Abstract
We have identified a four-generation family with 10 affected females manifesting one or more of the following features: osseous dysplasia involving the metacarpals, metatarsals, and phalanges leading to brachydactyly, camptodactyly, and other digital deformities; pigmentary defects on the face and scalp; and multiple frenula. There were no affected males. We performed X-inactivation studies on seven affected females, using a methylation assay at the androgen receptor locus; all seven demonstrated preferential inactivation of their maternal chromosomes carrying the mutation, and two unaffected females showed a random pattern. These findings indicate that this disorder is linked to the X chromosome. To map the gene for this disorder, we analyzed DNA from nine affected females and five unaffected individuals, using 40 polymorphic markers evenly distributed throughout the X chromosome. Two-point and multipoint linkage analyses using informative markers excluded most of the X chromosome and demonstrated linkage to a region on the long arm between DXS548 and Xqter. A maximum LOD score of 3.16 at recombination fraction 0 was obtained for five markers mapping to Xq27.3-Xq28. The mapping data should facilitate the identification of the molecular basis of this disorder.
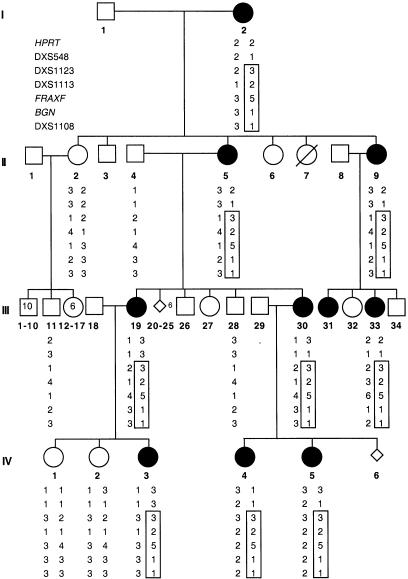
We identified a novel limb-malformation syndrome in a four-generation family (fig. 1). The syndrome is characterized by abnormal and delayed ossification of bones in the hands and feet, leading to brachydactyly, camptodactyly, and clinodactyly of the digits, severe limb deformities, and joint contractures. In addition, the affected individuals have pigmentary skin lesions on the face and scalp, dysmorphic features such as hypertelorism, and multiple frenula. The spectrum of phenotypic manifestations in this pedigree is reminiscent of those described by Horii et al. (1998) and Bloem et al. (1974) in a few sporadic cases.
Figure 1.
Family pedigree with haplotype analysis of seven representative markers in Xq26-Xqter region. The represented markers are shown, left, in generation I. The identical haplotype shared by affected females is boxed.
Because only females in the family are apparently affected and the number of male offspring from affected women is reduced, we hypothesized that this is an X-linked dominant, male-lethal disease. (Unaffected women had a male:female offspring ratio of 1.25:1. The affected women, however, had a ratio of 1:3.4.) We obtained blood samples from 15 family members and isolated genomic DNAs either from lymphoblast cell lines or peripheral blood leukocytes (PBLs), using procedures described elsewhere (Ellison et al. 1992).
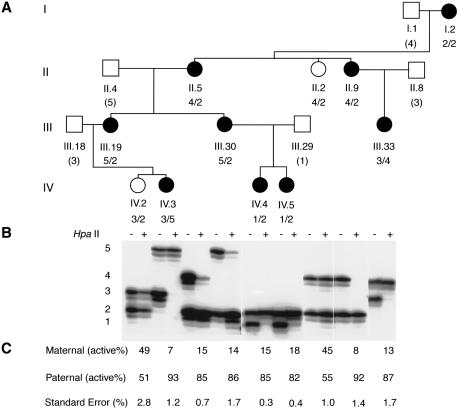
We performed X-inactivation studies with PBL DNA from seven affected and two unaffected females, using the previously described methylation assay at the androgen-receptor (AR) locus (Allen et al. 1992). Results were analyzed as described elsewhere (Pegoraro et al. 1994) and are shown in figure 2.
Figure 2.
Analysis of X-inactivation patterns using the methylation assay at the androgen receptor. A, Partial pedigree of the family, showing the members analyzed by the AR assay. Individuals are numbered according to the full pedigree in figure 1. The genotypes at the AR locus are shown beneath the symbol for each individual, with inferred genotypes in parentheses. B, Autoradiographs of labeled PCR products. PBL DNA from each individual was either used directly as a template (− lanes) or digested with HpaII prior to PCR amplification (+ lanes). For each individual, the two alleles were present in the undigested samples. In the HpaII-digested samples of the affected females, the alleles from the maternal X chromosomes were overrepresented, indicating preferential inactivation of these X chromosomes. C, Percentage of paternal and maternal active alleles. Ratios of active X chromosomes are determined with use of a correction factor to account for preferential amplification of the smaller allele (Pegoraro et al. 1994). The numbers shown here represent the means calculated from at least two separate experiments, with standard errors shown below.
