The luteinizing hormone (LH) receptor (LHR) is a heptahelical receptor present primarily in the ovaries of females and the testes of males. This same receptor can bind with high affinity either pituitary LH or the nearly identical placental hormone human chorionic gonadotropin (hCG). In both males and females, the levels of LH remain quite low during childhood years, until puberty, at which time the hypothalamic-pituitary-gonadal axis matures. After puberty, the functions of LH are critical to normal reproductive function. In postpubertal males, LH stimulates testosterone synthesis in the Leydig cells of the testes, which, in turn, is necessary for both formation of male secondary sexual characteristics and spermatogenesis. In nonpregnant postpubertal females, LH plays several roles. During the follicular phase of the ovarian cycle, LH stimulates theca cells to synthesize androgens, which are then aromatized into estradiol in granulosa cells under the influence of follicle-stimulating hormone (FSH). The midcycle surge of LH induces follicular maturation and ovulation. Subsequently, during the luteal phase, LH induces the formation of the corpus luteum and stimulates progesterone synthesis. In the pregnant female, placental hCG binds to the LHR on ovarian luteal cells and causes the corpus luteum, which otherwise undergoes atresia, to be maintained and to continue steroid synthesis, which is necessary for the continuation of pregnancy. During pregnancy, if the fetus is male, placental hCG also stimulates fetal testicular Leydig cells to produce testosterone, which, in turn, mediates the differentiation of the external genitalia and induces the descent of the testes (see Roberts et al. 1999 [in this issue]).
Clearly, the LHR plays a critical role in reproductive physiology in both males and females. The importance of the LHR signal-transduction pathway in normal reproductive functioning has been further underscored by the discovery, in recent years, of naturally occurring mutations of the human LHR gene, hLHR.
Structure and Signaling Properties of the LHR
The hLHR gene is composed of 11 exons, and the gene has been mapped to 2p21 (Rousseau-Merck et al. 1990; Segaloff and Ascoli 1993; Latronico et al. 1997). The final, 11th exon of the gene encodes the entire carboxyl-terminal half of the receptor, including all seven transmembrane helices, the three interconnecting extracellular loops, the three interconnecting intracellular loops, and the cytoplasmic tail. This carboxyl half of the receptor shares homology with other members of the superfamily of rhodopsin-like G protein–coupled receptors. The first 10 exons of the hLHR gene encode a large amino-terminal extracellular domain that contains a number of leucine-rich repeat motifs likely to be involved in protein-protein interactions. It has been shown that the extracellular domain of the LHR, when expressed in isolation in transfected cells, binds hCG with the same high affinity as does the full-length receptor. Therefore, although low-affinity interactions between the carboxyl half of the receptor and the hormone may also occur, clearly the high-affinity binding of the hormone is mediated by the extracellular N-terminal domain of the receptor.
Ultimately, the binding of LH or hCG to the LHR causes it to be stabilized in an active conformation that can interact with and activate the appropriate heterotrimeric G proteins. The LHR has been shown to stimulate phosphatidylinositol phosphate (PIP) production in cultured cells, but the physiological significance of this signaling pathway is debatable, since PIP accumulates only when both the receptor and the hormone are present at extremely high levels. In contrast, there is little or no dispute that the primary pathway activated by hormone occupancy of the LHR is the Gs/adenylyl cyclase/cAMP pathway.
Naturally occurring mutations in G protein–coupled receptor genes can cause human disease by producing either gain- or loss-of-receptor function. The elucidation of the cDNA sequence and genomic organization of the hLHR have made it possible to identify hLHR mutations that can be directly linked to specific reproductive disorders (see table 1).
Table 1.
Clinical Manifestations of Activating and Inactivating Mutations of the hLHR Gene in 46,XY and 46,XX Individuals
| hLHR Mutation | 46,XY Individuals | 46,XX Individuals |
| Activating | Familial or sporadic pseudoprecocious puberty | Asymptomatic |
| Inactivating: | ||
| Severe defect | Leydig-cell hypoplasia with complete female external genitalia | Menstrual disorders, cystic ovaries, and infertility |
| Mild defect | Micropenis and/or hypospadias | Not described |
Activating Mutations of the hLHR: Identification and Clinical Presentation
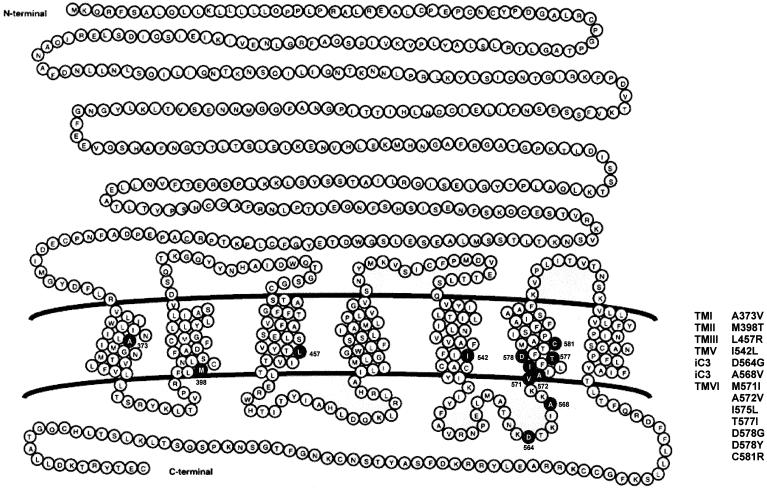
