Summary
The factors influencing the tissue-specific pattern of somatic mosaicism in CAG-repeat diseases have not yet been fully resolved. We performed a detailed analysis of the degree of somatic mosaicism in various tissues from 20 patients with spinal and bulbar muscular atrophy (SBMA), including 4 who were deceased. The most outstanding feature was the prominent somatic mosaicism observed in the cardiac and skeletal muscles, composed predominantly of postmitotic cells, and in the skin, prostate, and testis. The CNS tissues, liver, and spleen showed the least mosaicism. The tissue distribution of somatic mosaicism in patients with SBMA was markedly different from that in patients with Huntington disease (HD) and from that in patients with dentatorubral-pallidoluysian atrophy (DRPLA). The degree of somatic mosaicism correlated with the CAG-repeat number but not with age at examination. Furthermore, tissues with a higher mosaicism level corresponded well to those with a higher expression level of androgen receptor protein. The tissue-specific pattern of somatic mosaicism related not only to cell composition with different cell turnover rates but to repeat size and gene expression levels, and postnatal cell division is unlikely to be a major cause of somatic mosaicism probably because of the relative stability of CAG repeat in SBMA.
Introduction
Spinal and bulbar muscular atrophy (SBMA [MIM 313200]) is an X-linked, recessive, inherited neurodegenerative disorder characterized by motor-neuron loss in the spinal cord and brain stem and is associated with a less extensive loss of sensory neurons and chronic denervation atrophy in the skeletal muscles (Kennedy et al. 1968; Sobue et al. 1989).
SBMA was first proved to be caused by unstable CAG-repeat expansions of the androgen receptor (AR) gene (La Spada et al. 1991). The number of CAG-repeat units of normal alleles in the AR gene is 14–32, whereas that of expanded alleles is 40–55 (Tanaka et al. 1996a). Recently, affected tissue-specific intranuclear inclusions of the mutant protein have been detected in CAG-repeat diseases, including SBMA (Li et al. 1998a). However, the latest reports indicate that nuclear aggregation of ataxin-1 and huntingtin are not required for initiation of pathogenesis of spinocerebellar ataxia type 1 (SCA1) in transgenic mice (Klement et al. 1998) or cultured neurons of Huntington disease (HD) (Saudou et al. 1998). Therefore, at present, the pathogenic role of intranuclear inclusions of mutant protein mediated by polyglutamine-directed aggregation remains unknown.
The triplet-repeat instability in somatic cells within an individual is known as “somatic mosaicism.” The first evidence of heterogenic somatic mosaicism of the CAG repeat in various tissues was provided by Telenius et al. (1994). In subsequent reports on SCA1 (Chong et al. 1995; Lopes-Cendes et al. 1996; Hashida et al. 1997), dentatorubral-pallidoluysian atrophy (DRPLA) (Ueno et al. 1995; Takano et al. 1996; Tanaka et al. 1996b; Hashida et al. 1997), and Machado-Joseph disease (MJD) (Lopes-Cendes et al. 1996; Tanaka et al. 1996b; Hashida et al. 1997), several features of somatic mosaicism were described despite their distinct neuropathology. Unlike the mutant allele in other regions, a shortening, by several repeat units, of the mutant allele in the cerebellar cortex is common to these CAG-repeat diseases (Telenius et al. 1994; Chong et al. 1995; Ueno et al. 1995; Lopes-Cendes et al. 1996; Takano et al. 1996; Tanaka et al. 1996b; Hashida et al. 1997). Such a common pattern of somatic mosaicism, irrespective of different CAG-repeat diseases, has drawn attention to the cellular mitotic potential as one possible factor influencing the degree of somatic mosaicism. Tissues that constantly undergo cell division would display a greater heterogeneity and larger expansion, whereas mitotically quiescent tissues, such as the cerebellar cortex, skeletal, and cardiac muscle, would show less heterogeneity and smaller expansion (Chong et al. 1995; Takano et al. 1996; Hashida et al. 1997).
