 women. No BRCA1 mutation was identified, and a single truncating mutation was identified in BRCA2 (6696delTC). Although the Jewish paternal aunt of the patient was also affected with early-onset ovarian cancer, further investigation of this family revealed that the mutation was inherited from the non-Jewish mother (fig. 6). Surprisingly the sister of the proband was diagnosed with early-onset bilateral cancer but was not a carrier of the 6696delT mutation.
Figure 6.
Pedigree of the family carrying the 6696delTC mutation. Circles denote women; squares denote men. Individuals affected with cancer (blackened symbols) and unaffected individuals (unblackened symbols) are both indicated. A diagonal line through a symbol indicates that the individual denoted is dead.
Discussion
We identified a very high proportion (41%) of hereditary ovarian cancers among unselected cases of epithelial ovarian cancer in Ashkenazi Jewish women from North America and Israel. This fraction is much higher than the hereditary proportions of other common adult cancers for any ethnic group studied to date. Ours is the first large survey of both BRCA1 and BRCA2 mutations in Jewish women with ovarian cancer who are unselected for age or family history. Muto et al. (1996) found that 6 of 31 (19.3%) unselected Jewish patients with ovarian cancer in Massachusetts carried a 185delAG mutation, and Modan et al. (1996) found that 15 of 79 (19.0%) Ashkenazi women with ovarian cancer in Israel carried a 185delAG mutation. For this single mutation, our estimate is similar (20.5%). Three other Israeli studies reported on the prevalence of BRCA1 and BRCA2 mutations in Israeli Jewish women with ovarian cancer (Abeliovich et al. 1997; Beller et al. 1997; Levy-Lehad et al. 1997). These studies focused on women selected either for early age at onset or a family history positive for cancer. Abeliovich et al. (1997) found 13 of 21 (62%) of women with ovarian cancer had one of three founding mutations. Because these patients included women referred from a cancer genetics counseling clinic, their estimate is higher than ours. No 5382insC mutation was found in this sample. Levy-Lehad et al. (1997) studied 22 incident cases of ovarian cancer among Ashkenazi women presenting to the Division of Gynecologic Oncology at the Shaare Zedek Medical Center in Jerusalem between September 1995 and May 1996. One of three founder mutations was present in 10 of 22 women (45%). The mean age at diagnosis in the three BRCA2 carriers (68.3 years) was much higher than that in the BRCA1 carriers (50.5 years). These patients are also described by Beller et al. (1997).
The prevalence of mutations in Ashkenazi Jews with ovarian cancer is many times higher than in non-Jewish patients. In a study of 374 women with ovarian cancer in the United Kingdom, Stratton et al. (1997) estimated that only 3.5% of all cancer cases carry a BRCA1 mutation. In a smaller study, Rubin et al. (1998) found one BRCA2 mutation and 10 BRCA1 mutations in 113 hospital-based cases of ovarian cancer, for an overall prevalence of 9.7%. Janezic et al. (1999) found a pathogenic BRCA1 mutation in only 2 of 107 unselected ovarian-cancer cases in California. The proportions of Jewish patients in these studies are not known.
The frequency of mutations in BRCA1 and BRCA2 in the Jewish population at large is ∼2% and is much greater than the frequency in non-Jews. This high prevalence accounts, to a large extent, for the greater population-attributable risk observed in the Jewish population; however, it is also possible that the risk of ovarian cancer associated with the Jewish founder mutations exceeds that of other BRCA1 or BRCA2 mutations. On the basis of the RR method, we estimate the penetrance of ovarian cancer to be 51% for carriers of the 185delAG mutation, 21% for carriers of the 5382insC mutation, and 14% for carriers of the 6174delT mutation. The risk estimates generated by the kin-cohort method were much lower—but these estimates were made on the basis of small numbers of cases in relatives, and the confidence limits are wide. Furthermore, the kin-cohort method depends on the accuracy of the probands' recollections. Struewing et al. (1997) also used the kin-cohort method to estimate penetrance in the Jewish population, but their estimates were made on the basis of only 11 observed ovarian cancers in 306 female relatives of carriers.
The Jewish founder mutations represent only three of the hundreds of mutations in BRCA1 or BRCA2 that have been identified to date. The risk of ovarian cancer is believed not to be the same for all BRCA2 mutations and varies according to the position of the mutation along the gene. In particular, the 6174delT mutation is in the ovarian-cancer cluster region of BRCA2 as defined by Gayther et al. (1997). BRCA2 mutations outside this region of exon 11 are believed to carry a much lower risk of ovarian cancer. This is consistent with the high risk of ovarian cancer associated with the 6174delT mutation in our study. Similarly, truncating mutations in the first two-thirds of the coding region of BRCA1 are thought to be associated with a higher risk of ovarian cancer than mutations in the last third of the gene (Gayther et al. 1995). The 185delAG mutation is in the extreme 5′ end of the gene, and the 5382insC mutation is in the extreme 3′ end of the gene. In support of this hypothesis, we found the ovarian-cancer risk to be moderately higher for carriers of the 185delAG mutation, compared with carriers of the 5382insC mutation (by the RR method).