Several dominant gain-of-function mutations in the hLHR gene have been found in males with sporadic or familial male-limited gonadotropin-independent pseudoprecocious puberty, also known as “testotoxicosis” (Kremer et al. 1993; Shenker et al. 1993; Yano et al. 1994, 1996; Kawate et al. 1995; Kosugi et al. 1995; Kraaij et al. 1995; Latronico et al. 1995, 1998; Laue et al. 1995a; Evans et al. 1996; Rosenthal et al. 1996; Gromoll et al. 1998). In the presence of a heterozygous activating mutation, boys with this condition display elevated levels of testosterone, although their GnRH and LH levels remain prepubertal (Shenker et al. 1993), suggesting that the LHR-signaling pathway in their Leydig cells is activated, even in the absence of hormonal stimulation. This disorder, which usually is present by age 1–4 years, is characterized by signs of puberty, rapid virilization, and linear-growth acceleration (Shenker et al. 1993; Kraaij et al. 1995; Latronico et al. 1995, 1998; Laue et al. 1995a; Gromoll et al. 1998). All gain-of-function missense mutations described to date have been found in the carboxyl half of the hLHR, with most of them clustering within the sixth transmembrane helix and third intracellular loop. Although this may indicate a prevalence of gain-of-function mutations in this region of the hLHR gene, it must be cautioned that in many of the earlier studies only a small portion of the 11th exon encompassing the third intracellular loop and sixth transmembrane helix had been examined for potential mutations. Indeed, activating mutations have also been described in helices I–V (fig. 1). Cells transfected with the cDNAs for the mutant hLHRs exhibit markedly increased cAMP production in the absence of agonist, suggesting that autonomous Leydig-cell activity in this form of male precocious puberty results from constitutive activation of the hLHR (Shenker et al. 1993). Interestingly, basal levels of cAMP in cells that express the constitutively active hLHRs are not as great as the levels attained in response to a saturating concentration of hormone. Therefore, in most cases, the constitutively active mutants still respond further to hormonal stimulation. However, of the 13 activating mutations of the hLHR described thus far, 3 (Leu457Arg, Ile542Leu, and Cys581Arg) cause elevated basal cAMP production but prevent the mutant receptors from responding to further stimulation by hCG (Laue et al. 1995a; Latronico et al. 1998).
Figure 1.
Gain-of-function mutations of the hLHR gene. The schematic representation of the hLHR indicates positions of constitutively activating mutations of the hLHR gene that cause sporadic or familial male-limited pseudoprecocious puberty.
46,XX female mothers or sisters of boys with male-limited pseudoprecocious puberty show normal ovarian function, despite carrying constitutively activating mutations of the hLHR gene in heterozygous form. Rosenthal et al. (1996) have evaluated the pituitary-gonadal axis of a mother of two sons who had familial male-limited precocious puberty due to the most common constitutively activating mutation (Asp578Gly) of the hLHR gene in the United States. The mother's dynamics of LH, FSH, and androgen secretion were normal in the basal state and after acute or chronic GnRH agonist or dexamethasone administration. Thus, activating hLHR mutations do not appear to cause functional ovarian hyperandrogenism in mature women. This may be because ovarian theca cells are less efficient than testicular Leydig cells in steroid biosynthesis at the level of 17,20-lyase, which is the rate-limiting step in androgen formation (Ehrman et al. 1995).
Molecular and Cellular Mechanisms Underlying Activating hLHR Mutations
As with other G protein–coupled receptors, activating mutations of the hLHR are thought to stabilize the receptor in an activated state. The current paradigm for G protein–coupled receptor activation is based on the revised ternary complex model (Samama et al. 1993; Bond et al. 1995). In this model, it is presumed that the unoccupied receptor exists in an equilibrium between inactive (R) and active (R*) conformations. The preferential binding of agonist to R* causes G-protein activation by shifting the equilibrium toward the active H-R* state. Mutations that cause ligand-independent activation of a G protein–coupled receptor are thought to do so by also shifting the equilibrium toward the R* state. Whether the R* state of a constitutively active mutant is structurally equivalent to the R* state of the agonist-occupied receptor is a question undergoing active investigation. Some studies have suggested that the R* state of a constitutively active mutant may be intermediate between R and the R* state of the agonist-occupied receptor and that there may be many R* states (Hjorth et al. 1998).
Studies with other G protein–coupled receptors support a general model, reviewed by Gether and Kobilka (1998), according to which receptor activation involves the increased movement of one or more helices, which opens the cytoplasmic cleft to expose specific sites for G-protein interaction and activation. In particular, the movement of helices III and VI relative to each other has been proposed to be the activation switch for G protein–coupled receptors (Baranski et al. 1999; Sheikh et al. 1999). Consistent with this model, studies of some activating mutations of the hLHR gene have suggested that the mutations introduce alterations in interhelical interactions (Kjelsberg et al. 1992; Kosugi et al. 1996, 1997, 1998). Studies of other G protein–coupled receptors suggest that, in the activated state, regions of the cytoplasmic loops in close juxtaposition to the plasma membrane form the G protein–binding pocket. Recent studies suggest that, at least for the hLHR, regions within the transmembrane helices themselves—in particular, helix VI—may also be directly involved in Gs activation (Abell and Segaloff 1997; Abell et al. 1998).
