Abstract
Juvenile-onset cataracts are distinguished from congenital cataracts by the initial clarity of the lens at birth and the gradual development of lens opacity in the second and third decades of life. Genomewide linkage analysis in a multigenerational pedigree, segregating for autosomal dominant juvenile-onset cataracts, identified a locus in chromosome region 3q21.2-q22.3. Because of the proximity of the gene coding for lens beaded filament structural protein–2 (BFSP2) to this locus, we screened for mutations in the coding sequence of BFSP2. We observed a unique C→T transition, one that was not observed in 200 normal chromosomes. We predicted that this led to a nonconservative R287W substitution in exon 4 that cosegregated with cataracts. This mutation alters an evolutionarily conserved arginine residue in the central rod domain of the intermediate filament. On consideration of the proposed function of BFSP2 in the lens cytoskeleton, it is likely that this alteration is the cause of cataracts in the members of the family we studied. This is the first example of a mutation in a noncrystallin structural gene that leads to a juvenile-onset, progressive cataract.
Congenital and juvenile-onset cataracts are phenotypically and genetically heterogeneous, with at least 15 distinct loci mapped to 14 unique chromosomal locations. Causative mutations have been identified in nine distinct genes, including α-, β-, and γ-crystallin, connexins 46 and 50, and the homeobox gene PITX (MacDonald et al. 1998; Francis et al. 1999). Cataracts due to mutations in genes coding for lens structural proteins, crystallins, and connexins have a reported congenital onset, with cataracts detected at birth or in infancy. We have identified a single large family (fig. 1) with many individuals who have juvenile-onset cataracts; we located the family through the Family Studies Center for Hereditary Eye Disorders, University of Pittsburgh. The family members we recruited gave written informed consent to participate in a genetic linkage study. Clinical information and blood specimens were obtained from family members. Family members ranged in age at onset from 9 years to late 20s. No individuals were diagnosed with cataracts at birth, nor did any individuals have nystagmus or impaired visual development as a result of the lens opacities. The earliest reported findings were a general haze of the lens with prominent sutures, and all of the individuals with more advanced cases demonstrated lamellar cataracts without discrete subcapsular or nuclear opacities. Two affected individuals (brothers) were reported to have nuclear, embryonic cataracts. Only one of these brothers participated in this study. The ages for cataract surgery were 12–50 years. The clinical data are summarized in table 1, and an example of a typical advanced cataract is shown in figure 2.
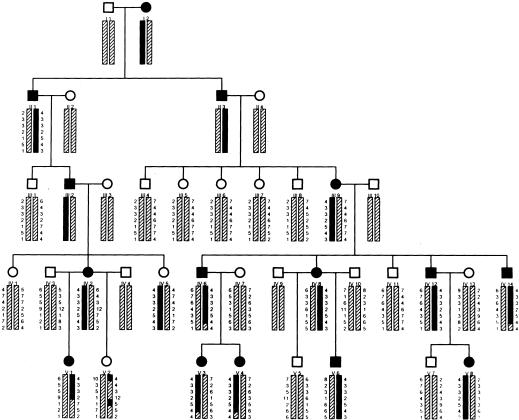
Figure 1.
Pedigree of a family with juvenile-onset, progressive cataract, showing seven locus genotypes and inferred haplotypes. Individuals designated as affected on the basis of family history or clinical records are indicated by blackened symbols. The affected haplotype is indicated by a dark vertical bar. Genotypes are shown in order from top to bottom: D3S1267, D3S1273, D3S1764, D3S3612, D3S3694, D3S1744, and D3S1763.
Table 1.
