Abstract
Background
A third DNA strand can bind into the major groove of a homopurine duplex DNA to form a DNA triple helix. Sequence specific triplex formation can be applied for gene targeting, gene silencing and mutagenesis.
Results
We have analyzed triplex formation of two polypurine triplex forming oligodeoxynucleotides (TFOs) using fluorescence resonance energy transfer (FRET). Under our conditions, the TFOs bind to their cognate double strand DNAs with binding constants of 2.6 × 105 and 2.3 × 106 M-1. Our data confirm that the polypurine TFO binds in an antiparallel orientation with respect to the polypurine DNA strand and that triplex formation requires Mg2+ions whereas it is inhibited by K+ions. The rate of formation of triple helices is slow with bimolecular rate constants of 5.6 × 104 and 8.1 × 104 min-1 M-1. Triplex dissociation was not detectable over at least 30 hours. Triplex formation is sequence specific; alteration of a single base pair within the 13 base pairs long TFOs prevents detectable triplex formation.
Conclusion
We have applied a FRET assay to investigate the specificity of DNA triple helix formation. This assay is homogeneous, continuous and specific, because the appearance of the FRET signal is directly correlated to triplex formation. We show that polypurine TFOs bind highly specifically to polypurine stretches in double stranded DNA. This is a prerequisite for biotechnical applications of triple helices to mediate sequence specific recognition of DNA.
Background
It has been discovered in 1957 that a homopyrimidine DNA strand (triplex forming oligonucleotide, TFO) can bind to a homopurine/homopyrimide DNA duplex in the major groove by forming Hoogsteen base pairs with the homopurine strand [1]. The Hoogsteen base pairing scheme mediates sequence specific recognition of the double stranded DNA by the TFO where an AT base pair is recognized by a T and a GC base pair by a C that is protonated at N3 (reviews: [2-4]). Later it was found that homopurine strands can also specifically form a DNA triplex in which the AT base pair is contacted by an A and the GC base pair by a G [5]. Triple helix formation with purine rich TFOs is less pH sensitive but requires divalent cations like Mg2+[6-8]. In either case, the two pyrimidine strands and the two purine strands must be arranged in an antiparallel orientation to form a stable triplex (reviews: [2-4]). Triple helix formation has been employed for various purposes in biotechnology like gene targeting, mutagenesis and inhibition of gene activity (reviews: [9-11]).
In this work, we investigate the sequence specificity of triple helix formation, which is the prerequisite for all biotechnological applications. It has been shown that triplex formation with polypyrimidine TFOs is very sequence specific. The energetic penalty of one single mismatch is similar to that observed in double stranded DNA [12-16]. Similar results were published for polypurine TFOs [17,18]. However, many analyses of triplex formation were carried out with assays like UV melting, CD melting, differential scanning calorimetry, gel electrophoresis, footprint analysis, affinity cleavage, chromatography and filter binding. Most of these assays are heterophasic and the signal often is not directly correlated to triplex formation. In contrast, Yang et al. have introduced a fluorescence resonance energy transfer (FRET) assay to analyze triple helix formation that is homophasic and allows to study the kinetics and thermodynamics of this process [19]. In this assay, the double stranded DNA is labeled with fluorescein and the TFO carries a rhodamine label. If a triplex is formed, FRET takes place between the fluorescein donor and the rhodamine acceptor (Fig. 1B). This FRET signal is directly correlated to the formation of triple helices. We have employed this method here to characterize triplex formation of polypurine TFOs and show that it is highly sequence specific.