Inactivating Mutations of the hLHR: Identification and Clinical Presentation
Loss-of-function mutations of the hLHR gene result in testicular resistance to LH, leading to Leydig-cell hypoplasia (Kremer et al. 1995; Latronico et al. 1996). During the first trimester of normal male embryogenesis, hCG induces the differentiation of testicular mesenchymal cells into Leydig cells and stimulates androgen production by these cells, which are responsible for virilization of the undifferentiated external genitalia. During the second and third trimesters, LH stimulates the Leydig cells to produce testosterone, which is responsible for penile growth. Leydig-cell hypoplasia is a rare form of autosomal recessive male pseudohermaphroditism, characterized by failure of fetal testicular Leydig-cell differentiation (Bertezene et al. 1976; Schwartz et al. 1981; Saldanha et al. 1987). Affected 46,XY males are impaired in this process and may display female external genitalia or a micropenis, sometimes accompanied by hypospadias (Bertezene et al. 1976; Schwartz et al. 1981; Kremer et al. 1995; Latronico et al. 1996). Affected individuals with complete female external genitalia usually have a female gender and, therefore, seek medical attention only when their breast development and menstrual periods fail to occur at the expected time of puberty. Müllerian derivatives are absent, but an epididymis and vas deferens may be identified histologically. Testes are inguinal or intraabdominal and reach near-normal size after puberty, with relatively preserved seminiferous tubules but no differentiated Leydig cells. Levels of LH are elevated, and levels of testosterone and its precursors are low and fail to increase with hCG administration.
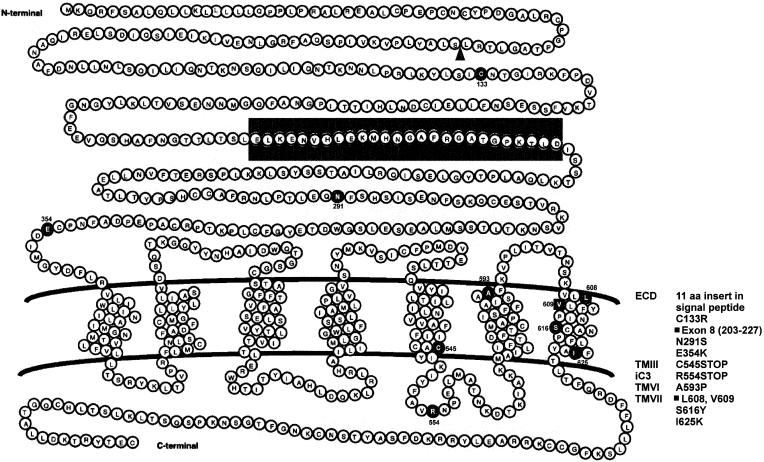
Eleven distinct mutations of the hLHR gene have been described among nine unrelated kindreds with Leydig-cell hypoplasia (see fig. 2) (Kremer et al. 1995; Laue et al. 1995b, 1996; Latronico et al. 1996, 1997; Misrahi et al. 1997; Martens et al. 1998; Stavrou et al. 1998; Wu et al. 1998). Two partial deletions, one insertion, and seven single-base-pair substitutions impair or eliminate hormone-stimulated signal transduction when expressed in cultured cells (Laue et al. 1995b, 1996; Latronico et al. 1997; Misrahi et al. 1997; Martens et al. 1998; Stavrou et al. 1998; Wu et al. 1998). Although Leydig-cell hypoplasia generally results from a homozygous inactivating hLHR gene mutation, it can also result from compound heterozygous loss-of-function mutations (Laue et al. 1996; Wu et al. 1998).
Figure 2.
Loss-of-function mutations of the hLHR gene. The schematic representation of the hLHR indicates positions of hLHR mutations in males and females with LH/hCG resistance. The blackened area indicates the extracellular region that is deleted on deletion of exon 8. The triangle indicates the position of an 11-amino-acid insertion.
The first of the severe hLHR mutations that cause male pseudohermaphroditism associated with Leydig-cell hypoplasia was reported by Kremer et al. (1995), who identified a homozygous Ala593Pro mutation in the sixth transmembrane domain of the hLHR in two 46,XY siblings with female external genitalia. Cells expressing the recombinant form of this hLHR mutant do not induce cAMP production in response to hCG (Kremer et al. 1995). A different homozygous nonsense mutation (Arg554Stop) was subsequently found in the third intracellular loop of the hLHR in three male pseudohermaphrodite siblings with female phenotypes and Leydig-cell hypoplasia and also in a 46,XX sister with secondary amenorrhea (Latronico et al. 1996). Although it is not known whether the mutant mRNA is stably expressed or degraded, if it were expressed, the premature stop codon would eliminate a large portion of the receptor (Latronico et al. 1996). Recently, another severe hLHR inactivating mutation was identified as a homozygous microdeletion of Leu608 and Val609 in the seventh transmembrane region of the hLHR. This mutation was identified in two related patients, a male pseudohermaphrodite with female external genitalia and his sister with oligoamenorrhea and infertility (Latronico et al. 1997). Cells expressing this hLHR mutant are do not respond to hCG with increased cAMP production (Latronico et al. 1997).
