Summary
The tumor-suppressor activity of the retinoblastoma protein (RB) is encoded within a protein-binding (“pocket”) domain that is targeted for mutations in all cases of familial retinoblastoma and in many common adult cancers. Although familial retinoblastoma is a paradigm for a highly penetrant, recessive model of tumorigenesis, the molecular basis for the phenotype of incomplete penetrance of familial retinoblastoma is undefined. We studied the RB pocket-binding properties of three independent, mutant RB alleles that are present in the germline of 12 kindreds with the phenotype of incomplete penetrance of familial retinoblastoma. Each arises from alterations of single codons within the RB pocket domain (designated “Δ480,” “661W,” or “712R”). Under the same conditions, we studied the properties of wild-type (WT) RB, an RB point mutant isolated from a lung carcinoma sample (706F) and an adjacent, in vitro–generated point mutant (707W). The Δ480, 661W, and 712R mutants lack pocket protein-binding activity in vitro but retain the WT ability to undergo cyclin-mediated phosphorylation in vivo. Each of the low-penetrant RB mutants exhibits marked enhancement of pocket protein binding when the cells are grown at reduced temperature. In contrast, in this temperature range, no change in binding activity is seen with WT RB, the 706F mutant, or the 707W mutant. We have demonstrated that many families with incomplete penetrance of familial retinoblastoma carry unstable, mutant RB alleles with temperature-sensitive pocket protein-binding activity. The variable frequency for tumor development in these families may result from reversible fluctuations in a threshold level of RB pocket-binding activity.
Introduction
The isolation of the retinoblastoma protein gene (RB) (Friend et al. 1986) validated the two-hit hypothesis for tumorigenesis (Knudson 1971) and, unexpectedly, revealed that inactivation of a complex pathway, referred to as the “RB/cyclin–dependent kinase” pathway, underlies the development of both the rare retinoblastoma cancer syndromes (MIM 180200) and a wide range of common adult malignancies (Harbour et al. 1988; Otterson et al. 1994; Weinberg 1995). In the case of familial retinoblastoma, a defective RB copy is transmitted from one affected parent to half of the offspring. These infants subsequently developed highly penetrant, multifocal (bilateral) tumors consistent with mathematical modeling of mutational rates within susceptible retinal cells, which are required to inactivate the remaining wild-type (WT) allele (Knudson 1971). A striking exception, however, has been the occasional families that present with the clinical phenotype of incomplete penetrance of familial retinoblastoma. This phenotype is characterized by the absence of clinical disease in obligate carriers or the presence of children with unifocal tumors that are more characteristic of sporadic retinoblastoma. In an effort to quantitate these clinical observations, a disease-eye ratio (DER) was proposed that scored for each family the ratio of the sum of the number of eyes with retinal tumors over the number of obligate carriers (Lohmann et al. 1994). Kindreds with classic familial retinoblastoma characteristically show a DER score that approaches 2.0, whereas families with incomplete penetrance had DER scores <1.5.
Although early hypotheses had proposed immune-mediated or epigenetic mechanisms to explain the phenotype of incomplete penetrance (Char et al. 1974; Greger et al. 1989), an important clue was the report of families with incomplete penetrance that carried germline mutations within regulatory sequences of the RB promoter (Sakai et al. 1991; Cowell et al. 1996; Huang et al. 1998). Recent studies have shown that germline mutations from other families with incomplete penetrance can encode altered RB proteins that retain several parameters of WT function (Kratzke et al. 1994; Otterson et al. 1997; Sellers et al. 1998). These data, therefore, demonstrate that the phenotype of incomplete penetrance can arise either from mutations that lead to reduced or deregulated levels of WT protein (class 1 low-penetrant mutants) or from subtle mutations within the RB coding sequence that result in defective proteins, which retain partial WT activity (class 2 mutants) (Otterson et al. 1997). Although these observations suggested that the molecular basis for incomplete penetrance is linked with a unique type of RB mutation, they did not provide a working model to explain the unpredictably reduced risk of developing retinal tumors within these families. We had previously shown that two low-penetrant missense mutations localized to the RB pocket (Δ480 and 661W) encoded a unique type of protein that exhibited defective protein binding but that retained the ability to undergo cyclin-mediated phosphorylation (Otterson et al. 1997). We have now studied the functional properties of a novel RB-pocket missense mutant (712R) linked with the phenotype of low penetrance. We observed that it shares similar biochemical properties with Δ480 and 661W, including the ability to undergo cyclin-mediated phosphorylation in vivo. Furthermore, we have shown that each of these three pocket missense mutants, which have now been identified in the germline of 12 different families with incomplete penetrance of familial retinoblastoma, encode unstable, temperature-sensitive pocket protein-binding activity that can fluctuate from null to an almost WT protein-binding pattern.