This study suggests that the three founder mutations account for the most of the heritability of ovarian cancer in the Jewish population. Of 87 identified mutations, 86 were founder mutations, and the single nonfounder mutation was inherited from a non-Jewish ancestor. Furthermore, we found only a modest, nonsignificant excess risk of ovarian cancer in the relatives of the mutation-negative women. This suggests that most of the familial clustering of ovarian cancer in the Jewish population is attributable to these three founding mutations. If there are ovarian cancer–susceptibility genes other than BRCA1 and BRCA2, their contribution will be relatively minor in the Jewish population.
It has been reported that the risk of breast and ovarian cancer appears to be increasing from generation to generation in some families whose members carry mutations (Narod et al. 1993). The present study provided the opportunity to directly evaluate the possibility of a cohort effect among the carriers. We compared the risk of breast and ovarian cancer in the mothers and sisters of the carriers. Under the assumption that cancer risk is increasing with birth year, we would expect that the risk in sisters should exceed that of mothers. This was not found, and our data set does not support the phenomenon of genetic anticipation or a cohort effect.
A family history of pancreatic cancer was found to be a risk factor for ovarian cancer. This relationship is largely due to the impact of BRCA2. In a previous study, BRCA2 mutations were found to be present in 3 of 26 unselected Jewish cases of pancreatic cancer (Ozcelik et al. 1997). Our data also support the hypothesis that male carriers of BRCA1 or BRCA2 mutations are at increased risk of prostate cancer (Warner et al. 1999), and we recommend prostate specific antigen screening of men with BRCA1 or BRCA2 mutations from age 50 years. The association of BRCA1 and endometrial cancer has not been reported previously and warrants further attention.
We did not see an increase in the risk of colon cancer in the relatives of the BRCA1 or BRCA2 carriers, compared with noncarriers or healthy controls. No excess of colon cancer was seen in the family members of BRCA2 carriers in the study of the Breast Cancer Linkage Consortium (1999). Since the original paper by Ford et al. (1994), colon cancer has not been found to be a feature of the BRCA1 spectrum.
Rubin et al. (1996) reported that ovarian cancer patients with BRCA1 mutations have a better likelihood of 10-year survival than do women without mutations, after adjustments are made for the stage of cancer. This study has been criticized on the basis that the selection criteria favored long-term survivors in carriers (Brunet et al. 1997; Modan 1997). However, a recent study based on pathology specimens confirmed the previous results (Boyd et al. 1999). Our data indirectly support the hypothesis of Rubin and his colleagues that BRCA1-associated ovarian cancer has a better prognosis. We found an increasing prevalence of mutations with time elapsed since diagnosis—consistent with the notion that BRCA1 mutations are overrepresented in long-term ovarian-cancer survivors. It was surprising, therefore, to find that the BRCA1 carriers commonly presented with high-grade tumors. It may be that the high-grade, BRCA1-positive tumors lack the capacity to repair DNA damage and are therefore particularly sensitive to the cytotoxic effects of chemotherapy.
We feel that our data support the position that Jewish women with ovarian cancer should be offered testing for BRCA1 and BRCA2 mutations. Factors that predict the presence of a mutation include tumor grade, age at onset, histology, and family history. Tumor grade appears to be the most discriminating of the four predictive factors. A mutation was found in 28% of the ovarian-cancer cases that had no family history of breast or ovarian cancer and in 64% of patients with a family history of ovarian cancer. On the basis of these data, we expect that the majority of Jewish women who develop ovarian cancer in the context of a screening program for women at high familial risk will also carry a BRCA1 or a BRCA2 mutation. Because of the high risk of ovarian cancer associated with each of these mutations and because of the limitations of ovarian cancer screening, we feel that the option of prophylactic oophorectomy should be raised with women who carry the mutation who have not yet developed cancer. Struewing et al. (1995b) found a 45% reduction in the risk of ovarian or peritoneal carcinoma following prophylactic oophorectomy in high-risk women. Prospective data on the residual risk of cancer and on the complications of premenopausal surgical oophorectomy are needed. Data are emerging that prophylactic oophorectomy may also be effective in reducing the risk of breast cancer (Rebbeck et al. 1999). On the basis of the age distributions of the mutation-positive ovarian cancers (table 1), it may be reasonable to wait until the time of the natural menopause to perform oophorectomy for BRCA2 carriers, but, for maximum protection, the operation should be performed at a much lower age in women who carry BRCA1 mutations; 31 of 57 (54%) of the ovarian cancers in carriers of BRCA1 mutations were diagnosed before age 50 years.