Milder inactivating mutations of the hLHR have been described that permit partial LH function in males (Latronico et al. 1996; Laue et al. 1996; Misrahi et al. 1997; Martens et al. 1998). A homozygous substitution, Ser616Tyr, in the seventh transmembrane helix of the hLHR gene was first reported in a boy from Puerto Rico who had micropenis, bilaterally descended testes, and no response to exogenous hCG (Latronico et al. 1996). The same mutation was carried by the asymptomatic parents of the patient, who were heterozygous for this amino acid substitution, suggesting that one defective hLHR allele causes no abnormality in either sex (Latronico et al. 1996). A more recent report described a compound-heterozygous mutation of the hLHR gene in another Puerto Rican boy with Leydig-cell hypoplasia, who had micropenis associated with severe perineoscrotal hypospadias and cryptorchidism (Laue et al. 1996). This individual carries both a deletion of the entire exon 8 in one hLHR allele and two different missense mutations in the other allele. Martens et al. (1998) have reported the homozygous mutation Ile625Lys, located at the border between the seventh transmembrane helix and the cytoplasmic tail of the hLHR, in three other brothers with micropenis. All mutations described above resulted in impaired hCG-stimulated cAMP production in cells expressing the mutant receptors, suggesting a clear correlation between the severity of the clinical phenotype of patients and overall receptor signal capacity, which reflects both cell-surface expression levels and coupling efficiency (Laue et al. 1996; Martens et al. 1998).
In normal women, LH stimulates the theca cells to produce androgen precursors for aromatization to estradiol by granulosa cells during the follicular phase of the menstrual cycle. Subsequently, during its midcycle surge, LH promotes follicular maturation and ovulation, and, during the luteal phase, LH induces the formation of the corpus luteum and stimulates progesterone secretion. Thus, abnormalities in the LH receptor would be expected to result in partial ovarian failure, characterized by defective folliculogenesis, anovulation, and the absence of progesterone secretion during the second phase of the menstrual cycle. Such abnormalities would be predicted to cause delayed or incomplete feminization at puberty, amenorrhea, and infertility.
To date, only four genetic females have been described with hLHR inactivating mutations (Latronico et al. 1996, 1997; Toledo et al. 1996; Stavrou et al. 1998). These women, sisters of 46,XY individuals with Leydig-cell hypoplasia, carry homozygous or compound-heterozygous inactivating mutations of the hLHR gene. Women with ovarian resistance to LH exhibit normal female external genitalia and experience normal breast development and pubic hair growth at puberty but are amenorrheic or have menstrual irregularities and, as expected, are infertile (Toledo et al. 1996; Arnhold et al. 1997). Plasma LH levels in these woman are elevated, with a high LH:FSH ratio, and their estradiol concentrations are within the normal late-follicular-phase range, although their progesterone concentrations do not reach postovulatory levels (Arnhold et al. 1997, 1999). In some cases, the uterus is hypoplastic and the ovaries are enlarged and contain several cysts. Ovarian biopsy reveals antral follicles with proliferative activity of granulosa and theca cells but no corpora lutea or albicans (Arnhold et al. 1997).
The normal pubertal feminization in women with inactivating mutations of the hLHR suggests that LH is not essential for female pubertal development. Instead, LH appears to be essential to stimulate the ovaries to secrete normal preovulatory estrogen levels, to induce ovulation, to cause corpus luteum formation, and to sustain the function of the corpus luteum through the luteal phase of the cycle.
Molecular and Cellular Mechanisms Underlying Inactivating hLHR Mutations
Several distinct alterations in intracellular events may account for the loss of LH/hCG responsiveness in gonadal cells that carry loss-of-function hLHR mutations. In contrast to gain-of-function mutations, which cause an increase in the constitutive basal activity of the hLHR, a loss-of-function mutation may or may not be due to a decrease in the intrinsic signaling properties of the hLHR. Thus, cell-surface hLHRs may be unresponsive to LH/hCG as a result of decreased hormone-binding affinity or impaired ability of the receptor to activate Gs. Furthermore, decreased gonadal-cell responsiveness may be due to a decrease in the number of cell-surface hLHRs expressed. This situation may arise if a mutation alters the total amount of receptor expressed, by decreasing either mRNA or receptor-protein levels. Concomitantly or independently, there may be a reduction in the percentage of receptor that is processed properly and targeted to the plasma membrane. This can occur if a mutation causes the hLHR to be improperly folded and retained in the endoplasmic reticulum (Rozell et al. 1995; Latronico et al. 1997). These events are not mutually exclusive. Thus, one loss-of-function hLHR mutation (ΔL608,V609), appears to reduce target-cell responsiveness, by a combination of mechanisms (Latronico et al. 1997). Cells expressing this mutant receptor exhibit only 10% of the cell-surface receptors as cells expressing the wild-type receptor, both because of a decrease in the total amount of hLHR expressed and because of an increased intracellular retention of the hLHR mutant. Moreover, those receptors that reach the cell surface bind hCG with high affinity but are unable to mediate increased cAMP production.
Because the basal as well as hormone-stimulated levels of cAMP production are dependent on the number of cell-surface receptors, the signaling properties of cells expressing a hLHR mutant can be assessed only in comparison with those of cells that express comparable levels of wild-type hLHR. However, in many reports that identify loss-of-function mutations of the hLHR gene, the expression of the mutant receptor is much lower than that of the wild-type receptor, so it is not valid to conclude that these mutants are impaired in signaling per se. Nonetheless, the decreased levels of cell-surface mutant hLHR could be sufficient to account for the decreased gonadal responsiveness to LH/hCG.
Perspective
The identification and characterization of naturally occurring mutations of the hLHR gene have considerably advanced our understanding of the actions of this receptor in reproductive physiology. Not surprisingly, however, these studies prompt additional questions and the need for further investigation.