Subjects, Material, and Methods
Kindreds
For our analysis, we modified the definition of the DER score (Lohmann et al. 1994) to distinguish between nonproliferating retinal scars (retinomas/retinocytomas) that are detected incidentally in asymptomatic obligate carriers (not scored as retinal tumors) and bona fide retinal tumors that represent a clonal expansion and require clinical intervention.
Δ480
A summary of the incomplete-penetrant pedigree carrying a germline Δ480 RB mutation (in-frame deletion of RB codon 480, resulting from a triplet nucleotide deletion) has been reported elsewhere (Lohmann et al. 1994). The DER score for this family was 1.0, with five obligate carriers: one unaffected, three having unilateral disease, and one having bilateral disease.
661W
Nine families with incomplete penetrance of familial retinoblastoma and carrying a 661W mutation (arginine-to-tryptophan substitution at RB codon 661, resulting from a single nucleotide substitution) have been identified (Onadim et al. 1992; Lohmann et al. 1994; Seminara and Dryja 1994; Cowell and Bia 1998; Huang et al. 1998). Sufficient clinical information was available from seven of these families to calculate the DER scores: 0.6, 1.0, 0.6, 1.0, 0.33, 0.83, and 0.2.
712R
Two separate families with incomplete penetrance and carrying a germline 712R mutation (cysteine-to-arginine substitution at RB codon 712, resulting from a single nucleotide change) have been reported. No clinical information is available for one kindred (Gallie et al. 1995). The DER score for the other family was 0.50, with eight obligate carriers: four unaffected and four having unilateral disease (Cowell and Bia 1998).
Protein Analysis
A mutant cDNA clone representing the 712R was generated by use of a PCR mutagenesis strategy with paired sets of oligonucleotides incorporating the corresponding mutation, to anchor the first of a two-step PCR reaction. The amplification products were then purified and subcloned into the eukaryotic expression plasmid, pCI-neo (Promega), and were confirmed by nucleotide sequencing. The construction of vectors representing WT RB and the mutants Δ480, 661W, 706F, and 707W have been reported elsewhere (Kaye et al. 1990; Kratzke et al. 1992, 1994; Otterson et al. 1997). In vivo cyclin-mediated phosphorylation was assayed by cotransfecting the RB expression plasmids with members of the cyclin D or cyclin E family into an RB (−/−) cell line (H2009 non–small-cell lung cancer cells) (Kratzke et al. 1993). The cells were lysed 72 h after transfection, and extracts were subjected to sequential α-RB immunoprecipitation and α-RB immunoblot analysis, as described elsewhere (Otterson et al. 1997). Pocket protein binding of the mutant and WT RB constructs was assessed by incubating [35S]-methionine-labeled in vitro–translated RB proteins with a recombinant glutathione S-transferase (GST)-E2F1 fusion protein that had been prepared and purified as described elsewhere (Kaelin et al. 1991). Bound proteins were washed, separated by SDS-PAGE, and subjected to autoradiography.