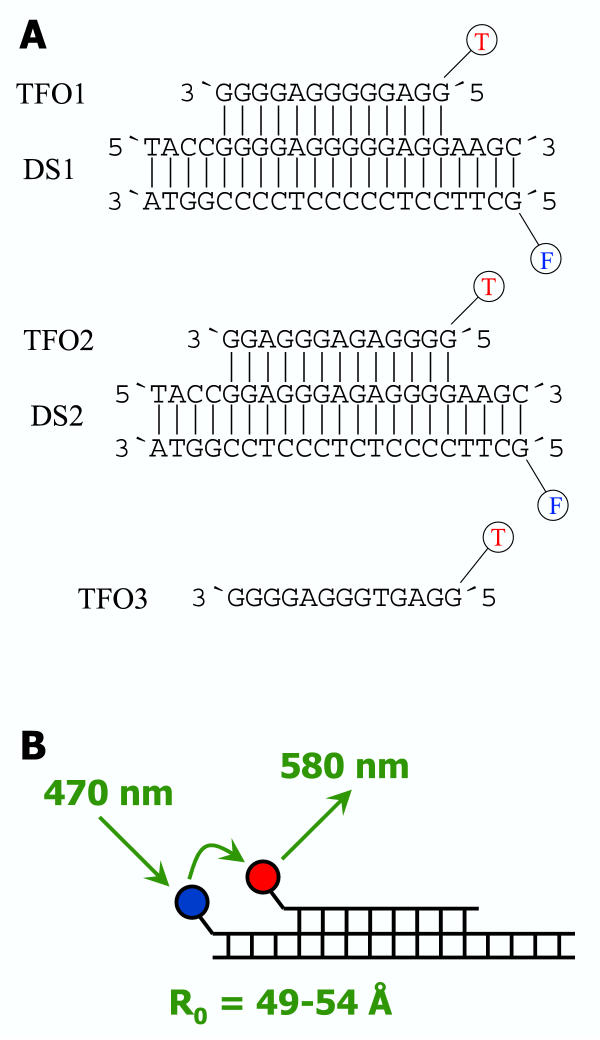
Figure 1.
Oligonucleotides used in this study. A) Sequences of the TFOs and DNAs used in this study. B) Principle of the FRET assay used in this study. F denotes for 6-FAM, T for TAMRA; the R0-value of this pair for fluorophors is 49–54 Å.
Results and Discussion
Detection of triple helix formation by FRET
We have investigated the biochemical and biophysical properties of a stable purine(purine-pyrimidine) triplex [7], and checked for its sequence specificity. We used a 21 mer double stranded DNA, DS1, that contains a homopurine stretch of 16 base pairs (Fig. 1). The pyrimidine strand carries a 6-carboxyfluorescein (FAM) label on its 5' end. The corresponding 13 mer TFO1 is 5' labeled with 5-carboxytetramethylrhodamine (TAMRA). FAM and TAMRA form a FRET pair with an R0 of 49–54 Å [20]. After triplex formation, the donor and acceptor groups are separated by 4 base pairs which according to computer modeling corresponds to a distance of approximately 15 Å. Therefore, a direct interaction of the fluorophors, which is unfavorable for the assay, cannot occur, but the probes are well within a distance that should lead to highly efficient FRET.
After excitation of DS1 alone at 470 nm, a strong fluorescence of the FAM-acceptor group is detected at 520 nm (Fig. 2A). In contrast, the emission of the TAMRA group was almost not detectable (580 nm) if TFO1 alone was excited at 470 nm. After mixing DS1 and the TFO1 and annealing at 55°C for 10 min, FRET from FAM to TAMRA occurs as illustrated by a decrease in FAM fluorescence and concomitant increase in the intensity of the emission of the TAMRA group. In the absence of Mg2+ no FRET signal was detectable (Fig. 3) confirming the finding in the literature that triplex formation with polypurine TFOs requires divalent cations [6-8]. We also did not observe a FRET signal in the presence of K+ions (data not shown) which are known to inhibit triple helix formation of G-rich TFOs by stabilization of quadruplex structures of the TFO [8,21]. These results show that the FRET assay is well suited to detect formation of DNA triple helices.
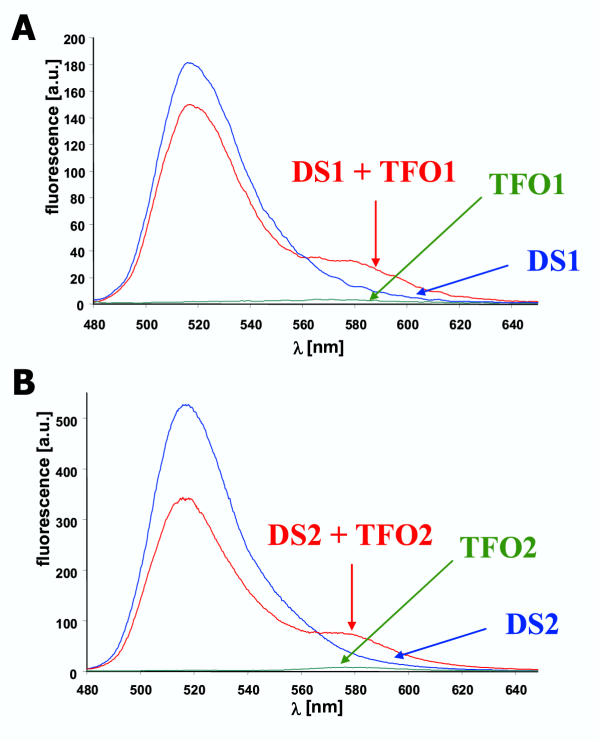
Figure 2.