Characteristics of Affected Individuals in Pedigree 1[Note]
| Individual | Visual Acuities(Just Before Surgery) | Age at Onseta (Age at Surgery)(years) | Type of Cataract | Other Findings |
| II:1 | No records available | |||
| II:2 | No records available | |||
| III:2 | (20/30 OD, 20/40 OS) | (36 OD, 34 OS) | Not specified in records | Cystoid macular edema OS; aphakia OU; retinal detachment OS at age 45 years |
| III:9 | Not available | (50 OU) | Not specified in records | Secondary implants OU; postoperative glaucoma |
| IV:2 | No records available | |||
| IV:6 | 20/40 OU (age 16 years) (20/200 OD, 20/100 OS) | 16 (35 OD, 31 OS) | Nuclear embryonic; central snowflake | Retinal detachment OD at age 45 years |
| IV:9 | (20/70 OU) | 25 (36 OD, 35 OS) | Cortical | |
| IV:12 | (20/30 OD, 20/50 OS) | 12 (35 OD, 33 OS) | Scattered lens opacities | |
| IV:14 | 20/80 OD, 20/40 OS | (29 OD, 28 OS) | Not specified in records | |
| V:1 | 20/40 OU | 13 | Not specified in records | Glare visual acuities 20/200 OU |
| V:3 | 25/50 OU (age 18 years) | 10 | Postsubcapsular OU, cortical OS | Glare visual acuities LP, 20/200 |
| V:4 | (20/200 OD, 20/200 OS) | 10 (12 OU) | Prominent suture lines, slightly nuclear haze at onset, cortical OD (later) | Glare visual acuities 20/200 OU |
| V:6 | 20/30 OU | 16 | Lamellar (anterior) | |
| V:8 | 20/80 OU | 8 | Lamellar | |
| V:9 | 20/200 OD, 20/50 OS | 17 | Lamellar | Optic nerve deformity with amblyopia OD |
Note.— OD = right eye; OS = left eye; OU = both eyes; LP = light perception.
Earliest age of documented cataracts, in years.
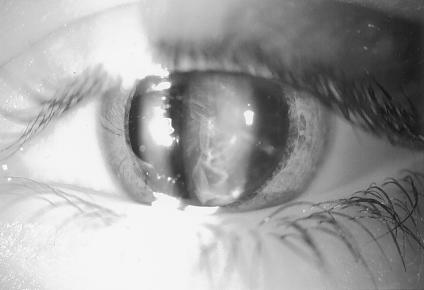
Figure 2.
Slit-lamp photograph of left eye of subject V:8 at age 8 years, with 20/80 visual acuity. The black-and-white image was generated from a color slide by use of the red color channel, to highlight the lens opacities.
Initially, 16 members of this family were genotyped, for 386 microsatellite markers spanning the human genome at an autosomal resolution of 9.5 cM, by the National Heart, Lung, and Blood Institute (NHLBI) Mammalian Genotyping Service. Two-point linkage analysis under the assumption of a fully penetrant autosomal dominant model gave a single LOD score >2.0 for marker D3S1744 (Z=2.77) at recombination fraction (θ) 0, and flanking markers D3S1764 (cen) and D3S1763 (tel) gave LOD scores of 1.06 (θ=0) and 1.34 (θ=0.08), respectively. No other suggestive LOD scores were observed. Thirteen additional family members were recruited, and additional markers flanking D3S1744 were genotyped in the pedigree by use of standard protocols (Ferrell et al. 1998). Final two-point linkage gave LOD scores >3.0 for markers D3S1744 (Z=3.67; θ=0.07), D3S1267 (Z=3.51; θ=0.03), and D3S3612 (Z=3.15; θ=0). Seven-locus inferred haplotypes were constructed by use of SIMWALK2 (Sobel et al. 1995; Weeks et al. 1995; Sobel and Lange 1996), under the assumption of the following order (superscripts refer to genetic distances, in cM): cen139.12–D3S12677.48–D3S12736.02–D3S17641.12–D3S36120–D3S36947.30–D3S174415.5–D3S1763 tel. Figure 1 shows individual genotypes and estimated haplotypes. Multipoint linkage analysis (O'Connell and Weeks 1995) gave a most likely location of the gene within an estimated 14-cM interval flanked by markers D3S1267 and D3S3612.
Searches of gene-expression databases revealed that the gene for the lens-specific beaded filament structural protein–2 (BFSP2, also called phakinin, CP47, CP49, and LIFL-L [MIM 603212]) had been mapped to human chromosome 3q21-q25 by use of human-rodent somatic cell hybrids (Hess et al. 1995). Unique sequence-amplification primers were designed flanking each exon and the intron-exon junctions of BFSP2 (Hess et al. 1996; GenBank accession numbers NM003571 and HSU48224). Direct sequencing of amplified exons revealed a single C→T transition at nucleotide position (np) 859 (NM-003571) of exon 4, which is predicted to lead to an arginine-to-tryptophan amino acid substitution at residue 287 of the mature gene product. No other exonic sequence variants were observed in the four affected and two unaffected family members we sequenced. This nucleotide substitution leads to loss of an MspI restriction enzyme–cleavage site in the genomic DNA. Amplification, with forward primer 3′-CTG GCT AGA ATT CTA TGC CA-5′ and reverse primer 3′-CTG CAC ATT GAC TAG TTT GC-5′ (1.5 mM MgCl2, annealing temperature 52°C), and MspI digestion of exon 4 from family members and from a sample of 96 unrelated individuals from the population showed that this mutation was present only in affected and at-risk family members and was not present in unrelated spouses or in the general population. Comparison of the predicted amino acid sequences of human, chicken, and mouse (GenBank accession numbers GGRNACP49 and 555549) revealed that R287 is conserved in these species.
