Summary
The mtDNA variation of 50 Spanish and 4 Cuban families affected by nonsyndromic sensorineural deafness due to the A1555G mutation in the 12S rRNA gene was studied by high-resolution RFLP analysis and sequencing of the control region. Phylogenetic analyses of haplotypes and detailed survey of population controls revealed that the A1555G mutation can be attributed to ⩾30 independent mutational events among the 50 Spanish families and that it occurs on mtDNA haplogroups that are common in all European populations. This indicates that the relatively high detection rate of this mutation in Spain is not due to sampling biases or to a single major founder event. Moreover, the distribution of these mutational events on different haplogroups is compatible with a random occurrence of the A1555G mutation and tends to support the conclusion that mtDNA backgrounds do not play a significant role in the expression of the mutation. Overall, these findings appear to indicate that the rare detection of this mutation in other populations is most likely due to inadequacy in patient ascertainment and molecular screening. This probable lack of identification of the A1555G mutation in subjects affected by sensorineural hearing loss implies that their maternally related relatives are not benefiting from presymptomatic detection and information concerning their increased risk of ototoxicity due to aminoglycoside treatments.
Introduction
The A1555G mutation in the 12S rRNA gene has been the first mtDNA mutation associated with nonsyndromic sensorineural deafness (MIM 580000 and MIM 561000) (Fischel-Ghodsian et al. 1993; Hutchin 1999; Prezant et al. 1993). This mutation was initially described mainly in subjects who became deaf after aminoglycoside exposure, thus indicating that, because of this mutation, patients' mitochondrial ribosomes had become targets of aminoglycosides, in a manner similar to that seen in the evolutionarily related bacterial ribosomes (for a review, see Fischel-Ghodsian 1998, 1999). However, more recently this mutation also has been found in numerous deaf subjects who apparently were never exposed to aminoglycosides (El-Schahawi et al. 1997; Estivill et al. 1998; Sarduy et al. 1998). The A1555G mutation has been reported in affected families of different ethnic origin (Hutchin et al. 1993; Prezant et al. 1993; Matthijs et al. 1996; Fischel-Ghodsian et al. 1997; Gardner et al. 1997; Pandya et al. 1997), and haplotype and phylogenetic studies also have shown that affected families do not generally share the A1555G mutation by descent but that it has arisen independently numerous times (Hutchin and Cortopassi 1997; Abe et al. 1998; Estivill et al. 1998). In addition, although initial studies had suggested that only a small proportion of familial sensorineural hearing loss was due to the A1555G mutation, several studies have now proposed that this mutation could be rather common (Fischel-Ghodsian et al. 1997; Usami et al. 1997; Estivill et al. 1998; Sarduy et al. 1998; Hutchin 1999). In particular, two independent studies of Spanish families detected high incidence of the A1555G mutation among pedigrees affected with sensorineural hearing loss—in 19 of 70 pedigrees in one study (Estivill et al. 1998) and in 32 of 134 in another study (I. del Castillo and F. Moreno, unpublished data). The identification of such a large number of families in Spain, relative to the few detected in other European populations (Casano et al. 1998), has led us to investigate the haplotype and phylogenetic relationships of the Spanish A1555G mtDNA samples, to determine whether this higher frequency is due to a particular history/origin of the mutation in Spanish affected families and/or to some particular features of mtDNA variation in the general Spanish population. A hypothetical role of mtDNA haplogroups in disease expression, as well as the possible existence of putative founder effects, were also investigated.
Subjects and Methods
Subjects
mtDNA variation was analyzed in 54 subjects (50 from Spain and 4 from Cuba) affected by nonsyndromic sensorineural deafness who previously had been found to be positive for the presence of the A1555G mutation in the 12S rRNA gene. Appropriate informed consent was obtained from these subjects. All of these individuals were from families that, according to interview of family members, were maternally unrelated at least within the preceding four generations. The maternal origins of the Spanish subjects represented a wide range of regions, including Old and New Castile, Estremadura, Catalonia, Andalusia, the Basque Country, and the Balearic Islands. Of the 50 Spanish subjects, 2 had been described previously by El-Schahawi et al. (1997), 19 by Estivill et al. (1998), and 5 by Sarduy et al. (1998). Genomic DNA was extracted from buffy coats by standard procedures. The molecular screening for the mutation A1555G was performed as described by Estivill et al. (1998) or in Sarduy et al. (1998), and the mutation was always found to be homoplasmic, except in one patient, as reported by El-Schahawi et al. (1997).