Several problems involving the somatic mosaicism of CAG repeats, however, have not been resolved. First, why is it that tissues with a higher cell-turnover rate, such as blood, liver, and colon, do not necessarily show a higher degree of mosaicism? Second, if the tissue-specific postnatal cell-turnover rate is the major factor governing tissue-specific mosaicism, why is there no common pattern of somatic mosaicism present among the triplet-repeat diseases? Third, how is the degree of somatic mosaicism related to the degree of CAG-repeat size or expression level of the responsible gene?
In this study, we performed a detailed analysis of the degree of somatic mosaicism in various tissues from 20 patients with SBMA, including 4 who were deceased. The most outstanding finding was that the most prominent somatic mosaicism was observed in the cardiac and skeletal muscles, composed predominantly of postmitotic cells, as well as in the skin, prostate, and testis. These tissues corresponded well to those with a higher expression level of the AR gene, which may provide new insights into the molecular basis of somatic mosaicism of CAG-repeat diseases.
Patients, Material, and Methods
Patients and Samples
Tissue samples from 20 subjects with SBMA, age 42–74 years (mean ± SD = 55.1±8.7), were studied. The four deceased patients had the typical clinical progression of SBMA, with onset of proximal-limb weakness followed by proximal-limb wasting, tongue atrophy, generally depressed tendon reflexes, mild loss of vibration sense, and gynecomastia. These four patients died of respiratory failure at the ages of 64 years (patient 1), 74 years (patient 2), 52 years (patient 3), and 59 years (patient 4).
We investigated the degree of somatic mosaicism in 18 different neural and nonneural tissues of patient 1, 41 of patient 2, 20 of patient 3, 23 of patient 4, and 12 of the control subject. To assess the possible correlation of the somatic mosaicism level with age and CAG-repeat number, we further examined 17 muscle and 10 skin samples obtained at biopsy or autopsy of our 20 patients with SBMA. Those tissue samples were snap-frozen in liquid nitrogen and were stored at −80°C. A part of each tissue sample was fixed in 10% formalin solution and was processed for histopathological assessment, as described elsewhere (Sobue et al. 1989; Li et al. 1995).
For comparison with our findings on patients with SBMA, we analyzed the somatic mosaicism of four patients with HD, one of whom was deceased (patient 1—54 years, CAG 47; patient 2—40 years, CAG 48; patient 3—51 years, CAG 37; and patient 4—60 years, CAG 42), and of one deceased patient who had had DRPLA (45 years, CAG 69). Informed consent was obtained from all living patients for biopsy and DNA analysis, and from the family members of deceased patients and deceased control subject for DNA analysis.
Analysis of CAG Repeat
By means of careful dissection, 50–100 mg of frozen tissue was obtained from each region. The homogenized tissues were incubated with lysis solution containing Proteinase K at a final concentration of 100 μg/ml at 55°C for 16 h, and the DNA was gently extracted three times with phenol/chloroform. A sufficient removal of contaminating proteins was confirmed by an A260/280 ratio of >1.8 of the isolated DNA samples.