In Vivo Two-Hybrid–Binding Assay
RB cDNAs encoding the pocket domain (RB codons 398–792) were subcloned in-frame into the Gal4 DNA–binding domain plasmid, pGBT9 (Clontech). The yeast strain SFY526 was transformed with the pGBT9-RB plasmids and with a plasmid encoding the simian virus-40 large T antigen fused to the Gal4 activation domain (pTD1; Clontech) and was plated onto selective media. Transformants were then either replica plated onto fresh (−)Leu/Trp plates, for the qualitative assay, or into liquid selective media and were grown at the indicated temperatures overnight. The qualitative β-galactosidase assays were performed as recommended by the manufacturer (Clontech). Two independent transformants for each mutant were assayed simultaneously, and each experiment was repeated three times. O-nitrophenyl β-D-galactopyranoside (ONPG) (Sigma) was used as the substrate for the β-galactosidase quantitative assay. One unit of β-galactosidase activity is defined as the amount that hydrolyzes 1 mmol of ONPG to 0-nitrophenol and D-galactose per minute per cell: β-galactosidase units = 1,000 × OD420/time (min) × volume × concentration factor × OD600. Duplicate measurements of three independent yeast transformants were assayed for each RB mutant.
Results
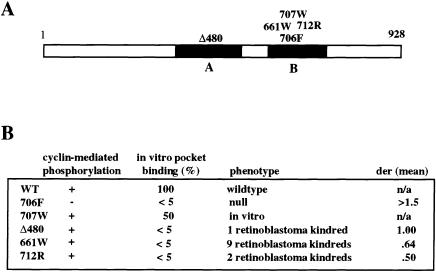
We recently demonstrated that two low-penetrant RB alleles, Δ480 (a deletion of codon 480) and 661W (an arginine-to-tryptophan substitution at codon 661), retained the ability to undergo cyclin-dependent, kinase-mediated phosphorylation but showed defective RB pocket binding in vitro (Otterson et al. 1997). We have now tested a novel RB missense mutation, 712R (cysteine-to-arginine substitution at codon 712), that also localizes to the RB-pocket domain and that was identified within the germline of two independent families with incomplete penetrance of familial retinoblastoma (Gallie et al. 1995; Cowell and Bia 1998). Transient transfection of an RB (−) carcinoma cell line with a mammalian expression plasmid containing the 712R substitution showed evidence for RB phosphorylation when the 712R plasmid was cotransfected with a plasmid encoding either cyclin D2, cyclin D3, or cyclin E, although the degree of hyperphosphorylation was less than that observed for WT RB (fig. 1A). In contrast, a plasmid expressing the 706F substitution (Kaye et al. 1990) was unable to undergo cyclin-mediated phosphorylation. Although the 712R protein retained the ability to undergo cyclin-mediated phosphorylation in vivo, it demonstrated defective RB-pocket binding activity in vitro by means of recombinant E2F1 as a target (fig. 1B). These findings show that three independent RB alleles, Δ480, 661W, and 712R, localized to the pocket domain, share similar biochemical properties that are distinct from WT RB and from classic RB null mutations found in familial retinoblastoma or sporadic adult carcinomas (fig. 2).
Figure 1.
A, In vivo phosphorylation analysis. Plasmids encoding WT RB (lanes 1–4), 706F mutant RB (lanes 5–8), and the incomplete-penetrant mutant 712R (lanes 9–12) were cotransfected with the indicated cyclin plasmids (D2, D3, and E) into the RB (−/−) tumor cell line H2009 and were subjected to α-RB immunoblotting. Phosphorylation is indicated by the slight retardation in migration of the RB protein signal (ppRB). Protein-size marker is shown on the left. B, In vitro E2F1 binding assay. cDNA plasmids encoding WT or the 706F and 712R mutants were in-vitro translated with [35S]-methionine and were subjected to SDS-PAGE analysis before (1 μl of lysate; lanes 1, 4, and 7) or after precipitation with either GST alone (10 μl of lysate; lanes 2, 5, and 8) or GST-E2F1 fusion proteins (10 μl of lysates; lanes 3, 6, and 9).