Acknowledgments
We thank all of the patients and their families, without whose kind cooperation this study would not have been possible. We thank Michelle Kelly-Dicanio, Myrna Ben Yaashay, Aimee Wonderlick, and Elaine Ball for their assistance in obtaining blood samples and epidemiological information on some of the patients. Technical assistance by John Abrahamson, Jalil Hakimi, Elaine Jack, Elaine Kwan, and Graciela Kuperstein is much appreciated. We also thank J. M. Friedman, Meri Klein, Marianna Gorbataia, Elizabeth Hoodfar, Alexander Liede, and Eva Weinroth. This study was supported by grants from the NIH (RO1CA 63678-05), the United States Army (DAMD 17-94-J-4299), the Canadian Genetic Disease Network, and the Canadian Breast Cancer Research Initiative.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for inherited breast cancer type 1 and ovarian cancer [MIM 113705] and inherited breast cancer type 2 [MIM 600185])
References
- Abeliovich D, Kaduri L, Lerer I, Weinberg N, Amir G, Sagi M, Zlotogora J, et al (1997) The founder mutations 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 appear in 60% of ovarian cancer and 30% of early-onset breast cancer patients among Ashkenazi women. Am J Hum Genet 60:505–514 [PMC free article] [PubMed] [Google Scholar]
- Amos CI, Struewing JP (1993) Genetic epidemiology of epithelial ovarian cancer. Cancer 71:566–572 [DOI] [PubMed] [Google Scholar]
- Beller U, Halle D, Catane R, Kaufman B, Hornereich G, Levy-Lahad E (1997) High frequency of BRCA1 and BRCA2 germline mutations in Ashkenazi Jewish ovarian cancer patients, regardless of family history. Gynecol Oncol 67:123–126 [DOI] [PubMed] [Google Scholar]
- Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, Barakat RR, et al (1999) Clinical and pathologic features of hereditary ovarian cancers associated with germline mutations of BRCA1 or BRCA2. Gynecol Oncol 72:444 [Google Scholar]
- Breast Cancer Linkage Consortium (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91:1310–1316 [DOI] [PubMed] [Google Scholar]
- Brunet JS, Narod SA, Tonin P, Foulkes WD (1997) Survival rates in BRCA1 carriers with ovarian cancer. N Engl J Med 336:1256 [PubMed] [Google Scholar]
- Easton D, Ford D, Bishop D, Breast Cancer Linkage Consortium (1995) Breast and ovarian cancer incidence in BRCA1 mutation carriers. Am J Hum Genet 56:265–271 [PMC free article] [PubMed] [Google Scholar]
- Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE, Breast Cancer Linkage Consortium (1994) Risks of cancer in BRCA1-mutation carriers. Lancet 343:692–695 [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, et al (1998) Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 62:676–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayther SA, Mangion J, Russell P, Seal S, Barfoot R, Ponder BAJ, Stratton MR, et al (1997) Variation of risks of breast and ovarian associated with different germline mutations of the BRCA2 gene. Nat Genet 15:103–105 [DOI] [PubMed] [Google Scholar]
- Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiana M, Seal S, et al (1995) Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet 11:428–433 [DOI] [PubMed] [Google Scholar]
- Janezic SA, Ziogas A, Krumroy LM, Krasner M, Plummer SJ, Cohen P, Gildea M, et al (1999) Germline BRCA1alterations in a population-based series of ovarian cancer cases. Hum Mol Genet 8:889–898 [DOI] [PubMed] [Google Scholar]
- Levy-Lehad E, Catane R, Eisenberg S, Kauffman B, Hornreich G, Lishinksy E, Shohat M, et al (1997) Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel: frequency and differential penetrance in ovarian cancer and in breast cancer families. Am J Hum Genet 60:1059–1067 [PMC free article] [PubMed] [Google Scholar]
- Modan B (1997) BRCA1 mutations and survival in women with ovarian cancer. N Engl J Med 336:1255 [PubMed] [Google Scholar]
- Modan B, Gak E, Sade-Baruchim R, Hirsch-Yechezkel G, Theodor L, Lubin F, Ben-Baruch G, et al (1996) High frequency of BRCA1 185delAG mutation in ovarian cancer in Israel. JAMA 276:1823–1825 [PubMed] [Google Scholar]