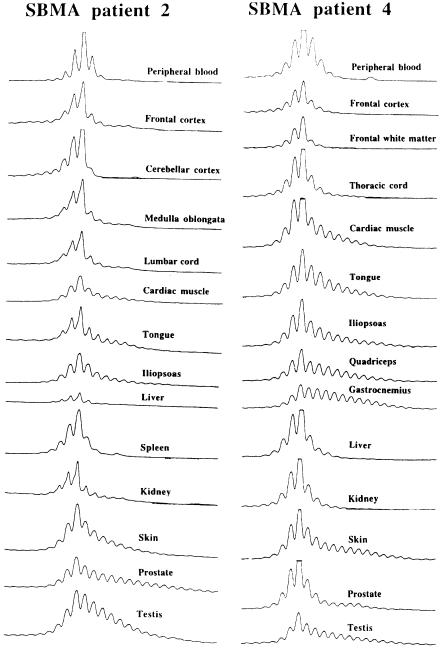
PCR amplification of the CAG repeat in the AR gene was performed with reaction volumes of 10 μl containing 150 ng of purified genomic DNA, 0.4 μM of each fluorescein-labeled primer (5′-TCCAGAATCTGTTCCAGAGCGTGC-3′) and (5′-CCTGTGGGGCCTCTACGATGGGCTTG-3′), 1 × PCR buffer (50 mM KCl, 10 mM Tris-HCl [pH 8.3]), 1.5 mM MgCl2, 200 μM each of dATP, dCTP, dGTP, and dTTP, and 0.1 U of Taq polymerase (Takara). PCR conditions were 35 cycles of denaturation at 95°C for 60 s, annealing at 65°C for 90 s, and elongation at 72°C for 120 s, with a final extension of 7 min. Two microliters of PCR products were combined with 4 μl of 95% formamide loading dye and then were heated to 95°C for 5 min before separation by electrophoresis in 6% HydroLink LongRanger gels (AT Biochem) by an autoread sequencer (ALFred; Pharmacia). Their sizes were determined by comparison with M13 DNA dideoxy sequencing ladders. The range of expanded alleles was defined as the number of peaks giving >5% of the height of the main peak (the highest peak) (fig. 1).
Figure 1.
Somatic mosaicism of the CAG repeats in SBMA tissues (patients 2 and 4). The main peak of the expanded CAG allele showed invariably the same size—46 (patient 2) and 50 (patient 4)—among all examined tissues, including those of the cerebellum. The expanded alleles showed a considerable variation in CAG-repeat ranges of different tissues, indicating the presence of tissue-specific somatic mosaicism. The widest variation in the CAG-repeat range was observed in the cardiac muscle, skeletal muscles, skin, prostate, and testis. Overall, the CNS tissues displayed a narrower range of expanded alleles than did the tissues described above. Of the visceral organs, the liver and the spleen showed the least mosaicism.
The correlation of the somatic mosaicism level with age and CAG-repeat number was statistically analyzed by the Pearson-correlation-coefficient procedure. For this purpose, only the number of peaks with a repeat size larger than that of the main peak was used as the index of the mosaicism level (degree of mosaicism). PCR conditions for the CAG repeats of HD and DRPLA genes are as described elsewhere (The Huntington's Disease Collaborative Research Group 1993; Nagafuchi et al. 1994b), and the methods of electrophoresis and analysis are the same as described above.
Immunohistochemistry of Tissue Samples
Cryostat 8-μm sections were prepared from snap-frozen tissue samples of the deceased patients with SBMA. For tissue and cellular localization analysis of the AR protein, we used PG-21 (rabbit polyclonal antibody [IgG]; Affinity BioReagents) and AR(N-20) (rabbit polyclonal antibody [IgG]; Santa Cruz Biotechnology), which recognize 21- and 20-amino-acid residues of the N-terminus of the AR as the primary antibody. Details of the procedure are as described elsewhere (Li et al. 1998a, 1998b).
Immunoblot Analysis
Two hundred milligrams of each tissue sample (frontal cortex, thoracic cord, liver, spleen, kidney, cardiac muscle, tongue, iliopsoas, prostate, skin, and testis) collected from a deceased patient who had had SBMA (patient 4) was homogenized in 50 mM Tris-HCl (pH 7.4), 1% (vol/vol) Nonidet P-40, 0.1% (vol/vol) sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 1 mM EDTA, and 10 μg aprotinin/ml. Homogenates were spun at 15,000 g, and the supernatants were used for analysis of protein expression. Protein concentration was determined by means of the Bradford test (Bio-Rad Protein Assay; Bio-Rad Laboratories). Fifty micrograms of protein extracts were separated on a 10% SDS-PAGE (Mini-PROTEAN II Ready Gels; Bio-Rad Laboratories), and immunoblots were prepared and probed with PG-21 (1:250 dilution) by means of procedures described elsewhere (Li et al. 1998a). After the detection of immunoreactivity, the signal intensity for each tissue was evaluated by National Institutes of Health (NIH) image version 1.61 software (W. Rasband, NIH).