Figure 2.
A, Schematic representation of the RB protein. The black rectangles represent the A and B domains of the RB pocket, which is required for large T binding and for tumor suppression. The approximate locations of the missense mutations tested in this study are indicated. B, Activities of phenotypes. Null refers to the inactivating RB mutations that characterize familial retinoblastoma and selected common adult cancers. The DER is calculated as the sum of the number of eyes clinically involved with retinoblastoma, divided by the number of obligate carriers for each kindred, as described in the Subjects, Material, and Methods section. Clinical data sufficient to determine the mean DER score shown were available from only seven families (661W) or one family (712R). References for these kindreds are indicated in the Subjects, Material, and Methods section.
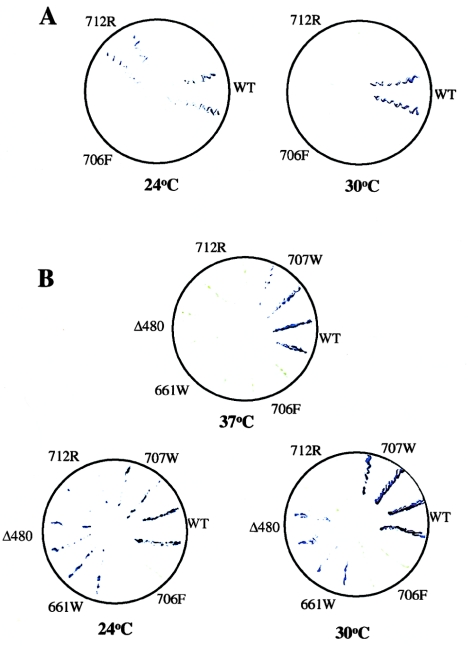
To further study these low-penetrant alleles, we have developed a yeast two-hybrid assay and have shown that the Δ480 and 661W mutants retain in vivo a basal level of binding activity to SV40 T antigen that is significantly reduced but consistently greater than the null binding activity of the 706F RB mutant (Otterson et al. 1997). To examine whether the new low-penetrant mutant, 712R, also exhibited a reduced but detectable pocket-binding activity for large T antigen, we plated independent yeast colonies that coexpressed large T antigen with the 712R mutant fused to a Gal4 DNA-binding domain and performed a qualitative filter assay for pocket binding (fig. 3A). In contrast with our earlier data with Δ480 and 661W (Otterson et al. 1997), both the 712R and the null 706F mutants had undetectable pocket binding at the physiological temperature for yeast growth, 30°C. A duplicate plate, however, where the yeast cells had grown overnight at 24°C showed evidence for an interaction of the 712R mutant with large T antigen, although the null binding of the 706F mutant was unaffected.
Figure 3.
A, β-Galactosidase filter analysis for WT and for the 706F and 712R mutants, assayed after growth of cells at either 24°C or 30°C, as indicated. Two independent transformants are shown for each RB cDNA. B, β-Galactosidase filter analysis of WT or the five different RB point mutants, as indicated. Two independent transformants are shown for each RB cDNA, and assays were performed after growth of cells at either 37°C, 30°C, or 24°C. The yellow-green signal indicates the streaked yeast colonies transferred to the nitrocellulose filter. The purple-blue signal represents β-galactosidase activity as a reporter for a protein-binding interaction between the RB clones and large T antigen.
To extend this observation, we repeated this assay after an incubation, at 24°C, 30°C, and 37°C, of two independent yeast transformants that coexpressed large T antigen with either WT RB, the null 706F mutant, an adjacent in vitro mutant designated “707W” (Kratzke et al. 1992), or each of the three low-penetrant RB mutants, Δ480, 661W, and 712R (fig. 3B). We observed that all three low-penetrant alleles demonstrated a temperature-sensitive pattern of pocket binding to large T antigen, with loss of specific binding at 37°C and a suggestion of enhanced binding, as compared with WT RB at 24°C. In contrast, the binding of the 706F mutant and the 707W in vitro mutant were unaffected by incubation at the different temperatures. The reduced binding of the 712R product is consistent with our observation that this low-penetrant mutant exhibited a reduced level of cyclin-mediated hyperphosphorylation as compared with WT RB and with the other low-penetrant mutants (fig. 1; data not shown).