Triple helix formation analyzed by the FRET assay A) Fluorescence emission spectra were recorded for DS1 (0.5 μM), TFO1 (2.5 μM) and DS1 annealed to TFO1 in binding buffer. B) Same experiments with DS2 and TFO2. In both panels, a decrease in FAM fluorescence at 520 nm and an increase in TAMRA fluorescence at 580 nm is observed that is indicative of FRET.
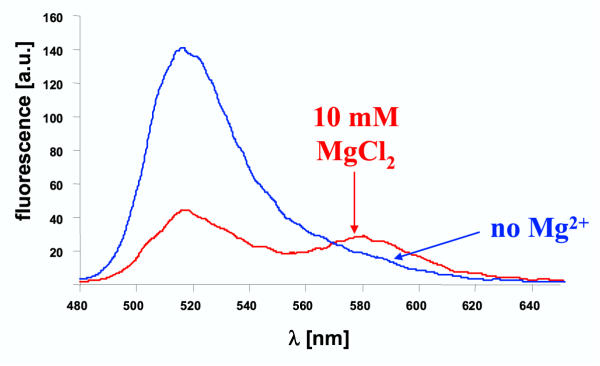
Figure 3.
Dependence of triplex formation on divalent cations To analyze the dependence of triplex formation on divalent cations, DS1 (0.5 μM) was annealed to TFO (10 μM) in binding buffer (20 mM HEPES pH 7.5, 50 mM Na-acetate, 10 mM MgCl2) and in buffer not containing Mg2+-ions (20 mM HEPES pH 7.5, 50 mM Na-acetate). In the Mg2+ free buffer, no triplex formation is detectable.
To check the general feasibility of this technique, we have used an additional pair of DNA and TFO. To this end, the sequence of TFO1 was changed at four positions to create TFO2 that should specifically bind to DS2. Triple helix formation of TFO2 and DS2 has not been studied so far. As shown in Fig. 2B, the results obtained with the TFO2/DS2 pair are very similar to the TFO1/DS1 pair. This finding demonstrates that triplex formation with polypurine TFOs is a general phenomenon not restricted to certain sequences.
Determination of the biophysical properties of the different triple helices
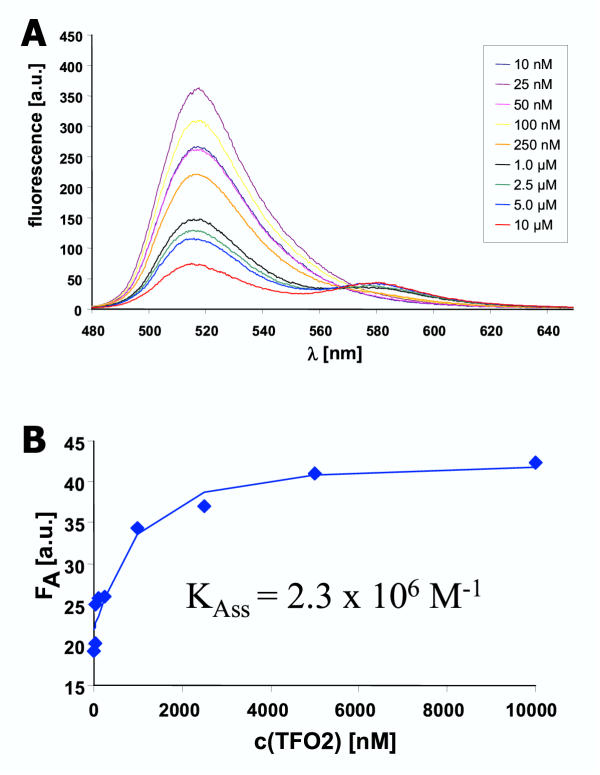
Titrations were carried out with both pairs of double stranded DNA and TFOs in order to measure the binding constants of the TFOs to the DNA. In these experiments, increasing amounts of TFO were added to a constant amount of double stranded DNA. As shown in Fig. 4, higher concentrations of the TFO led to an increase in the efficiency of FRET as illustrated by a reduction of the donor fluorescence and increasing acceptor fluorescence. Control experiments were carried out to confirm that even at the highest concentrations the TFO alone does not show significant fluorescence (data not shown). The fluorescence of the TAMRA group was analyzed with respect to the equilibrium binding constants of the TFOs to the corresponding DNA. A quantitative analysis of these data shows that TFO1 binds to DS1 with a binding constant of 2.6 × 105 M-1 whereas TFO2 binds to DS2 with Kass = 2.3 × 106 M-1. The finding that triplex 2 is almost 10-fold more stable than triplex 1 suggests that the A-AT triple is thermodynamically favorable in comparison to the G-GC triple by approximately 5–6 kJ/mol. However, it is possible that quadruplex formation is more efficient with the more G-rich TFO1 [22-24]. Since quadruplex formation competes with triplex formation, this effect could contribute to the higher apparent stability of triplex 1.