Results
Tissue-Specific Pattern of Somatic Mosaicism in SBMA
Pathology examination of all four deceased patients showed typical features of SBMA (Kennedy et al. 1968; Sobue et al. 1989; Li et al. 1995), with severe neuronal loss and mild gliosis in the spinal anterior horn and brain-stem motor-neuron nuclei. The dorsal root–ganglion neurons were also mildly affected by the presence of residual nodules. The skeletal muscle showed marked chronic denervation atrophy with extensive fiber-type grouping. The CNS tissues other than lower motor neurons were well preserved. The details of histopathological involvement in each tissue are described in figure 2.
Figure 2.
Range of expanded alleles, histopathological findings, and immunohistochemical reactivity for AR in four deceased patients with SBMA. The range of expanded CAG-repeat sizes in each tissue is indicated where the main peak size is designated as “0.” There is no apparent relationship between the range of expanded alleles and the histopathology, although tissue-specific AR-gene expression level correlated well with tissue-specific pattern of somatic mosaicism of CAG repeats in SBMA. Histopathological findings were graded as follows: − = no pathological changes; + = detectable and mild pathology; ++ = moderate involvement; +++ = severe and advanced pathological changes. The grading system in our previous report (Sobue et al. 1989) serves as a basis for the grading system in this report. Immunohistochemical reactivity for AR was graded as described in the Patients, Material, and Methods section.
The normal CAG allele from a control subject showed a discrete major peak, invariably with the same size of 22 among all tissues examined, without detectable heterogeneity except for 1- or 2-repeat-smaller peaks of PCR artifacts (Litt et al. 1989) during the amplification of microsatellite repeats (data not shown).
The size of the main peak of the expanded alleles in SBMA was identical among all examined tissues including the cerebellum in each patient (patient 1, CAG 45; patient 2, CAG 46 (fig. 1); patient 3, CAG 47; and patient 4, CAG 50 (fig. 1). However, the expanded alleles showed a considerable variation in the CAG-repeat ranges of different tissues. The tissue-specific PCR patterns were highly reproducible within the patient even when different regions of the same tissue were analyzed or the primer set was changed (data not shown). Furthermore, the patterns were also reproducible between examined patients (figs. 1 and 2). Taken together, the repeat changes seen in the various tissues indicate the presence of tissue-specific somatic mosaicism (figs. 1 and 2). The ranges of expanded alleles in all tissues of these four patients with SBMA are schematically illustrated in figure 2.
The widest variation in the CAG-repeat range was observed in the cardiac muscle, skeletal muscles, skin, prostate, and testis (figs. 1 and 2). Although we examined a number of the skeletal muscles in the proximal and distal extremities as well as in the tongue and diaphragm, the expanded alleles ranged to a similar extent in all muscles independently of the pathological severity in one individual (figs. 1 and 2). Overall, CNS tissues displayed a narrower range of expanded alleles than the aforementioned visceral tissues with a wider range of CAG repeats (figs. 1 and 2). Among visceral organs, the adrenal, liver, and spleen showed the least mosaicism (figs. 1 and 2).
Comparison of Mosaicism Pattern between Patients with SBMA and Patients with either HD or DRPLA
To confirm whether such a tissue-specific pattern of mosaicism is specific to SBMA, we further analyzed tissue samples from one deceased individual who had had HD and from one deceased individual who had had DRPLA, as well as biopsied skin samples from three patients with HD, since the somatic mosaicism of nonneural tissues, such as cardiac muscle, skeletal muscles, skin, prostate, and testis, has not been well investigated in individuals with either HD or DRPLA. The expanded alleles differed extensively among tissues, and tissue-specific somatic mosaicism was more pronounced in the CNS tissue, except for the cerebellar cortex, than in peripheral blood, cardiac muscle, and skeletal muscles (fig. 3). In patients with HD, the pronounced CAG-repeat mosaicism was observed in the CNS—rather than in peripheral blood and cardiac muscle, as it is in patients with DRPLA. The liver showed a somewhat higher degree of heterogeneity than did the blood (fig. 3). As shown in figure 3, in patients with HD the degree of somatic mosaicism in skin did not differ from that in blood. Our observations of patients with either HD or DRPLA were markedly different from our observations of patients with SBMA, who had a higher degree of mosaicism in cardiac muscle, skeletal muscles, skin, prostate, and testis and a lower degree of mosaicism in CNS, liver, spleen, and peripheral blood.