We confirmed the finding of temperature-sensitive binding by using a quantitative yeast two-hybrid assay with six independent measurements for each RB mutant, to assay β-galactosidase activity per unit time (min) and per yeast cell (table 1). We observed, as reported elsewhere, that the 707W mutant had a moderate reduction in pocket binding (50% of WT binding activity) (Kratzke et al. 1992; Otterson et al. 1997), and the 706F showed undetectable binding at the 30°C incubation, whereas each of the low-penetrant mutants showed a reduced basal-protein binding, with a range of 1.9%–23.6% of WT binding (table 1). Simultaneous measurements after a 24°C incubation showed a marked enhancement of pocket binding for each of the low-penetrant mutants, representing a 2.7-fold increase to 64% of WT binding for the Δ480 mutant, a 6.1-fold increase to 57.4% for the 661W mutant, and a 10.8-fold increase to 20.6% for the 712R mutant. In contrast, no difference was detected for the 707W mutant (53.4%–46.1%) or for the 706F mutant (0.3%–0.4% of WT binding).
Table 1.
Temperature-Sensitive Binding of Mutant RB Alleles
|
Mean ± SD β-Galactosidase Activity ata(% of WT) |
||
| cDNA | 30°C | 24°C |
| WT RB | 100 ± 21.5 | 100 ± 24.8 |
| 706F | .3 ± .1 | .4 ± .4 |
| 707W | 53.4 ± 6.3 | 46.1 ± 4.8 |
| 661W | 9.4 ± 1.6 | 57.4 ± 9.6 |
| Δ480 | 23.6 ± 2.9 | 63.9 ± 4.1 |
| 712R | 1.9 ± .5 | 20.6 ± 5.4 |
One unit β-galactosidase hydrolyzes 1 μmol ONPG to O-nitrophenol and D-galactose/minute/cell.
Discussion
The identification of unique RB mutant alleles that are present in the germline of families with incomplete penetrance of familial retinoblastoma is the first example of a genotype-phenotype relationship within the RB gene that links clinical findings with biochemical analyses. From a clinical perspective, these findings emphasize the importance of cautious genetic counseling of patients who present with sporadic, unilateral retinoblastoma, since they may still harbor a germline RB mutation (Draper et al. 1992; Klutz et al. 1999). The occasional use of the phrase “mild expression” as a synonym for low penetrance of inherited retinoblastoma is premature, since there is no evidence suggesting that clonally expanded tumors arising within these families are biologically less aggressive. The key clinical question, therefore, is, Why do germline carriers of low-penetrant RB mutant alleles exhibit a reduced number of tumor foci that are otherwise indistinguishable from sporadic or inherited retinoblastoma? Inspection of the pedigrees from large families with incomplete penetrance typically shows an unpredictably variable frequency of retinal tumor development, even within carriers of the same mutant allele (Onadim et al. 1992; Lohmann et al. 1994; Seminara and Dryja 1994; Cowell and Bia 1998; Huang et al. 1998). Although this likely represents reduced retinal-cell tumorigenesis in these families, a component of tumor regression or a block-to-tumor progression may also participate in the phenotype of low penetrance. For example, ∼5%–10% of families with a child diagnosed with retinoblastoma will have at least one other member diagnosed with unifocal or multifocal retinomas, detected as a nonproliferating retinal scar (Balmer et al. 1991, 1992; Gunalp et al. 1996). In addition, ∼2%–3% of untreated retinoblastoma tumors are reported to undergo spontaneous regression (Lommatzsch et al. 1993). Although the presence of retinomas and/or regressed retinal tumors has been detected in otherwise unaffected, obligate carriers of low-penetrant mutant RB alleles (Onadim et al. 1992), these lesions have also been observed in classic familial retinoblastoma, and there is no evidence to date that links this interesting phenomenon with the phenotype of incomplete penetrance.