One intriguing outcome of these studies is the observation that precocious puberty is observed only in males carrying activating mutations of the hLHR gene and not in females. It has been suggested that females require the activation of both the hLHR and human follitropin receptor (hSHR) or predominantly the hFSHR for the induction of puberty—and that, thus, the constitutive activation solely of the hLHR would be insufficient to induce this process (Kremer et al. 1993; Rosenthal et al. 1996). (Although both hLHR and hFSHR activate Gs, their cellular and temporal expression in the ovary differs, and, thus, activation of one or the other receptor would have different physiological effects.) It is relevant that hCG-secreting tumors promote precocious puberty in boys but not in girls, supporting the idea that ovarian function in humans is dependent on activation of both hLHR and hFSHR. Consistent with this is the observation that both males and females exhibiting McCune-Albright syndrome (MAS) undergo precocious puberty. This syndrome arises from activating mutations of the GNAS1 gene, which encodes the α-subunit of the trimeric Gs protein. Individuals with MAS (who are mosaic for this defect, since these mutations are usually lethal in utero) thus have a postreceptor activation that includes both the hLHR and the hFSHR pathways. Interestingly, there may be a species difference with respect to whether activation of the LHR and/or of the FSHR causes puberty. Thus, transgenic female mice, but not male mice, harboring a transgene that causes overexpression of LH exhibit precocious puberty (Risma et al. 1997). Therefore, caution must be used in extrapolating from one species to another, with regard to the role that the LHR and the FSHR play in the induction of puberty.
Within the superfamily of rhodopsin-like G protein–coupled receptors, the LHR is most closely related to the FSHR and the thyrotropin receptor (TSHR), all of which have large hormone-binding extracellular domains and interact with Gs. As discussed herein, males with gonadotropin-independent precocious puberty have been a valuable resource for the identification of activating mutations of the hLHR gene. Similarly, the screening of individuals with thyroid adenomas has led to the identification of many activating mutations of the human TSHR gene, hTSHR (Epstein 1997). Because, in humans, activation of the hFSHR alone would not be expected to cause precocious puberty in males, individuals with gonadotropin-independent precocious puberty would not be a suitable choice for identification of activating mutations of the hFSHR gene. It had been hypothesized that constitutive activation of the hFSHR gene may underlie some granulosa-cell tumors; however, screening of these thus far has not yielded any activating mutations of the hFSHR gene. Only one putative constitutively activating mutation of the hFSHR gene has been reported (Gromoll et al. 1996). This mutation was originally identified in a hypophysectomized male who exhibited normal testes volume and fertility after testosterone treatment. In the initial report, the observed increase in basal cAMP in cells transfected with hFSHR(D567G) was quite low. A subsequent study by a different group was unable to demonstrate any elevation of basal cAMP in cells transfected with hFSHR(D576G) (Kudo et al. 1996). Therefore, the D576G substitution may represent a nonfunctional polymorphic mutation in the hFSHR—and a different, as yet unidentified, mutation may cause the phenotype of the patient in the original study. Alternatively, the D576G mutation may cause only a minor constitutive activation of the hFSHR, which might make it difficult to observe in a reproducible manner. In either case, the D576G mutation in the hFSHR is in marked contrast to both the comparable D564G mutation in the hLHR and the D633 substitution in the hTSHR, which cause significant constitutive activation of these receptors. Furthermore, two other mutations, one in the third intracellular loop and one in helix VI, which are known to induce constitutive activation of the hLHR, have been shown to have no effect on the hFSHR (Kudo et al. 1996). The hFSHR is not entirely refractory to mutation-induced constitutive activation, because a mutation of a highly conserved leucine in transmembrane helix III causes constitutive activation (Y. X. Tao, X. Liu, K. Nakamura, and D. L. Segaloff, unpublished data). Thus, although the hFSHR can be made constitutively active, it differs significantly from the hLHR, in terms of the role that residues in the sixth transmembrane helix and in the third intracellular loop play in maintenance of the inactive state.
As discussed earlier, the clustering of many of the activating mutations of the hLHR in helix VI may have arisen in part because of the sequencing, in earlier studies, of only that small portion of the gene. Nonetheless, the large preponderance of activating mutations of the hLHR in helix VI is certainly consistent with the proposed role of the movement of helix VI during the activation of other G protein–coupled receptors (Baranski et al. 1999; Sheikh et al. 1999) and with studies suggesting a possible interaction of hLHR helix VI with Gs (Abell and Segaloff 1997; Abell et al. 1998). On the other hand, the ability of mutations in many other hLHR helices to induce constitutive activation also underscores the complexity of the activation of G protein–coupled receptor activation and reflects the key role that changes in interhelical interactions play in this activation process.
The inactivating mutations of the hLHR are even more widespread throughout the gene, affecting regions not only in the carboxyl half of the receptor but also in the extracellular domain. This reflects the observation that most of the inactivating mutations of the hLHR result in decreased target-cell responsiveness, because of decreased expression of the mutants on the cell surface. From these studies, as well as mutagenesis studies on the rat LHR, it is clear that the LHR is very susceptible to mutations that cause misfolding and intracellular retention of the receptor in the endoplasmic reticulum (Rozell et al. 1995). Furthermore, mutations in any portion of the receptor may result in intracellular retention, and no pattern is apparent. These observations underscore the necessity of learning more about the folding and processing of the hLHR, if there ever is to be a strategy for “rescuing” otherwise functional but intracellularly retained mutants. The actual signaling properties of most inactivating hLHR mutants remain uncertain because of their poor expression and will require thorough study to determine which mutants would be functional if they could be induced to the cell surface. Such data would also provide further insight into the mechanism of activation of the hLHR.