Figure 3.
Somatic mosaicism of the CAG repeat in tissue samples from patients with either DRPLA or HD. Tissue-specific somatic mosaicism was more pronounced in the CNS tissues, except for the cerebellar cortex, than in the peripheral blood, cardiac muscle, and skeletal muscles and was markedly different from that of patients with SBMA.
Correlation of Somatic Mosaicism Level with Age and CAG-Repeat Number in Patients with SBMA
We further analyzed the correlation of the degree of somatic mosaicism to age and CAG-repeat number, in 17 muscle and 10 skin samples obtained at biopsy or autopsy from the 20 patients with SBMA. SBMA muscle and skin consistently revealed a higher degree of mosaicism than did peripheral blood. The degree of somatic mosaicism in muscles significantly correlated with the CAG-repeat number (n=17, r=.764, P<.001; fig. 4A) but not with age at examination (n=17, r=.344, P=.178; fig. 4B). In the skin, it also correlated with the CAG-repeat number (n=10, r=.715, P<.05; fig. 4C) but not with age at examination (n=10, r=.134, P=.712; fig. 4D).
Figure 4.
Correlation of the degree of somatic mosaicism with age and CAG-repeat number, in muscle and skin samples from patients with SBMA. The degree of somatic mosaicism in muscles correlated significantly with CAG-repeat number (n=17, r=.764, P<.001) (A) but not with age at examination (B). In the skin, it correlated with CAG-repeat number (n=10, r=.715, P<.05) (C) but not with age at examination (D).
Correlation of Somatic Mosaicism Level in SBMA to AR Gene Expression
In immunohistochemistry, the skeletal muscles, prostate, skin (including scrotal skin), and testis showed the strongest immunoreactivity for AR (fig. 2). The cardiac muscle also showed a considerably strong immunoreactivity. In CNS tissues, the immunoreactivity was also observed on neurons, but overall immunoreactive intensity in the tissue was lower than that in the aforementioned tissues (fig. 2). The liver and spleen were not immunoreactive for AR, or they were positive, if at all, at a very low level (fig. 2).
The result of immunoblot analysis, shown in figure 5, was generally compatible with that of immunohistochemistry. Strong signal intensity was observed in the cardiac muscle, tongue, iliopsoas, prostate, skin, and testis, as compared with the CNS, liver, and spleen (fig. 5).
Figure 5.
Western blot analysis of tissues from a patient (patient 4) with SBMA. Each lane contained 50 mg of protein extracts. PG-21 detected a 109-kD AR protein for examined tissues with variable expression levels. Each bar represents the signal intensity of AR immunoreactivity, expressed as a percentage of testis. Strong signal intensity was observed in the cardiac muscle, tongue, iliopsoas, prostate, skin, and testis, as compared with the CNS, liver, and spleen.
Such tissue-specific AR-gene expression levels assessed by immunohistochemistry and immunoblot analysis correlated well with the tissue-specific pattern of somatic mosaicism of CAG repeats in SBMA (fig. 2), whereas the distribution and severity of tissue-specific pathology did not correlate with the level of somatic mosaicism (fig. 2).