To address the molecular basis for low penetrance, we have studied the biochemical properties of three low-penetrant RB alleles (Δ480, 661W, and 712R) that target single amino acids localized to the RB-pocket domain and that map to the interface between the A and B domains, which is essential for stabilizing the binding-pocket cleft localized within the B domain (Lee et al. 1998). Each of these mutants is distinguished from WT RB by its link to the development of retinoblastoma in 12 families and by evidence of defective RB-pocket binding by means of standard in vitro pocket-binding assays (Kratzke et al. 1994; Otterson et al. 1997; see fig. 1B) and is distinguished from all other null RB mutants by the retention of several parameters of WT function, including the ability to undergo cyclin-dependent, kinase-mediated–phosphorylation in vivo assays (Kratzke et al. 1994; Otterson et al. 1997; see fig. 1A). In addition, the 661W mutant has been recently shown to suppress colony growth of RB (−/−) tumor cells and to induce a pattern of cellular differentiation in SAOS-2 osteosarcoma cells comparable to that in WT RB, while simultaneously behaving as a null mutant in experiments that measure its ability to induce a cell-cycle G1 arrest or to repress the E2F1 transcription-factor activity in vivo (Kratzke et al. 1994; Sellers et al. 1998). These data suggest, but do not prove, that the partial inactivation of low-penetrant mutants, such as the 661W allele, may reflect RB tumor suppression mediated by specific pocket binding to non-E2F1 cellular partners that are linked to a cellular-differentiation pathway. The tumor-suppressor activity of these alleles, however, is intermittently defective, as clinically manifested by the development of unifocal and multifocal retinoblastoma tumors in a subset of germline carriers. The demonstration that the pocket binding activity of each of these mutants can fluctuate 2.7-fold to 10.8-fold with changes in the ambient conditions of the incubated cells, whereas an adjacent point mutant (707W) or a null pocket mutant (706F) showed no fluctuations under the same conditions (table 1), suggests a model where the basal tumor-suppressor pocket activity of these unstable, single-codon RB mutants is near a threshold level of pocket protein binding in vivo, which may be impacted by minor variations in ambient conditions. It is unlikely, however, that thermal energy alone would be responsible for affecting protein stability, since eukaryotic stress responses are coordinated through a complex family of protein chaperones that can function to reversibly stabilize mutant proteins (Chen et al. 1996; Rutherford and Lindquist 1998). In addition, it is also unlikely that a single model will define the mechanism for the phenotype of incomplete penetrance, since there is already heterogeneity in the classification of underlying low-penetrant RB mutations. For example, two additional RB mutations that arise from large deletions outside the pocket domain have been recently identified in single kindreds with low penetrance (Dryja et al. 1993; Bremner et al. 1997). These two mutants, a deletion of exon 4 (Δex4) and a deletion of exons 24/25 (Δex24/25), exhibit a biochemical pattern that is distinct from the Δ480, 661W, and 712R mutants, including, in the case of Δ24/25, a pocket-binding pattern in vitro and with the yeast two-hybrid assay, which was indistinguishable from WT binding under all conditions of testing (data not shown). Although the underlying defect in the nonpocket, low-penetrant alleles (Δex 4 and Δex24/25) is different from that in the family of pocket, temperature-sensitive, low-penetrant mutants, it is possible that the tumor-suppressor activity exhibited by each of these mutant RB alleles will ultimately act through a similar cellular differentiation pathway. The observation that reversible fluctuations in a threshold level of RB-pocket binding activity can be demonstrated in a series of low-penetrant RB alleles suggests that this protein instability may contribute to the variable frequency of tumor development observed in many of these families with the phenotype of incomplete penetrance. The availability of primary retinoblastoma tumor material to examine the mechanism underlying the genetic inactivation of the remaining WT allele in these affected subjects, however, will also be important in the development of a working model to explain the molecular basis for incomplete penetrance. Ultimately, understanding the mechanism of tumor suppression for each of these low-penetrant mutants will help to define more precisely the functional properties of the RB tumor-suppressor gene and may impact the clinical management of these families in the future.