Acknowledgments
Work from the authors' laboratories was supported by Fundacao de Amparo a Pesquisa do Estado de São Paulo grants 96/2040-2 and 96/2021-1 to A.C.L., National Institutes of Health (NIH) grant HD22196 to D.L.S., and the Diabetes and Endocrinology Research Center of the University of Iowa (supported by NIH grant DK 25295). Because of space limits, not all of the relevant papers could be cited. Additional papers are noted in the Supplemental Reading List, which is available through the electronic version of this article.
Footnotes
*This article represents the opinion of the authors and has not been peer reviewed.
References
- Abell AN, McCormick D, Segaloff DL (1998) Certain activating mutations within helix 6 of the human luteinizing hormone receptor may be explained by alterations that allow transmembrane regions to activate Gs. Mol Endocrinol 12:1857–1869 [DOI] [PubMed]
- Abell AN, Segaloff DL (1997) Evidence for the direct involvement of transmembrane region six of the lutropin/choriogonadotropin receptor in activating Gs. J Biol Chem 272:14586–14591 [DOI] [PubMed]
- Arnhold IJP, Latronico AC, Batista MC, Carvalho FM, Chrousos GP, Mendonca BB (1997) Ovarian resistance to luteinizing hormone: a novel cause of amenorrhea and infertility. Fertil Steril 67:394–397 [DOI] [PubMed]
- Arnhold IJP, Latronico AC, Batista MC, Mendonca BB (1999) Menstrual disorders and infertility caused by inactivating mutations of the luteinizing hormone receptor gene. Fertil Steril 71:1–5 [DOI] [PubMed]
- Baranski TJ, Herzmark P, Lichtarge O, Gerber BO, Trueheart J, Meng EC, Iiri T, et al (1999) C5a receptor activation: genetic identification of critical residues in four transmembrane helices. J Biol Chem 274:15757–15765 [DOI] [PubMed]
- Bertezene F, Forest MG, Grimaud JA, Claustrat B, Mornex R (1976) Leydig cell agenesis: a cause of male pseudohermaphroditism. N Engl J Med 295:969–972 [DOI] [PubMed]
- Bond RA, Leff P, Johnson TD, Milano CA, Rockman HA, McMinn TR, Apparsundaram S, et al (1995) Physiological effects of inverse agonists in transgenic mice with myocardial overexpression of the β2-adrenoreceptor. Nature 374:272–276 [DOI] [PubMed]
- Ehrman DA, Barnes RB, Rosenfield RL (1995) Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev 16:322–353 [DOI] [PubMed]
- Epstein FH (1997) The thyrotropin receptor in thyroid diseases. N Engl J Med 337:1675–1681 [DOI] [PubMed]
- Evans BAJ, Bowen DJ, Smith PJ, Clayton PE, Gregory JW (1996) A new point mutation in the luteinising hormone receptor gene in familial and sporadic male limited precocious puberty: genotype does not always correlate with phenotype. J Med Genet 33:143–147 [DOI] [PMC free article] [PubMed]
- Gether U, Kobilka BK (1998) G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem 273:17979–17982 [DOI] [PubMed]
- Gromoll J, Partsch C-J, Simoni M, Nordhoff V, Sippell WG, Nieschlag E, Saxena BB (1998) A mutation in the first transmembrane domain of the lutropin receptor causes male precocious puberty. J Clin Endocrinol Metab 83:476–480 [DOI] [PubMed]
- Gromoll J, Simoni M, Nieschlag E (1996) An activating mutation of the follicle-stimulating hormone receptor autonomously sustains spermatogenesis in a hyophysectomized man. J Clin Endocrinol Metab 81:1367–1370 [DOI] [PubMed]
- Hjorth SA, Orskov C, Schwartz TW (1998) Constitutive activity of glucagon receptor mutants. Mol Endocrinol 12:78–86 [DOI] [PubMed]
- Kawate N, Kletter GB, Wilson BE, Netzloff ML, Menon KMJ (1995) Identification of constitutively activating mutation of the luteinising hormone receptor in a family with male limited gonadotrophin independent precocious puberty (testotoxicosis). J Med Genet 32:553–554 [DOI] [PMC free article] [PubMed]
- Kjelsberg MA, Cotecchia S, Ostrowski J, Caron MC, Lefkowitz RJ (1992) Constitutive activation of the a1B-adrenergic receptor by all amino acid substitutions at a single site. J Biol Chem 267:1430–1433 [PubMed]
- Kosugi S, Lin Z, Pearlstein R, Mori T, Shenker A (1997) Evidence that interhelical hydrogen bonding between Asp 578 and Asn 619 helps maintain the inactive conformation of the human lutropin receptor (LHR). Endocrine Society, Minneapolis [Google Scholar]
- Kosugi S, Mori T, Shenker A (1996) The role of Asp578 in maintaining the inactive conformation of the human lutropin/choriogonadotropin receptor. J Biol Chem 271:31813–31817 [DOI] [PubMed]
- ——— (1998) An anionic residue at position 564 is important for maintaining the inactive conformation of the human lutropin/choriogonadotropin receptor. Mol Pharmacol 53:895–901 [PubMed]