- Muto MG, Cramer DW, Tangir J, Berkowitz R, Mok S (1996) Frequency of the BRCA1 185delAG mutation among Jewish women with ovarian cancer and matched population controls. Cancer Res 56:1250–1252 [PubMed] [Google Scholar]
- Narod SA, Ford D, Devilee P, Barkardottir RB, Eyfjord J, Lenoir GM, Serova O, et al (1995a) Genetic heterogeneity of breast-ovarian cancer revisited. Am J Hum Genet 57:957 [PMC free article] [PubMed] [Google Scholar]
- Narod SA, Ford D, Devilee P, Barkardottir RB, Lynch HT, Smith SA, Ponder BAJ, et al (1995b) An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Am J Hum Genet 56:254–264 [PMC free article] [PubMed] [Google Scholar]
- Narod SA, Lynch H, Conway T, Watson P, Fuenteun J, Lenoir GM (1993) Increasing incidence of breast cancer in family with BRCA1 mutation. Lancet 341:1101 [DOI] [PubMed] [Google Scholar]
- Narod SA, Madlensky L, Bradley L, Cole D, Tonin P, Rosen B, Risch HA (1994) Hereditary and familial ovarian cancer in Southern Ontario. Cancer 74:2341–2346 [DOI] [PubMed] [Google Scholar]
- Oddoux C, Struewing JP, Clayton M, Neuhausen S, Brody LC, Kaback M, Haas B, et al (1996) The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi Jewish individuals is approximately 1%. Nat Genet 14:188–190 [DOI] [PubMed] [Google Scholar]
- Ozcelik HO, Schmocker B, Di Nicola N, Shi X-H, Langer B, Moore M, Taylor BR, et al (1997) Germline BRCA2 6174delT mutations in Ashkenazi Jewish pancreatic cancer patients. Nat Genet 16:17–18 [DOI] [PubMed] [Google Scholar]
- Parkin D, Muir C, Whelan SL, Ferlay J, Raymond L, Young J (1997) Cancer incidence in five continents, vol 7. IARC Scientific Publications, Lyon [Google Scholar]
- Rebbeck TR, Levin AM, Eisen A, Snyder C, Lynch HT, Chittenden A, Garber JE, et al (1999) Reduction in breast cancer risk following bilateral prophylactic mastectomy in BRCA1 carriers. J Natl Cancer Inst 91:1475–1479 [DOI] [PubMed] [Google Scholar]
- Risch HA (1998) Hormonal etiology of epithelial ovarian cancer with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 90:1774–1786 [DOI] [PubMed] [Google Scholar]
- Roa B, Boyd A, Volcik K, Richards C (1996) Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet 14:185–187 [DOI] [PubMed] [Google Scholar]
- Rubin SC, Benjamine I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, Berchuk A, et al (1996) Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med 335:1413–1416 [DOI] [PubMed] [Google Scholar]
- Rubin SC, Blackwood MA, Bandera C, Behbakht K, Benjamin I, Rebbeck TR, Boyd J (1998) BRCA1,BRCA2 and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population. Relationship to family history and implications for genetic testing. Am J Obstet Gynecol 178:670–677 [DOI] [PubMed] [Google Scholar]
- Stratton JF, Gayther SA, Russell PL, Dearden J, Gore M, Blake P, Easton D, et al (1997) Contribution of BRCA1 mutations to ovarian cancer. N Engl J Med 336:1125–1130 [DOI] [PubMed] [Google Scholar]
- Struewing J, Abeliovich D, Peretz T, Avishai N, Kaback MM, Collins FS, Brody LC (1995a) The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet 11:198–200 [DOI] [PubMed] [Google Scholar]
- Struewing J, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, et al (1997) The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med 336:1401–1408 [DOI] [PubMed] [Google Scholar]
- Struewing JP, Watson P, Easton DF, Ponder BA, Lynch HT, Tucker MA (1995b) Prophylactic oophorectomy in inherited breast/ovarian cancer families. J Natl Cancer Inst 17:33–35 [PubMed] [Google Scholar]
- Tonin P, Weber B, Offit K, Couch F, Rebbeck TR, Neuhasuen S, Godwin AK, et al (1996) Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med 2:1179–1183 [DOI] [PubMed] [Google Scholar]
- Wacholder S, Hartge P, Struewing JP, Pee D, McAdams M, Brody L, Tucker M (1998) The kin-cohort study for estimating penetrance. Am J Epidemiol 148:623–630 [DOI] [PubMed] [Google Scholar]
- Warner E, Goodwin P, Foulkes W, Meschino W, Goss P, Ozcelik H, Allingham-Hawkins D, et al (1999) Prevalence and penetrance of BRCA1 and BRCA2 mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91:1241–1247 [DOI] [PubMed] [Google Scholar]