Acknowledgments
We are grateful to Dr. Thaddeus P. Dryja for review of the manuscript and for providing the detailed pedigrees for several families carrying the 661W mutation.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man: http://www.ncbi.nlm.nih.gov/Omim (for retinoblastoma cancer syndromes [MIM 180200])
References
- Balmer A, Munier F, Gailloud C (1991) Retinoma: case studies. Ophthalmic Paediatr Genet 12:131–137 [DOI] [PubMed]
- ——— (1992) [Retinoma and phtisis bulbi: benign expression of retinoblastoma]. Klin Monatsbl Augenheilkd 200:436–439 [DOI] [PubMed]
- Bremner R, Du DC, Connolly-Wilson MJ, Bridge P, Ahmad KF, Mostachfi H, Rushlow D, et al (1997) Deletion of RB exons 24 and 25 causes low-penetrance retinoblastoma. Am J Hum Genet 61:556–570 [DOI] [PMC free article] [PubMed]
- Char DH, Ellsworth R, Rabson AS, Albert DM, Herberman RB (1974) Cell-mediated immunity to a retinoblastoma tissue culture line in patients with retinoblastoma. Am J Ophthalmol 78:5–11 [DOI] [PubMed]
- Chen CF, Chen Y, Dai K, Chen PL, Riley DJ, Lee WH (1996) A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol Cell Biol 16:4691–4699 [DOI] [PMC free article] [PubMed]
- Cowell JK, Bia B (1998) A novel missense mutation in patients from a retinoblastoma pedigree showing only mild expression of the tumor phenotype. Oncogene 16:3211–3213 [DOI] [PubMed]
- Cowell JK, Bia B, Akoulitchev A (1996) A novel mutation in the promotor region in a family with a mild form of retinoblastoma indicates the location of a new regulatory domain for the RB1 gene. Oncogene 12:431–436 [PubMed]
- Draper GJ, Sanders BM, Brownbill PA, Hawkins MM (1992) Patterns of risk of hereditary retinoblastoma and applications to genetic counselling. Br J Cancer 66:211–219 [DOI] [PMC free article] [PubMed]
- Dryja TP, Rapaport J, McGee TL, Nork TM, Schwartz TL (1993) Molecular etiology of low-penetrance retinoblastoma in two pedigrees. Am J Hum Genet 52:1122–1128 [PMC free article] [PubMed]
- Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP (1986) A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323:643–646 [DOI] [PubMed]
- Gallie BL, Hei YJ, Mostachfi H, Dunn JM (1995) Retinoblastoma: for the next generation. In: Cowell JK (ed) Molecular genetics of cancer. Bios Scientific, Oxford, pp 1–30 [Google Scholar]
- Greger V, Passarge E, Hopping W, Messmer E, Horsthemke B (1989) Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet 83:155–158 [DOI] [PubMed]
- Gunalp I, Gunduz K, Arslan Y (1996) Retinoblastoma in Turkey—treatment and prognosis. Jpn J Ophthalmol 40:95–102 [PubMed]
- Harbour JW, Lai SL, Whang-Peng J, Gazdar AF, Minna JD, Kaye FJ (1988) Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science 241:353–357 [DOI] [PMC free article] [PubMed]
- Huang Q, Dryja TP, Yandell DW (1998) Distinct Rb gene point mutations in families showing low penetrance of hereditary retinoblastoma (in Chinese). Chung Hua I Hsueh I Chuan Hsueh Tsa Chih 15:139–142 [PubMed]
- Kaelin W Jr, Pallas DC, DeCaprio JA, Kaye FJ, Livingston DM (1991) Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell 64:521–532 [DOI] [PubMed]