High-Resolution RFLP Analysis and Phylogenetic Analysis of RFLP Haplotypes
To determine high-resolution RFLP haplotypes, the entire mtDNA of each of the 54 subjects was amplified in nine overlapping fragments, by PCR and the primer pairs described by Torroni et al. (1997). Each of the nine PCR segments was then digested with 14 restriction endonucleases (AluI, AvaII, BamHI, DdeI, HaeII, HaeIII, HhaI, HincII, HinfI, HpaI, MspI, MboI, RsaI, and TaqI). In addition, all subjects were screened for the presence/absence of the BstOI site at nucleotide position 13704, the AccI sites at nucleotide positions 14465 and 15254, the BfaI site at nucleotide position 4914, the NlaIII sites at nucleotide positions 4216 and 4577, and the MseI site at nucleotide position 14766. The A→G sequence polymorphism at nucleotide position 12308 in the tRNALeu gene was also tested, by a mismatched primer that generates a HinfI site when the A12308G mutation is present (Torroni et al. 1996). Restriction fragments were resolved as described elsewhere (Torroni et al. 1997).
Phylogenetic relationships between RFLP haplotypes of the patients and those found in 237 European control subjects were inferred by parsimony analysis. The dendrograms were rooted on the basis of the mid-rooting point. Maximum parsimony (MP) trees were generated through random addition of sequences, by the tree-bisection-and-reconnection algorithm (PAUP 3.1.1; Swofford 1993).
Sequencing of the mtDNA Control Region (CR)
Sequencing of the mtDNA CR between nucleotide positions 16003 and 16490 was performed both for all mtDNA samples that, on the basis of RFLP analysis, had proved to be members of haplogroup H and for some of the non-H mtDNA samples, including those which shared an identical RFLP haplotype. The CR sequence between nucleotide positions 40 and 460 also was determined for a few of these mtDNA samples. Sequencing was performed as previously described by Torroni et al. (1998).
Results
RFLP Haplotype Analysis of mtDNA with the A1555G Mutation
Table 1 shows data on high-resolution RFLP haplotypes detected in the 50 Spanish patients (samples 1–50) and 4 Cuban patients (samples 51–54). Twenty-four RFLP haplotypes (haplotypes 1–15 and 17–25) were found among the Spanish patients, and two (haplotypes 16 and 26) were found among the Cuban patients. Overall, these haplotypes were defined by 56 polymorphic restriction sites.
Table 1.
RFLP and CR Haplotypes of 54 mtDNA Samples with the Deafness Mutation A1555G
| Haplogroup and Sample(s) | RFLP Haplotypea | RFLP Haplotype Designation | CR Haplotypeb | RFLP-CR Haplotype Designation |
| H: | ||||
| 36 | −7025a,−14766u | 1 | 176 | 1.1 |
| 2, 7, 10 | −7025a,−14766u,−16517e | 2 | 234 | 2.1 |
| 4 | −7025a,−14766u,+16517e | 2 | 188A | 2.2 |
| 8, 9, 27, 31, 34, 49 | −7025a,−14766u,+16517e | 2 | … | 2.3 |
| 30 | −7025a,−14766u,+16517e | 2 | 093, 129, 316 | 2.4 |
| 35 | −7025a,−14766u,+16517e | 2 | 320 | 2.5 |
| 44 | −7025a,−14766u,+16517e | 2 | 093 | 2.6 |
| 1, 29 | +3846c/−3849e,−7025a,−14766u,+16517e | 3 | 129 | 3.1 |
| 5 | −7025a,−14766u,−16303k | 4 | 304 | 4.1 |
| 13 | −7025a,−14766u,−16303k | 4 | 304,362 | 4.2 |
| 11, 12, 14, 16, 17, 21, 41, 43, 46 | +93e,−7025a,+8255k,−14766u,+16517e | 5 | … | 5.1 |
| 15 | +255f,−7025a,−14766u,+16217l,+16517e | 6 | 176, 219 | 6.1 |
| 20, 40 | −7025a,−8309c,+14465s,−14766u,+16517e | 7 | 172 | 7.1 |
| 22, 28, 39, 50 | −7025a,−8838e,−14766u | 8 | 335 | 8.1 |
| 37 | +379a,−7025a,−14766u,+16517e | 9 | 270 | 9.1 |
| 38 | −7025a,+9985j,+14223n/+14224f,−14766u,+16517e | 10 | … | 10.1 |
| 45 | −7025a,−14766u,−14869j,+16517e | 11 | … | 11.1 |
| 47 | +4724q,−7025a,−9380f,−14766u,+16478c,+16517e | 12 | 362, 482 | 12.1 |
| V: | ||||
| 3 | −4577q,−14766u | 13 | 291, 298 | 13.1 |
| U: | ||||
| 18 | +12308g,+16517e | 14 | ND | |
| 26 | +12308g,+16145e | 15 | ND | |
| 51–53 | +2349j,+3348j,+12308g,+16217l,−16310k,+16517e | 16 | 163, 172, 219, 311 | 16.1 |
| K: | ||||