By use of a combination of linkage analysis and positional candidate-gene analysis, we have identified a mutation in the lens BFSP2 gene, which cosegregates with disease risk in four generations of a large pedigree and was not observed in unrelated spouses or in a sample from the general population. This mutation, a C→T transition at np 859, is predicted to lead to a nonconservative amino acid substitution, R287W, in the central rod domain of the mature intermediate filament. The arginine at residue 287 is conserved in mouse, human, and chicken. The product of the BFSP2 locus encodes a major component of the cytoskeletal structure called the beaded filament, which is abundant in differentiated fiber cells of the human lens (Maisel and Perry 1972). Among intermediate filament (IF) proteins, homologs from different species show a high degree of sequence variation, whereas IF proteins within a species exhibit a much higher degree of sequence variation. Multiple levels of analysis, including primary sequence, secondary structure, gene structure, and patterns of expression, establish that IF proteins can be grouped into several classes or types. Assembly of IF proteins is a function of type: for example, type I and type II form obligatory heterodimers but will not assemble with IF proteins of other types. Phakosin (also known as CP49) has been shown by multiple levels of analysis to be a divergent member of the type I cytokeratins (Hess et al. 1996). We report here a mutation that converts a positively charged residue in phakosin to a bulky, nonpolar one. The Appendix shows a multiple alignment of human phakosin with homologs from cow, mouse, and chicken and with 24 other human IF proteins. This alignment established that this positive charge is stringently conserved among type I and type II IF proteins but is not present among other IF types. Strict type-specific conservation of an amino acid property suggests that this positive charge plays a critical role in the type I–type II recognition process. These observations lead us to conclude that this mutation is the cause of cataracts in this family.
Previously reported cataractogenic mutations in lens structural proteins, with the possible exception of a recently reported mutation in γ-D crystallin (Stephan et al. 1999), lead to a phenotype characterized by cataracts that are detectable at birth or that become apparent in infancy. In this family, cataracts were observed in family members when they were aged 8–25 years, and cataract surgery was performed as early as age 12 years in one individual and as late as age 50 years in another. This leads us to speculate that subtle mutations in BFSP2 might be a predisposing factor in more common, age-related cataracts. Recently, Kramer et al. (2000) have mapped a locus for autosomal dominant congenital cataract to chromosome 3q21-q22 by linkage in a single family. Many of the members of that family underwent bilateral cataract surgery in early childhood (age 3–5 years), with surgery being performed as late as the late teens. This is substantially earlier than the surgical histories of the family members we studied. Like some of the individuals in the family we studied, several of the family members in the Kramer et al. study have not undergone surgery, and, reportedly, there is a 20-year-old individual with sutural opacities and cortical changes compatible with 20/40–20/30 visual acuities. The description of the cataracts in this individual is very similar to those of several of the family members in our study. In the following article (Jakobs et al. 2000), the phenotype in that family is shown to be a 3-bp deletion in exon 3 of BFSP2. A comparison of the clinical features of the two BRSP2 mutation families indicates that allelic heterogeneity at this locus may be manifest as significant phenotypic heterogeneity.
Our initial impression was that the cataract phenotype in this family was fully penetrant; however, neither personal history nor limited examination history of individual IV:5 (age 29 years), despite listing best-corrected visual acuities of 20/40 and 20/25 for the right and left eyes, respectively, have confirmed the presence of cataracts. This individual carries the R287W mutation on the affected haplotype, and we are endeavoring to complete a comprehensive examination of this person. This individual's symptoms suggest that this mutation is not fully penetrant or that the age-at-onset spectrum in this family is quite broad.