All seven affected females examined had clearly skewed X-inactivation patterns, ranging from 82:18% (IV.5) to 93:7% (IV.3) (fig. 2). Furthermore, all of the affected females studied showed preferential inactivation of their maternal X chromosomes, which carried the mutated gene. These results are consistent with an X-linked dominant, male-lethal inheritance pattern. Two unaffected individuals (II.2 and IV.2) have random X-inactivation, as revealed by approximately equal intensity of the two amplified alleles from HpaII-digested DNA. To map the gene, we genotyped all 15 samples with 40 highly polymorphic markers that cover the entire X chromosome at intervals of 10–20 cM. PCR was performed by use of published primer sequences and conditions (Genome Database). Haplotype analysis demonstrated that all affected females, but no unaffected individuals, shared an identical haplotype on the distal part of Xq demarcated proximally by DXS548 (fig. 1). To confirm the linkage of the disease locus to this region, we performed two-point linkage analysis (Schaffer et al. 1994). A maximum LOD score of 3.16 with recombination fraction (θ) 0 was obtained for each of the five markers that had alleles cosegregating with the disease locus (table 1). The rest of the X chromosome was excluded on the basis of LOD scores <−2. Taken together, these results provide strong evidence for the existence of a gene responsible for terminal osseous dysplasia and pigmentary defects in the region flanked by marker DXS548 and Xqter.
Table 1.
LOD Scores of Two-Point Linkage Analysis for Selected Markers[Note]
|
LOD Score at θ = |
|||||||
| Marker | .00 | .01 | .05 | .10 | .20 | .30 | .40 |
| HPRT | −10.795 | −4.415 | −2.280 | −1.370 | −.539 | −.161 | −.003 |
| DXS548 | −4.135 | .228 | .791 | .915 | .836 | .604 | .308 |
| DXS1123 | 3.164 | 3.113 | 2.906 | 2.635 | 2.051 | 1.410 | .722 |
| DXS1113 | 3.164 | 3.113 | 2.906 | 2.635 | 2.051 | 1.410 | .722 |
| FRAXF | 3.164 | 3.113 | 2.906 | 2.635 | 2.051 | 1.410 | .722 |
| BGN | 3.164 | 3.113 | 2.906 | 2.635 | 2.051 | 1.410 | .722 |
| DXS1108 | 3.164 | 3.113 | 2.905 | 2.633 | 2.047 | 1.406 | .718 |
Note.— Markers are listed in order from Xpter to Xqter. Penetrance of 90% and disease-allele frequency of .0001 were used for calculation.
Although all affected females preferentially inactivated their maternal X chromosomes, there is no direct correlation between the severity of clinical phenotype and the degree of the skewing of X-inactivation. Within the range of skewing (93:7%–82:18%), there is phenotypic variability, most likely because of differing inactivation patterns between different tissues.
The candidate region spans Xq28, one of the most gene-rich regions in the human genome. Approximately 60 cDNAs (National Center for Biotechnology Information Human Gene Map), 37 characterized genes (Integrated X Chromosome Database, version 2.2), and a number of human disorders have been mapped to this 8.7-Mb region. Christian syndrome (CHRS, MIM 309620) causes limb abnormalities, along with mental retardation, short stature, ridging of the metopic suture, and vertebral anomalies (fusions, hemivertebrae, and scoliosis); patients with otopalatodigital syndrome type 1 (OPD1, MIM 311300) are short in stature and mentally retarded, and they have cleft palate and a variety of limb anomalies. Despite the overlap in limb abnormalities with the syndrome in our pedigree, most of the features of CHRS and OPD1 are not present, making it unlikely that these diseases are allelic with the syndrome we describe here.