Discussion
The observed tissue-specific size heterogeneity in triplet-repeat diseases has so far been explained by the postnatal cell-turnover rates of each tissue (Chong et al. 1995; Ueno et al. 1995; Lopes-Cendes et al. 1996; Takano et al. 1996; Hashida et al. 1997). The importance of postnatal instability is also confirmed by the observation that the degree of size heterogeneity increases with age in human DRPLA tissues (Takano et al. 1996) and in transgenic-mice HD tissues (Mangiarini et al. 1997).
The striking observation in this study was the relatively increased instability observed in cardiac muscle, skeletal muscle, skin, prostate, and testis in patients with SBMA. In other CAG-repeat diseases, such as HD and DRPLA, skeletal muscle and cardiac muscle have been shown to display the least mosaicism (Takano et al. 1996; Tanaka et al. 1996b; Mangiarini et al. 1997), as confirmed by our results (fig. 3). Furthermore, in HD, the prostate reveals the lowest degree of mosaicism, whereas the testis is at an intermediate level, similar to that of the blood (Telenius et al. 1994). As for the skin, our assessment of three patients with HD has shown no significant differences, in the degree of mosaicism, between the skin and the blood, in contrast with patients with SBMA (fig. 3). Taken together, increased instability in the cardiac muscle, skeletal muscle, skin, prostate, and testis in patients with SBMA is a unique feature of tissue-specific somatic mosaicism, as compared with patients with either HD or DRPLA. Furthermore, in contrast with the higher degree of mosaicism reported in CNS tissues in patients with either DRPLA (Takano et al. 1996) or HD (Telenius et al. 1994) (fig. 3), a relatively low degree of mosaicism was observed in CNS tissues in patients with SBMA (figs. 1 and 2). The exaggerated pattern of somatic mosaicism in SBMA muscle and skin as compared with blood was further confirmed by the biopsy specimens (fig. 4).
Although muscle cells are postmitotic, secondary cell division could occur in the proliferation of satellite cells with regeneration (Campion 1984) and could explain the increased instability in SBMA muscle. However, muscle-cell regeneration is rare in SBMA (data not shown), and cannot account for the relatively marked degree of mosaicism in muscle. Furthermore, if secondary pathological cell division has an important role, the degree of somatic mosaicism might differ, depending on the pathological severity, particularly among the proximal-limb muscles (more profoundly affected in patients with SBMA) and the distal-limb muscles (less affected). However, a similar degree of somatic mosaicism was observed in all muscles (figs. 1 and 2). In addition, lack of correlation between age and degree of mosaicism in muscles (fig. 4B) indicates that postnatal cell division is unlikely to be a significant factor in the increased instability in muscle. In patients with SBMA, the cardiac muscle consisting of postmitotic cells showed a higher degree of mosaicism, and the skin, one of the tissues with the highest cell-turnover rate, did not show an increased somatic mosaicism with age (fig. 4D). Thus, postnatal cell division is unlikely to be a major cause of somatic mosaicism, but it is strongly suggested that the degree of somatic mosaicism is mainly determined at the stage of embryogenesis and changes little postnatally in SBMA. This may be explained by the relative stability of the CAG-repeat tract in SBMA, among CAG-repeat diseases (Tanaka et al. 1996a, 1996b).
Another interesting observation on skeletal muscle and skin was that the degree of somatic mosaicism correlated not with age but with CAG-repeat size (fig. 4A and C). The correlations between the extent of repeat-size heterogeneity and age or CAG-repeat number vary among triplet-repeat diseases (Telenius et al. 1994; Takano et al. 1996; Maciel et al. 1997). In DRPLA, the degree of somatic mosaicism in the cerebral cortex, cerebral white matter, and cerebellar white matter correlates with age and not with the CAG-repeat number (Takano et al. 1996), whereas in MJD and SCA1, the degree of mosaicism is not correlated with age or CAG-repeat number (Maciel et al. 1997). In patients with HD, there is a trend toward increasing mosaicism with larger repeat size (Telenius et al. 1994). The most striking observation in this study was the higher degree of somatic mosaicism found in tissues with a higher expression level of the AR gene (figs. 2 and 5). In patients with SBMA, the AR gene is widely expressed throughout neural and nonneural tissues, but its expression levels are very different among different tissues (figs. 2 and 5). The AR protein level assessed by western blots and immunohistochemistry in this study was high in the cardiac muscle, skeletal muscle, skin, prostate, and testis, but low in the liver and spleen (figs. 2 and 5). AR staining of the CNS tissues was present (fig. 2), but the amount of AR immunoreactivity in whole tissues was much lower than that in the prostate, testis, skin, and muscle (fig. 5), and mosaicism levels were lower in CNS (fig. 2).