Figure 4.
Equilibrium binding constant of TFO2 to DS2 A) To measure the binding affinity of TFO2 to DS2, DS2 (0.5 μM) was incubated with different amounts of TFO2 (10 nM to 10 μM) in binding buffer for 10 hours and the fluorescence spectra recorded. B) The TAMRA fluorescence was determined and fitted to an equation describing a bimolecular binding equilibrium.
The total efficiency of the FRET process can be estimated at the highest concentration of the TFO where the DNA is almost saturated with TFO. Under these conditions, the donor fluorescence is quenched by more than 85% (Fig. 4). This result confirms that the TFO binds to the DNA in an antiparallel orientation with respect to the polypurine strand [5,25], because in a parallel orientation the distance between the donor and acceptor groups would not allow highly efficient FRET.
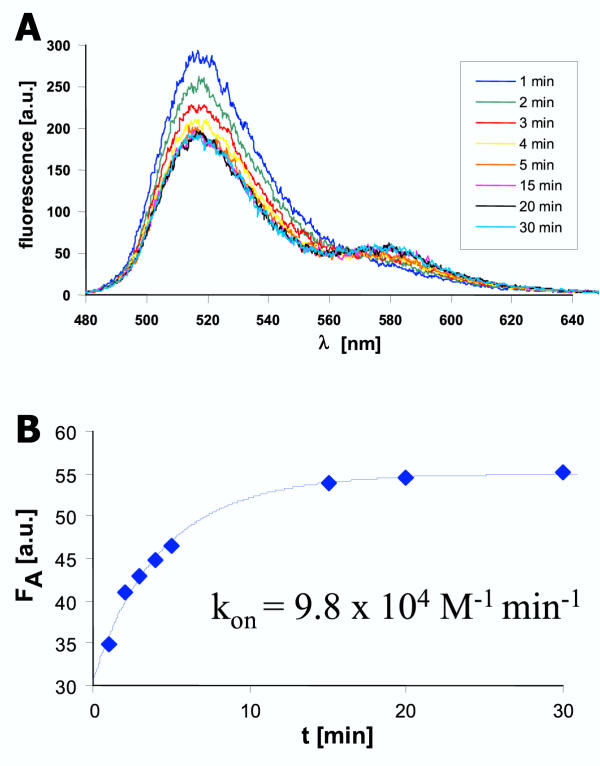
In addition, the kinetics of association and dissociation of both the triplexes were determined. For the association reactions the DNA and different amounts of TFO were mixed and incubated in the cuvette at 37°C. At defined time points, fluorescence spectra were recorded. The fluorescence of the TAMRA group was analyzed with respect to a bimolecular binding reaction. As shown in Fig. 5, association takes place within 30 min under these conditions. A quantitative analysis results in bimolecular rate constants of association of 5.6 × 104 min-1 M-1 for TFO1 and DS1 and 8.1 × 104 min-1 M-1 for TFO2 and DS2. These rate constants are in the range of results obtained with polypyrimidine TFOs and slightly higher than values previously determined for a polypurine TFO using a heterophasic assay [26]. This result shows that the 10-fold increase in the stability of triplex 2 is in part due to a faster rate of formation of this complex.
Figure 5.
Kinetics of association of TFO2 to DS2 A) Example of the association kinetics. DS2 (0.5 μM) and TFO2 (2.5 μM) were mixed in binding buffer and incubated at 37°C in the cuvette. The fluorescence emission spectra were recorded at various time points. B) The TAMRA fluorescence of the spectra shown in A was determined and the data fitted to an equation describing the kinetics of a bimolecular binding reaction.