Some of the clinical features seen in this family are comparable to features seen in tattered (Td), bare patches (Bpa), and striated (Str) mice. Td is an X-linked, semidominant mouse mutation associated with prenatal male lethality. Heterozygous females have patches of scarred skin at age 4–5 d. Because no hairs grow in the scarred areas, adults have tattered coats. The tattered coat may resemble the pigmented lesions and alopecia seen in the family described here, but it seems to be a generalized phenomenon that is not limited to the face and scalp as in our patients. Craniofacial defects and twisted toes have also been observed in some Td mice. Recently, the defect in Td mice has been identified as a mutation in a sterol isomerase encoded by the mouse emopamil-binding protein (Ebp) gene (Derry et al. 1999). Alterations in human EBP have been found in CDPX2 patients, whose phenotype bears similarity to that of the Td mouse (MIM 302960). The phenotypes of Bpa and Str mice are very similar to that of Td mice and are caused by mutations in the NAD(P)H steroid dehydrogenase–like gene (Nsdhl), which also encodes an enzyme involved in sterol biosynthesis (Liu et al. 1999). EBP maps to Xp11.23-p11.22 and NSDHL is located in Xq28. NSDHL is thus a candidate gene, and it will be interesting to examine sterol metabolism in our patients.
Although hundreds of human disorders involving defects in limb development have been described, only ∼25 have been mapped (Bamshad et al. 1999). We report the molecular studies and genetic mapping of a novel X-linked dominant, male-lethal disorder. We demonstrated a skewed pattern of X-inactivation in all affected females examined and mapped the gene responsible for this disorder to an 8.7-Mb region telomeric to DXS548 on the distal part of Xq. This will allow screening of candidate genes in this region for mutations. Identification of additional cases in the extended pedigrees of this and other families may help to further refine the critical interval.
Acknowledgments
We thank the family for their willingness to participate in this study; Jill Dahle for preparing genomic DNAs; V. Brandt for editorial comments; and the cores of the Mental Retardation Research Center at the Baylor College of Medicine for technical assistance. This work was supported by grant K08-HD 01171 from the National Institutes of Health (to I.BV.) and grant FY99-233 from the March of Dimes Birth Defects Foundation (to I.B.V.). H.Y.Z is an Investigator and W.Z. is a Postdoctoral Research Associate with the Howard Hughes Medical Institute.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Genome Database, http://gdbwww.gdb.org/ (for PCR primers and conditions of the markers used)
- Integrated X Chromosome Database, version 2.2, http://ixdb.mpimg-berlin-dahlem.mpg.de/ (for markers and genes from DXS548 to Xqter)
- National Center for Biotechnology Information Human Gene Map, http://www.ncbi.nlm.nih.gov/SCIENCE96/ (for cDNAs in DXS548-Xqter)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for OPD1 [MIM 311300], CHRS [MIM 309620], and CDPX2 [MIM 302960])
References
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW (1992) Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239 [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Watkins WS, Dixon ME, Le T, Roeder AD, Kramer BE, Carey JC, et al (1999) Reconstructing the history of human limb development: lessons from birth defects. Pediatr Res 45:291–299 [DOI] [PubMed] [Google Scholar]
- Bloem JJ, Vuzevski VD, Huffstadt AJ (1974) Recurring digital fibroma of infancy. J Bone Joint Surg [Br] 56-B:746–751 [DOI] [PubMed]
- Derry JM, Gormally E, Means GD, Zhao W, Meindl A, Kelley RI, Boyd Y, et al (1999) Mutations in a Δ8-Δ7 sterol isomerase in the tattered mouse and X-linked dominant chondrodysplasia punctata. Nat Genet 22:286–290 [DOI] [PubMed] [Google Scholar]
- Ellison KA, Fill CP, Terwilliger J, DeGennaro LJ, Martin-Gallardo A, Anvret M, Percy AK, et al (1992) Examination of X chromosome markers in Rett syndrome: exclusion mapping with a novel variation on multilocus linkage analysis. Am J Hum Genet 50:278–287 [PMC free article] [PubMed] [Google Scholar]
- Horii E, Sugiura Y, Nakamura R (1998) A syndrome of digital fibromas, facial pigmentary dysplasia, and metacarpal and metatarsal disorganization. Am J Med Genet 80:1–5 [DOI] [PubMed] [Google Scholar]
- Liu XY, Dangel AW, Kelley RI, Zhao W, Denny P, Botcherby M, Cattanach B, et al (1999) The gene mutated in bare patches and striated mice encodes a novel 3β-hydroxysteroid dehydrogenase. Nat Genet 22:182–187 [DOI] [PubMed] [Google Scholar]
- Pegoraro E, Schimke RN, Arahata K, Hayashi Y, Stern H, Marks H, Glasberg MR, et al (1994) Detection of new paternal dystrophin gene mutations in isolated cases of dystrophinopathy in females. Am J Hum Genet 54:989–1003 [PMC free article] [PubMed] [Google Scholar]
- Schaffer AA, Gupta SK, Shriram K, Cottingham RW Jr (1994) Avoiding recomputation in linkage analysis. Hum Hered 44:225–237 [DOI] [PubMed] [Google Scholar]