In patients with either DRPLA or HD, gene products atrophin-1 and huntingtin are widely expressed in various tissues, but the expression level in CNS tissues is significantly higher than that in non-CNS tissues (Strong et al. 1993; Nagafuchi et al. 1994a), and the somatic mosaicism in CNS tissues is more pronounced than that in non-CNS tissues (fig. 3) (Telenius et al. 1994; Takano et al. 1996). In HD- and SBMA-model mice, an absence of somatic instability was noted in transgenic lines that failed to express the mutant transgene (Mangiarini et al. 1997; La Spada et al. 1998), implying that expression of the responsible gene is correlated with somatic instability of the expanded repeats.
These findings in SBMA and other CAG-repeat diseases and transgenic mice raise the possibility that gene expression plays an important role in modulation of the gene-specific and tissue-specific patterns in somatic mosaicism. The underlying molecular mechanism of the correlation between gene expression and somatic instability is unknown. In summary, the tissue-specific pattern of somatic mosaicism is related to repeat size and gene expression level, and postnatal cell division is unlikely to be a major cause of somatic mosaicism in individuals with SBMA.
Acknowledgments
This study was supported by grants from the Ministry of Health and Welfare of Japan and a COE grant from the Ministry of Education, Science and Culture of Japan.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for SBMA [MIM 313200])
References
- Campion DR (1984) The muscle satellite cell: a review. Int Rev Cytol 87:225–251 [DOI] [PubMed]
- Chong SS, McCall AE, Cota J, Subramony SH, Orr HT, Hughes MR, Zoghbi HY (1995) Gametic and somatic tissue-specific heterogeneity of the expanded SCA1 CAG repeat in spinocerebellar ataxia type 1. Nat Genet 10:344–353 [DOI] [PubMed]
- Hashida H, Goto J, Kurisaki H, Mizusawa H, Kanazawa I (1997) Brain regional differences in the expansion of a CAG repeat in the spinocerebellar ataxias: dentatorubral-pallidoluysian atrophy, Machado-Joseph disease, and spinocerebellar ataxia type 1. Ann Neurol 41:505–511 [DOI] [PubMed]
- Huntington's Disease Collaborative Research Group, The (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72:971–983 [DOI] [PubMed]
- Kennedy WR, Alter M, Sung JH (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. Neurology 18:671–680 [DOI] [PubMed]
- Klement IA, Skinner PJ, Kaytor MD, Yi H, Hersch SM, Clark HB, Zoghbi HY, et al (1998) Ataxin-1 nuclear localization and aggregation: role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95:41–53 [DOI] [PubMed]
- La Spada AR, Peterson KR, Meadows SA, McClain ME, Jeng G, Chmelar RS, Haugen HA, et al (1998) Androgen receptor YAC transgenic mice carrying CAG 45 alleles show trinucleotide repeat instability. Hum Mol Genet 7:959–967 [DOI] [PubMed]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352:77–79 [DOI] [PubMed]
- Li M, Miwa S, Kobayashi Y, Merry DE, Yamamoto M, Tanaka F, Doyu M, et al (1998a) Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol 44:249–254 [DOI] [PubMed]
- Li M, Nakagomi Y, Kobayashi Y, Merry DE, Tanaka F, Doyu M, Mitsuma T, et al (1998b) Nonneural nuclear inclusions of androgen receptor protein in spinal and bulbar muscular atrophy. Am J Pathol 153:695–701 [DOI] [PMC free article] [PubMed]