For the dissociation reactions, triplex formation was performed by annealing at 55°C in a small volume of buffer. Then, the triplex was diluted into a solution containing a large excess of TFO not carrying the TAMRA label. Fluorescence was determined over 30 hours, but no significant change in the FRET efficiency was observed, indicating that dissociation occurs very slowly under these conditions. In contrast, rapid dissociation of the triplex was observed after addition of 20 mM EDTA to the binding buffer confirming that the experimental setup was suited to detect triplex dissociation (data not shown).
Sequence specificity of triplex formation
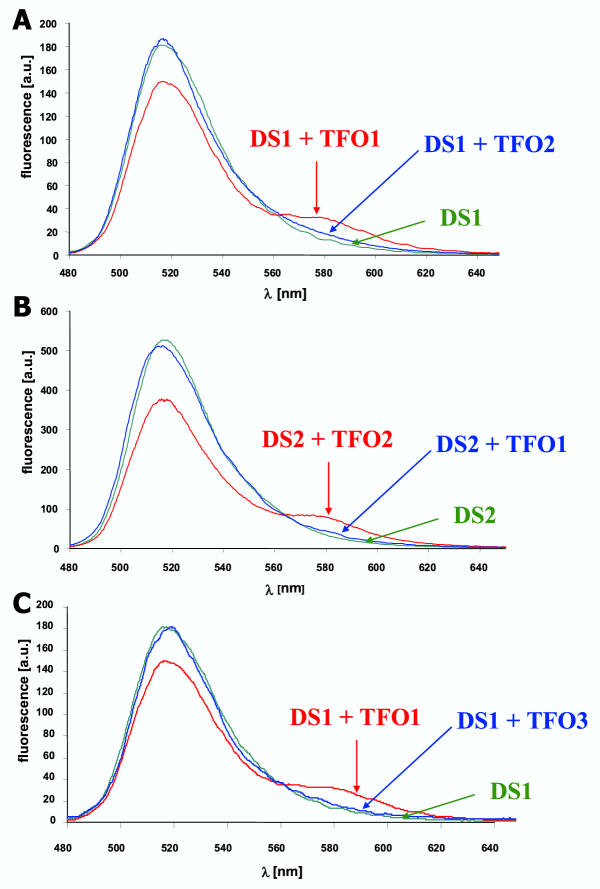
The sequences of TFO1 and TFO2 differ in 5 of 13 positions. To check for sequence specificity of triplex formation, we have investigated if TFO1 can also bind to DS2 and vice versa. As shown in Fig. 6, non-specific triplex formation does not occur indicating that triplex formation is sequence specific. To analyze the degree of specificity, we have used TFO3 that is identical in sequence to TFO1 with the only exception that one G has been changed to T thereby creating a T-GC base triple. Our results show that TFO3 cannot form a triplex on DS1 indicating that triplex formation is very specific, because introduction of one mismatch base abrogates triplex formation. They confirm similar results obtained by Beal and Dervan using the affinity cleavage method for a different TFO sequence [17].
Figure 6.
Specificity of triplex formation To determine the specificity of triplex formation, different pairs of non-matching TFOs and DNAs were tested for their ability to form a triplex. A) DS1 (0.5 μM) was annealed with 2.5 μM TFO1 or TFO2. B) DS2 (0.5 μM) was annealed with 2.5 μM TFO1 or TFO2. C) DS1 (0.5 μM) was annealed with 2.5 μM TFO1 or TFO3. Triplex formation between non-matching TFOs and DNAs was not detectable indicating a very high sequence specificity of this process.
Conclusions
In this work we have investigated formation and specificity of DNA triplexes using a FRET assay. This assay has many important advantages over alternative assay systems to monitor triplex formation: 1) It is very fast, convenient and requires only small amounts of sample. 2) It is a direct assay, because only triplex formation can lead to the specific FRET signal. 3) It is homophasic and does not depend on the separation of free and bound TFO, which always tends to shift the equilibrium. 4) It allows continuous measurements and thus is suited to follow kinetics of triplex formation and dissociation. We show that triplex formation is highly specific, because exchange of one base pair in the double stranded DNA or in the TFO prevents triplex formation. This high specificity is a prerequisite for the usage of triple helices in biotechnology, e.g. for gene targeting, reduction of gene expression or mutagenesis.