- Kosugi S, Van Dop C, Geffner ME, Rabl W, Carel JC, Chaussain JL, Mori T, et al (1995) Characterization of heterogeneous mutations causing constitutive activation of the luteinizing hormone receptor in familial male precocious puberty. Hum Mol Genet 4:183–188 [DOI] [PubMed]
- Kraaij R, Post M, Kremer H, Milgrom E, Epping W, Brunner HG, Grootegoed AJ, et al (1995) A missense mutation in the second transmembrane segment of the luteinizing hormone receptor causes familiar male precocious puberty. J Clin Endocrinol Metab 80:3168–3172 [DOI] [PubMed]
- Kremer H, Kraaij R, Toledo SPA, Post M, Fridman JB, Hayashida CY, van Reen M, et al (1995) Male pseudohermaphroditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet 9:160–164 [DOI] [PubMed]
- Kremer H, Mariman E, Otten BJ, Moll GW Jr, Stoelinja GB, Wit JM, Jansen M, et al (1993) Cosegregation of missense mutations of the luteinizing hormone receptor gene with familial male-limited precocious puberty. Hum Mol Genet 2:1779–1783 [DOI] [PubMed]
- Kudo M, Osuga Y, Kobilka BK, Hsueh AJW (1996) Transmembrane regions V and VI of the human luteinizing hormone receptor are required for constitutive activation by a mutation in the third intracellular loop. J Biol Chem 271:22470–22478 [DOI] [PubMed]
- Latronico AC, Abell AN, Arnhold IJP, Liu X, Lins TSS, Brito VN, Billerbeck AE, et al (1998) A unique constitutively activating mutation in the third transmembrane helix of the luteinizing hormone receptor causes sporadic male gonadotropin independent precocious puberty. J Clin Endocrinol Metab 83:2435–2440 [DOI] [PubMed]
- Latronico AC, Anasti J, Arnhold IJ, Mendonca BB, Domenice S, Albano MC, Zachman K, et al (1995) A novel mutation of the luteinizing hormone receptor gene causing male gonadotropin-independent precocious puberty. J Clin Endocrinol Metab 80:2490–2494 [DOI] [PubMed]
- Latronico AC, Anasti J, Arnhold IJP, Rapaport R, Mendonca BB, Bloise W, Castro M, et al (1996) Testicular and ovarian resistance to luteinizing hormone caused by homozygous inactivating mutations of the luteinizing hormone receptor gene. N Engl J Med 334:507–512 [DOI] [PubMed]
- Latronico AC, Chai Y, Arnhold IJ, Liu X, Mendonca BB, Segaloff DL (1998) A homozygous microdeletion in helix 7 of the luteinizing hormone receptor associated with familial testicular and ovarian resistance is due to both decreased cell surface expression and impaired effector activation by the cell surface receptor. Mol Endocrinol 12:442–450 [DOI] [PubMed]
- Laue L, Chan W-C, Hsueh A, Kudo M, Hsu SY, Wu S, Blomberg L, et al (1995a) Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc Natl Acad Sci USA 92:1906–1910 [DOI] [PMC free article] [PubMed]
- Laue LL, Wu SM, Kudo M, Bourony CJ, Cutler GB, Hsueh AJW, Chan WY (1996) Compound heterozygous mutations of the luteinizing hormone receptor gene in Leydig cell hypoplasia. Mol Endocrinol 10:987–997 [DOI] [PubMed]
- Laue L, Wu SM, Kudo M, Hsueh AJW, Cutler GB Jr, Griffin JE, Wilson JD, et al (1995b). A nonsense mutation of the human luteinizing hormone receptor gene in Leydig cell hypoplasia. Hum Mol Genet 4:1429–1433 [DOI] [PubMed]
- Martens JWM, Verhoef-Post M, Abelin N, Ezabella M, Toledo SPA, Brunner HG, Themmen APN (1998) A homozygous mutation of the luteinizing hormone receptor causes partial Leydig cell hypoplasia: correlation between receptor activity and phenotype. Mol Endocrinol 12:775–784 [DOI] [PubMed]
- Misrahi M, Meduri G, Pissard S, Bouvattier C, Beau I, Loosfelt H, Jolivet A, et al (1997) Comparison of immunocytochemical and molecular features with the phenotype in a case of incomplete male pseudohermaphroditism associated with a mutation of the luteinizing hormone receptor. J Clin Endocrinol Metab 82:2159–2165 [DOI] [PubMed]
- Risma KA, Hirshfield AN, Nilson JH (1997) Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology 138:3540–3547 [DOI] [PubMed]
- Roberts LM, Shen J, Ingraham HA (1999) New solutions to an ancient riddle: defining the differences between Adam and Eve. Am J Hum Genet 65:933–942 (in this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal IM, Refetoff S, Rich B, Barnes RB, Sunthornthepvarakul T, Parma J, Rosenfield RL (1996) Response to challenge with gonadotropin-releasing hormone agonist in a mother and her two sons with a constitutively activating mutation of the luteinizing hormone receptor—a clinical research center study. J Clin Endocrinol Metab 81:3802–3806 [DOI] [PubMed]
- Rousseau-Merck MF, Misrahi M, Atger M, Loosfelt H, Milgrom E, Berger R (1990) Localization of the human luteinizing hormone/choriogonadotropin receptor gene (LHCGR) to chromosome 2p21. Cytogenet Cell Genet 54:77–79 [DOI] [PubMed]