- Li M, Sobue G, Doyu M, Mukai E, Hashizume Y, Mitsuma T (1995) Primary sensory neurons in X-linked recessive bulbospinal neuronopathy: histopathology and androgen receptor gene expression. Muscle Nerve 18:301–308 [DOI] [PubMed]
- Litt M, Luty JA (1989) A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet 44:397–401 [PMC free article] [PubMed]
- Lopes-Cendes I, Maciel P, Kish S, Gaspar C, Robitaille Y, Clark HB, Koeppen AH, et al (1996) Somatic mosaicism in the central nervous system in spinocerebellar ataxia type 1 and Machado-Joseph disease. Ann Neurol 40:199–206 [DOI] [PubMed]
- Maciel P, Lopes-Cendes I, Kish S, Sequeiros J, Rouleau GA (1997) Mosaicism of the CAG repeat in CNS tissue in relation to age at death in spinocerebellar ataxia type 1 and Machado-Joseph disease patients. Am J Hum Genet 60:993–996 [PMC free article] [PubMed]
- Mangiarini L, Sathasivam K, Mahal A, Mott R, Seller M, Bates GP (1997) Instability of highly expanded CAG repeats in mice transgenic for the Huntington's disease mutation. Nat Genet 15:197–200 [DOI] [PubMed]
- Nagafuchi S, Yanagisawa H, Ohsaki E, Shirayama T, Tadokoro K, Inoue T, Yamada M (1994a) Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nat Genet 8:177–182 [DOI] [PubMed]
- Nagafuchi S, Yanagisawa H, Sato K, Shirayama T, Ohsaki E, Bundo M, Takeda T, et al (1994b) Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet 6:14–18 [DOI] [PubMed]
- Saudou F, Finkbeiner S, Devys D, Greenberg ME (1998) Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95:55–66 [DOI] [PubMed]
- Sobue G, Hashizume Y, Mukai E, Hirayama M, Mitsuma T, Takahashi A (1989) X-linked recessive bulbospinal neuropathy: a clinicopathological study. Brain 112:209–232 [DOI] [PubMed]
- Strong TV, Tagle DA, Valdes JM, Elmer LW, Boehm K, Swaroop M, Kaatz KW, et al (1993) Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nat Genet 5:259–265 [DOI] [PubMed]
- Takano H, Onodera O, Takahashi H, Igarashi S, Yamada M, Oyake M, Ikeuchi T, et al (1996) Somatic mosaicism of expanded CAG repeats in brains of patients with dentatorubral-pallidoluysian atrophy: cellular population-dependent dynamics of mitotic instability. Am J Hum Genet 58:1212–1222 [PMC free article] [PubMed]
- Tanaka F, Doyu M, Ito Y, Matsumoto M, Mitsuma T, Abe K, Aoki M, et al (1996a) Founder effect in spinal and bulbar muscular atrophy (SBMA). Hum Mol Genet 5:1253–1257 [DOI] [PubMed]
- Tanaka F, Sobue G, Doyu M, Ito Y, Yamamoto M, Shimada N, Yamamoto K, et al (1996b) Differential pattern in tissue-specific somatic mosaicism of expanded CAG trinucleotide repeat in dentatorubral-pallidoluysian atrophy, Machado-Joseph disease, and X-linked recessive spinal and bulbar muscular atrophy. J Neurol Sci 135:43–50 [DOI] [PubMed]
- Telenius H, Kremer B, Goldberg YP, Theilmann J, Andrew SE, Zeisler J, Adam S, et al (1994) Somatic and gonadal mosaicism of the Huntington's disease gene CAG repeat in brain and sperm. Nat Genet 6:409–414 [DOI] [PubMed]
- Ueno S, Kondoh K, Kotani Y, Komure O, Kuno S, Kawai J, Hazama F, et al (1995) Somatic mosaicism of CAG repeat in dentatorubral-pallidoluysian atrophy (DRPLA). Hum Mol Genet 4:663–666 [DOI] [PubMed]