Methods
Purified oligodeoxynucleotides were purchased from MWG (Eberberg, Germany). The homogeneity of the oligonucleotides was checked on denaturing polyacrylamide gels. Triplex formation was assayed in binding buffer (20 mM HEPES pH 7.5, 50 mM Na-acetate, 10 mM MgCl2). For most experiments, 500 nM double stranded DNA and 2500 nM TFO were mixed, annealed by heating to 55°C for 10 min and equilibrated at 37°C. Fluorescence was measured using a Hitachi F4500 spectrofluorometer using 50 μl fluorescence cuvettes. Excitation was at 470 nm, emission was determined between 480 and 690 nm. Excitation and emission slits were at 5 and 2.5 mm, respectively. Spectra were recorded at a scanning speed of 2400 nm/min, usually 9–16 spectra were averaged to improve the signal to noise ratio. To calculate the efficiency of FRET, we used the fluorescence of the acceptor group (TAMRA) which was obtained by averaging the fluorescence emission spectra between 576 and 584 nm. To measure the binding constant of the TFOs to their corresponding double stranded DNA increasing amounts of TFO (10 nM – 10 μM) were added to a constant amount of DNA (500 nM). The samples were incubated for 10 hours at 37°C and the fluorescence analyzed. To determine the equilibrium binding constants, data were fitted as described [27]. Association kinetics were determined using three different concentrations of TFO (1, 2.5 and 4 μM) and a constant amount of double stranded DNA (500 nM). TFO and DNA were mixed, immediately placed in the spectrofluorometer and temperature maintained at 37°C. Fluorescence was scanned at defined time points for 30 min. To determine the rate constant of association all data sets were globally fitted to a bimolecular association reaction as described [27].
Acknowledgments
Acknowledgements
Thanks are due to A. Pingoud, H. Gowher and K. Eisenschmidt for discussions and B. Kleiber for technical assistance. This work has been supported by the BMBF Biofuture program and the European Union.
Contributor Information
Sabine Reither, Email: seadaisy@gmx.net.
Albert Jeltsch, Email: albert.jeltsch@chemie.bio.uni-giessen.de.
References
- Felsenfeld G, Davies DR, Rich A. Formation of a three-stranded polynucleotide molecule. J Am Chem Soc. 1957;79:2023–2024. [Google Scholar]
- Frank-Kamenetskii MD, Mirkin SM. Triplex DNA structures. Annu Rev Biochem. 1995;64:65–95. doi: 10.1146/annurev.bi.64.070195.000433. [DOI] [PubMed] [Google Scholar]
- Sun JS, Garestier T, Helene C. Oligonucleotide directed triple helix formation. Curr Opin Struct Biol. 1996;6:327–33. doi: 10.1016/S0959-440X(96)80051-0. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan I, Patel DJ. DNA triplexes: solution structures, hydration sites, energetics, interactions, and function. Biochemistry. 1994;33:11405–16. doi: 10.1021/bi00204a001. [DOI] [PubMed] [Google Scholar]
- Beal PA, Dervan PB. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science. 1991;251:1360–3. doi: 10.1126/science.2003222. [DOI] [PubMed] [Google Scholar]
- Svinarchuk F, Bertrand JR, Malvy C. A short purine oligonucleotide forms a highly stable triple helix with the promoter of the murine c-pim-1 proto-oncogene. Nucleic Acids Res. 1994;22:3742–7. doi: 10.1093/nar/22.18.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svinarchuk F, Paoletti J, Malvy C. An unusually stable purine(purine-pyrimidine) short triplex. The third strand stabilizes double-stranded DNA. J Biol Chem. 1995;270:14068–71. doi: 10.1074/jbc.270.23.14068. [DOI] [PubMed] [Google Scholar]
- Floris R, Scaggiante B, Manzini G, Quadrifoglio F, Xodo LE. Effect of cations on purine.purine.pyrimidine triple helix formation in mixed-valence salt solutions. Eur J Biochem. 1999;260:801–9. doi: 10.1046/j.1432-1327.1999.00219.x. [DOI] [PubMed] [Google Scholar]
- Praseuth D, Guieysse AL, Helene C. Triple helix formation and the antigene strategy for sequence-specific control of gene expression. Biochim Biophys Acta. 1999;1489:181–206. doi: 10.1016/S0167-4781(99)00149-9. [DOI] [PubMed] [Google Scholar]