Acknowledgments
This work was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York City, New York, the Eye and Ear Foundation of Pittsburgh (M.B.G.), the Pennsylvania Lions Sight Conservation, the Eye Research Foundation (M.B.G.), and NIH grant EY08747 (P.G.F.). The genomewide screening was conducted by the NHLBI Mammalian Genotyping Service at the Marshfield Medical Research Foundation under the direction of Dr. James Weber. We thank the members of the family we studied, whose participation made this study possible, and we thank Dr. Paul Keverline, who initially identified this family and provided invaluable clinical records and photographs for this study.
Appendix
The identified mutation converts the positively charged R residue (underlined in the hum49 sequence) to an uncharged W. Positively charged residues are conserved at this site among all phakosin homologs and all but one of the type I and tII cytokeratins. Conversely, no other IF proteins exhibit a positively charged residue at this site. All sequences that are not phakosin homologs are human: hk1–10, 13–15, and 17–20 are type I and type II cytokeratins of the same number. Type III IF proteins: hvim = vimentin; hdes = desmin; hper = peripherin; hgfap = glial acidic fibrillary protein. Neurofilament proteins: hnfl = neurofilament protein light; hnfm = neurofilament protein medium; hnfh = neurofilament heavy.
Table A1.
Alignment of Human Phakosin Sequence (hum49) with the Bovine (bov49), Mouse (mou49), and Chicken (chi49) Homologs, and 24 Other Intermediate Filament Proteins
| hum49 | LETIRIQWER | DVEKNRVEAG | ALLQAKQQAE | VAHMSQTQEE | KLAAALRVEL |
| bov49 | LETIRIHWER | DVEKNRLQAG | ALLQAKQQAE | LARRAQTQEE | KLAAALRVEL |
| mou49 | LETIRVQWER | DVEKNRAEAG | ALLQAKQQTE | VVHVSQTQEE | KLAAALSVEL |
| chi49 | LEKIRIHWER | DIEKNRAEAG | ALLRTKQQAE | ATAAVRSQEE | ELVEGLRTEF |
| hk1 | IAEVKAQNED | IAQKSKAEAE | SLYQSKY.EE | LQITAGRHGD | SVRNS.KIEI |
| hk2 | IAEVRTQYEE | IAQRSKSEAE | ALYQTKL.GE | LQTTAGRHGD | DLRNT.KSEI |
| hk3 | IAEVGAQYED | IAQRSKAEAE | ALYQTKL.GE | LQTTAGRHGD | DLRNT.KSEI |
| Hk4 | AEVRAQYEE | IAQRSKAEAE | ALYQTKVQQ. | LQISVDQHGD | NLKNT KSEI |
| hk5 | IAEVKAQYEE | ANRSRTEAE | SWYQTKY.EE | LQQTAGRHGD | DLRNT.KHEI |
| hk6 | IAEVKAQYEE | IAQRSRAEAE | SWYQTKY.EE | LQVTAGRHGD | DLRNT.KQEI |
| hk7 | IAEVKAQYEE | MAKCSRAEAE | AWYQTKF.ET | LQAQAGKHGD | DLRNT.RNEI |
| hk8 | IAEVKAQYED | IANRSRAEAE | SMYQIKY.EE | LQSL.GKHGD | DLRRT.KTEI |
| hk9 | LNDMRQEYEQ | LIAKNRKDIE | NQYETQI.TQ | IEHEVSSSGQ | EVQSS.AKEV |
| hk10 | LNNMRSQYEQ | LAEQNRKDAE | AWFNEKS.KE | LTTEIDNNIE | QISSY.KSEI |
| hk13 | LAEMREQYEA | MAERNRRDAE | EWFHAKS.AE | LNKEVSTNTA | MIQTS.KT.I |
| hk14 | LNEMRDQYEK | MAEKNRKDAE | EWFFTKT.EE | LNREVATNSE | LVQ.G.KSEI |
| hk15 | LAEMREQYEA | MAEKNRRDVE | AWFFSKT..E | LNKEVASNTE | MIQTS.KTEI |
| hk17 | LNEMRDQYEK | MAEKNRKDAE | DWFFSKT.EE | LNREVATNSE | LVQSG.KSEI |
| hk18 | MADIRAQYDE | LARKNREELD | KYWSQQI.EE | STTVVTTQSA | EVGAA.ETTL |
| hk19 | LSDMRSQYEV | MAEQNRKDAE | AWFTSRT.EE | LNREVAGHTE | QLQMS.RSEV |
| hk20 | MNEMRQKYEV | MAQKNLQEAK | EQFERQT.AV | LQQQVTVNTE | ELKGT.EVQL |
| hvim | LRDVRQQYES | VAAKNLQEAE | EWYKSKF.AD | LSEAANRNND | ALRQA.KQES |