- Kaye FJ, Kratzke RA, Gerster JL, Horowitz JM (1990) A single amino acid substitution results in a retinoblastoma protein defective in phosphorylation and oncoprotein binding. Proc Natl Acad Sci USA 87:6922–6926 [DOI] [PMC free article] [PubMed]
- Klutz M, Horsthemke B, Lohmann DR (1999) RB1 gene mutations in peripheral blood DNA of patients with isolated unilateral retinoblastoma. Am J Hum Genet 64:667–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68:820–823 [DOI] [PMC free article] [PubMed]
- Kratzke RA, Otterson GA, Hogg A, Coxon AB, Geradts J, Cowell JK, Kaye FJ (1994) Partial inactivation of the RB product in a family with incomplete penetrance of familial retinoblastoma and benign retinal tumors. Oncogene 9:1321–1326 [PubMed]
- Kratzke RA, Otterson GA, Lin AY, Shimizu E, Alexandrova N, Zajac-Kaye M, Horowitz JM, et al (1992) Functional analysis at the Cys706 residue of the retinoblastoma protein. J Biol Chem 267:25998–26003 [PubMed]
- Kratzke RA, Shimizu E, Geradts J, Gerster JL, Segal S, Otterson GA, Kaye FJ (1993) RB-mediated tumor suppression of a lung cancer cell line is abrogated by an extract enriched in extracellular matrix. Cell Growth Differ 4:629–635 [PubMed]
- Lee JO, Russo AA, Pavletich NP (1998) Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature 391:859–865 [DOI] [PubMed]
- Lohmann DR, Brandt B, Hopping W, Passarge E, Horsthemke B (1994) Distinct RB1 gene mutations with low penetrance in hereditary retinoblastoma. Hum Genet 94:349–354 [DOI] [PubMed]
- Lommatzsch PK, Zimmermann W, Lommatzsch R (1993) Spontaneous growth inhibition in retinoblastoma (in German). Klin Monatsbl Augenheilkd 202:218–223 [DOI] [PubMed]
- Onadim Z, Hogg A, Baird PN, Cowell JK (1992) Oncogenic point mutations in exon 20 of the RB1 gene in families showing incomplete penetrance and mild expression of the retinoblastoma phenotype. Proc Natl Acad Sci USA 89:6177–6181 [DOI] [PMC free article] [PubMed]
- Otterson GA, Chen W, Coxon AB, Khleif SN, Kaye FJ (1997) Incomplete penetrance of familial retinoblastoma linked to germ-line mutations that result in partial loss of RB function. Proc Natl Acad Sci USA 94:12036–12040 [DOI] [PMC free article] [PubMed]
- Otterson GA, Kratzke RA, Coxon A, Kim YW, Kaye FJ (1994) Absence of p16INK4 protein is restricted to the subset of lung cancer lines that retains wildtype RB. Oncogene 9:3375–3378 [PubMed]
- Rutherford SL, Lindquist S (1998) Hsp90 as a capacitor for morphological evolution. Nature 396:336–342 [DOI] [PubMed]
- Sakai T, Ohtani N, McGee TL, Robbins PD, Dryja TP (1991) Oncogenic germ-line mutations in Sp1 and ATF sites in the human retinoblastoma gene. Nature 353:83–86 [DOI] [PubMed]
- Sellers WR, Novitch BG, Miyake S, Heith A, Otterson GA, Kaye FJ, Lassar AB, et al (1998) Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev 12:95–106 [DOI] [PMC free article] [PubMed]
- Seminara SB, Dryja TP (1994) Unbiased transmission of mutant alleles at the human retinoblastoma locus. Hum Genet 93:629–634 [DOI] [PubMed]
- Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81:323–330 [DOI] [PubMed]