- Vasquez KM, Wilson JH. Triplex-directed modification of genes and gene activity. Trends Biochem Sci. 1998;23:4–9. doi: 10.1016/S0968-0004(97)01158-4. [DOI] [PubMed] [Google Scholar]
- Knauert MP, Glazer PM. Triplex forming oligonucleotides: sequence-specific tools for gene targeting. Hum Mol Genet. 2001;10:2243–51. doi: 10.1093/hmg/10.20.2243. [DOI] [PubMed] [Google Scholar]
- Roberts RW, Crothers DM. Specificity and stringency in DNA triplex formation. Proc Natl Acad Sci U S A. 1991;88:9397–401. doi: 10.1073/pnas.88.21.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergny JL, Sun JS, Rougee M, Montenay-Garestier T, Barcelo F, Chomilier J, Helene C. Sequence specificity in triple-helix formation: experimental and theoretical studies of the effect of mismatches on triplex stability. Biochemistry. 1991;30:9791–8. doi: 10.1021/bi00104a031. [DOI] [PubMed] [Google Scholar]
- Fossella JA, Kim YJ, Shih H, Richards EG, Fresco JR. Relative specificities in binding of Watson-Crick base pairs by third strand residues in a DNA pyrimidine triplex motif. Nucleic Acids Res. 1993;21:4511–5. doi: 10.1093/nar/21.19.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AJ, Van Dyke MW. Oligodeoxyribonucleotide length and sequence effects on intermolecular purine-purine-pyrimidine triple-helix formation. Nucleic Acids Res. 1994;22:4742–7. doi: 10.1093/nar/22.22.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RW, Crothers DM. Prediction of the stability of DNA triplexes. Proc Natl Acad Sci U S A. 1996;93:4320–5. doi: 10.1073/pnas.93.9.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal PA, Dervan PB. The influence of single base triplet changes on the stability of a pur.pur.pyr triple helix determined by affinity cleaving. Nucleic Acids Res. 1992;20:2773–6. doi: 10.1093/nar/20.11.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardenbol P, Van Dyke MW. Sequence specificity of triplex DNA formation: Analysis by a combinatorial approach, restriction endonuclease protection selection and amplification. Proc Natl Acad Sci U S A. 1996;93:2811–6. doi: 10.1073/pnas.93.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ghosh SS, Millar DP. Direct measurement of thermodynamic and kinetic parameters of DNA triple helix formation by fluorescence spectroscopy. Biochemistry. 1994;33:15329–37. doi: 10.1021/bi00255a014. [DOI] [PubMed] [Google Scholar]
- Wu P, Brand L. Resonance energy transfer: methods and applications. Anal Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- Cheng AJ, Van Dyke MW. Monovalent cation effects on intermolecular purine-purine-pyrimidine triple-helix formation. Nucleic Acids Res. 1993;21:5630–5. doi: 10.1093/nar/21.24.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathias VM, Bolton PH. Determinants of DNA quadruplex structural type: sequence and potassium binding. Biochemistry. 1999;38:4355–64. doi: 10.1021/bi982604+. [DOI] [PubMed] [Google Scholar]
- Poon K, Macgregor RB., Jr Formation and structural determinants of multi-stranded guanine-rich DNA complexes. Biophys Chem. 2000;84:205–16. doi: 10.1016/S0301-4622(00)00106-X. [DOI] [PubMed] [Google Scholar]
- Hardin CC, Perry AG, White K. Thermodynamic and kinetic characterization of the dissociation and assembly of quadruplex nucleic acids. Biopolymers. 2000;56:147–94. doi: 10.1002/1097-0282(2000/2001)56:3<147::AID-BIP10011>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kohwi Y, Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988;85:3781–5. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xodo LE, Pirulli D, Quadrifoglio F. A kinetic study of triple-helix formation at a critical R × Y sequence of the murine c-Ki-ras promoter by (A,G)- and (G,T) oligonucleotides. Eur J Biochem. 1997;248:424–32. doi: 10.1111/j.1432-1033.1997.00424.x. [DOI] [PubMed] [Google Scholar]
- Pingoud A, Urbanke C, Hogget J, Jeltsch A. Biochemical Methods, Wiley-VCH, Weinheim,; 2002. Quantitative Analysis of Biochemical Data. [Google Scholar]