- Rozell TG, Wang H, Liu X, Segaloff DL (1995) Intracellular retention of mutant gonadotropin receptors results in loss of hormone binding activity of the follitropin receptor but not the lutropin/choriogonadotropin receptor. Mol Endocrinol 9:1727–1736 [DOI] [PubMed]
- Saldanha PH, Arnhold IJP, Mendonca BB, Bloise W, Toledo SPA (1987) A clinic-genetic investigation of Leydig cell hypoplasia. Am J Med Genet 26:337–344 [DOI] [PubMed]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ (1993) A mutation-induced activated state of the β2-adrenergic receptor: extending the ternary complex model. J Biol Chem 268:4625–4636 [PubMed]
- Schwartz M, Imperato-McGinley A, Peterson RE, Cooper E, Morris PL, MacGillivrary M, Hensle T (1981) Male pseudohermaphroditism secondary to an abnormality in Leydig cell differentiation. J Clin Endocrinol Metab 53:123–127 [DOI] [PubMed]
- Segaloff DL, Ascoli M (1993) The lutropin/choriogonadotropin (LH/CG) receptor-4 years later. Endocr Rev 14:324–347 [DOI] [PubMed]
- Sheikh SP, Vilardarga J-P, Baranski TJ, Lichtarge O, Iiri T, Meng EC, Nissenson RA, et al (1999) Similar structures and shared switch mechanisms of the β2-adrenoceptor and the parathyroid hormone receptor: Zn(II) bridges between helices III and VI block activation. J Biol Chem 274:17033–17041 [DOI] [PubMed]
- Shenker A, Laue L, Kosugi S, Merendino JJ Jr, Minegishi T, Cutler GB Jr (1993) A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature 365:652–654 [DOI] [PubMed]
- Stavrou SS, Zhu Y-S, Cai LQ, Katz MD, Herrara C, DeFillo-Ricart M, Imperato-McGinley J (1998) A novel mutation of the human luteinizing hormone receptor in 46XY and 46XX sisters. J Clin Endocrinol Metab 83:2091–2098 [DOI] [PubMed]
- Toledo SPA, Brunner HG, Kraaij R, Post M, Dahia PLM, Hayashida CY, Kremer H, et al (1996) An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46,XX female. J Clin Endocrinol Metab 81:3850–3854 [DOI] [PubMed]
- Wu SM, Hallermeier KM, Laue L, Brain C, Berry C, Grant DB, Griffin J, et al (1998) Inactivating of the luteinizing hormone/chorionic gonadotropin receptor by an insertional mutation in Leydig cell hypoplasia. Mol Endocrinol 12:1651–1660 [DOI] [PubMed]
- Yano K, Hidaka A, Saji M, Polymeropoulos MH, Okuno A, Kohn LD, Cutler GB (1994) A sporadic case of male-limited precocious puberty has the same constitutively activating point mutation in luteinizing hormone/choriogonadotropin receptor gene as familial cases. J Clin Endocrinol Metab 79:1818–1823 [DOI] [PubMed]
- Yano K, Kohn LD, Saji M, Kataoka N, Okuno A, Cutler GB Jr (1996) A case of male-limited precocious puberty caused by a point mutation in the second transmembrane domain of the luteinizing hormone choriogonadotropin receptor gene. Biochem Biophys Res Commun 220:1036–1042 [DOI] [PubMed]
Supplemental Reading List
- Baldwin JM, Schertler GF, Unger VM (1997) An alpha-carbon template for the transmembrane helices in the rhodopsin family of G-protein-coupled receptors. J Mol Biol 272:144–164 [DOI] [PubMed]
- Bourne HR (1997) How receptors talk to trimeric G proteins. Curr Opin Cell Biol 9:134–142 [DOI] [PubMed]
- Gudermann T, Birnbaumer M, Birnbaumer L (1992a) Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2+ mobilization: studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem 267:4479–4488 [PubMed]
- Gudermann T, Nichols C, Levy FO, Birnbaumer M, Birnbaumer L (1992b) Ca2+ mobilization by the LH receptor expressed in Xenopus oocytes independent of 3′,5′-cyclic adenosine monophosphate formation: evidence for parallel activation of two signaling pathways. Mol Endocrinol 6:272–278 [DOI] [PubMed]
- Hsu SY, Liang SG, Hsueh AJ (1998) Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol Endocrinol 12:1830–1845 [DOI] [PubMed]
- Jia XC, Oikawa M, Bo M, Tanake T, Ny T, Boime I, Hsueh AJ (1991) Expression of human luteinizing hormone (LH) receptor: interaction with LH and chorionic gonadotropin from human but not equine, rat, and ovine species. Mol Endocrinol 5:759–768 [DOI] [PubMed]
- Lin Z, Shenker A, Pearlstein R (1997) A model of the lutropin/choriogonadotropin receptor: insights into the structural and functional effects of constitutively activating mutations. Protein Eng 10:501–510 [DOI] [PubMed]
- McFarland KC, Sprengel R, Phillips HS, Kohler M, Rosemblit N, Nikolics K, Segaloff DL, et al (1989) Lutropin-choriogonadotropin receptor: an unusual member of the G protein-coupled receptor family. Science 245:494–499 [DOI] [PubMed]
- Minegishi T, Nakamura K, Takakura Y, Miyamoto K, Hasegawa Y, Ibuki Y, Igarashi M (1990) Cloning and sequencing of human LH/hCG receptor cDNA. Biochem Biophys Res Commun 172:1049–1054 [DOI] [PubMed]
- Zhu X, Gilbert S, Birnbaumer M, Birnbaumer L (1994) Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol 46:460–469 [PubMed]