| hdes | LRDIRAQYET | IAAKNISEAE | EWYKSKV.SD | LTQAANKNND | ALRQA.KQEM |
| hper | LRDIRAQYES | IAAKNLQEAE | EWYKSKY.AD | LSDAANRNHE | ALRQA.KQEM |
| hgfap | LKEIRTQYEA | MASSNMHEAE | EWYRSKF.AD | LTDAAARNAE | LLRQA.KHEA |
| hnfl | LKDIRAQYEK | LAAKNMQNAE | EWFKSRF.TV | LTESAAKNTD | AVRAA.KDEV |
| hnfm | LKEIRSQLES | HSDQNMHQAE | EWFKCRY.AK | LTEAAEQNKE | AIRSA.KEEI |
| hnfh | LREIRAQLEG | HAVQSTLQSE | EWFRVRL.DR | LSEAAKVNTD | AMRSA.QEEI |
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for BFSP2 [MIM 603212])
- GenBank, http://www2.ncbi.nlm.nih.gov/genbank
References
- Ferrell RE, Levinson KL, Esman JH, Kimak MA, Lawrence EC, Barmada MM, Finegold DN (1998) Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum Mol Genet 7:2073–2078 [DOI] [PubMed] [Google Scholar]
- Francis PJ, Berry V, Moore AT, Bhattacharya S (1999) Lens biology: development and human cataractogenesis. Trends Genet 15:191–196 [DOI] [PubMed] [Google Scholar]
- Hess JF, Casselman JT, FitzGerald PG (1995) Chromosomal locations of the genes for the beaded filament proteins CP115 and CP47. Curr Eye Res 14:11–18 [DOI] [PubMed] [Google Scholar]
- Hess JF, Casselman JT, FitzGerald PG (1996) Gene structure and cDNA sequence identify the beaded filament protein CP49 as a highly divergent type I intermediate filament protein. J Biol Chem 271:6729–6735 [DOI] [PubMed] [Google Scholar]
- Jakobs PM, Hess JF, FitzGerald PG, Kramer P, Weleber RG, Litt M (2000) Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am J Hum Genet 66:1432–1436 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PL, LaMorticella D, Schilling K, Billingslea A, Weleber R, Litt M (2000) A new locus for autosomal dominant congenital cataract maps to chromosome 3. Invest Ophthalmol Vis Sci 41:36–39 [PubMed] [Google Scholar]
- MacDonald IM, Haney PM, Musarella MA (1998) Summary of ocular genetic disorders and inherited systemic conditions with eye findings. Ophthalmic Genet 19:1–17 [DOI] [PubMed] [Google Scholar]
- Maisel H, Perry M (1972) Electron microscope observations on some structural proteins of the chick lens. Exp Eye Res 14:7–12 [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE (1995) The VITESSE algorithm for rapid exact linkage analysis via genotype set-recording and fuzzy inheritance. Nat Genet 11:402–408 [DOI] [PubMed] [Google Scholar]
- Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet 58:1323–1327 [PMC free article] [PubMed] [Google Scholar]
- Sobel E, Lange K, O'Connell JR, Weeks DE (1995) Haplotyping algorithms. In: Speed T, Waterman M (eds) IMA volumes in mathematics and its applications. Vol 81: Genetic mapping and DNA sequencing. Springer, New York, pp 89–110 [Google Scholar]
- Stephan DA, Gillanders E, Vanderveen D, Fraes-Lutz D, Wistow G, Baxevanis AD, Robbins CM, et al (1999) Progressive juvenile-onset punctate cataracts caused by mutation of the gamma-D-crystallin gene. Proc Natl Acad Sci USA 96:1008–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks DE, Sobel E, O'Connell JR, Lange K (1995) Computer programs for multilocus haplotyping of general pedigrees. Am J Hum Genet 56:1506–1507 [PMC free article] [PubMed] [Google Scholar]