| 32 | −9052n/−9053f,+10394c,+12308g,−16310k,+16517e | 17 | ND | |
| 42 | +4643k,−9052n/−9053f,+10289e,+10394c,−11922j,+12308g,−15254s,+15945c,−16310k,+16517e | 18 | ND | |
| 48 | −9052n/−9053f,+10289e,+10394c,−11922j,+12308g,+15945c,−16310k | 19 | ND | |
| T: | ||||
| 25 | +4216q,+4914r,+10746c,+13366m/−13367b/+13367j,+15606a,−15925i,−16303k,+16517e | 20 | ND | |
| J: | ||||
| 19 | +4216q,−6509a,+10394c,−13704t,−16065g | 21 | ND | |
| 24 | +4216q,+10394c,−13704t,+14923c,−16065g | 22 | ND | |
| I: | ||||
| 33 | −1715c,−4529n,+8249b/−8250e,+10032a,+10394c,+15754c,+16389m/−16390b/+16390j,+16517e | 23 | ND | |
| L2: | ||||
| 6 | +3592h,+10394c,+13803e,+16389g/−16390b,+16517e | 24 | 092, 189, 223, 278, 294, 309, 390 | 24.1 |
| L1: | ||||
| 23 | +185l,+2349j,−2758k,+3592h,−3693j,−7055a,+10394c,+10806g,+16517e | 25 | ND | |
| 54 | +185l,−1715c,+2349j,−2758k,+3592h,−3693j,+4216q,−7055a,+10394c,+10806g,+16254a,+16517e | 26 | ND |
Sites are numbered from the first nucleotide of the recognition sequence. A plus sign (+) indicates the presence of a restriction site, a minus sign (−) the absence. The explicit indication of the presence/absence of a site implies the absence/presence in haplotypes not so designated. The restriction enzymes used in the analysis are designated by single-letter code as follows: a = AluI; b = AvaII; c = DdeI; e = HaeIII; f = HhaI; g = HinfI; h = HpaI; i = MspI; j = MboI; k = RsaI; l = TaqI; m = BamHI; n = HaeII; o = HincII; q = NlaIII; r = BfaI; s = AccI; t = BstOI; u = MseI. A slash (/) separating states indicates the simultaneous presence or absence of restriction sites that can be correlated with a single nucleotide substitution. States diagnostic of RFLP haplogroups are underlined.
Nucleotide positions (−16000), between 16003 and 16490, that are different from the Cambridge Reference Sequence (Anderson et al. 1981). Mutations are transitions (T↔C and A↔G), unless the base change is explicitly specified. ND = not determined.
All the observed haplotypes were characterized by specific combinations of mutations (underlined in table 1) that previous studies had shown to define major monophyletic mtDNA clusters or haplogroups (Torroni et al. 1994, 1996, 1998; Chen et al. 1995; Macaulay et al. 1999). Thirty-eight (76.0%) of the Spanish mtDNA samples harbored the RFLP motif −7025 AluI,−14766 MseI, which is characteristic of the most common European-specific haplogroup, haplogroup H (Torroni et al. 1996, 1998; Macaulay et al. 1999). Although haplogroup H represented the majority of Spanish mtDNA samples, most of the other haplogroups specific to Europeans also were observed. Indeed, haplogroups V, T, and I were each represented by one subject (2.0%). Haplogroup-J and -U mtDNA samples were each observed in two subjects (4.0%), and haplogroup K was represented by three individuals (6.0%). The two remaining Spanish mtDNA samples (4.0%) were found to harbor RFLP motives that are characteristic of the African-specific haplogroups L1 and L2 (Chen et al. 1995). The presence of African-specific mtDNA in Spain has been described elsewhere (Côrte-Real et al. 1996) and probably reflects the arrival of north Africans during the Mesolithic (8,000 b.c.) and/or during the recent Arabic rule, which started ∼800 a.d. (Arnaiz-Villena et al. 1997). The mtDNA samples from the four Cuban patients belonged to two haplogroups. One of these mtDNA samples was a member of the African haplogroup L1, and the remaining three showed an identical haplotype (haplotype 16) belonging to haplogroup U.
The 38 Spanish haplogroup-H mtDNA samples were encompassed by 12 different RFLP haplotypes (RFLP haplotypes 1–12) (table 1). Haplotypes 2 and 5 were very common, being represented by 13 and 9 of the Spanish families, respectively. Other haplogroup-H haplotypes also were observed in more than one family. These included haplotype 8 (four families) and haplotypes 3, 4, and 7 (two families each). All other Spanish RFLP haplotypes were found in a single family.
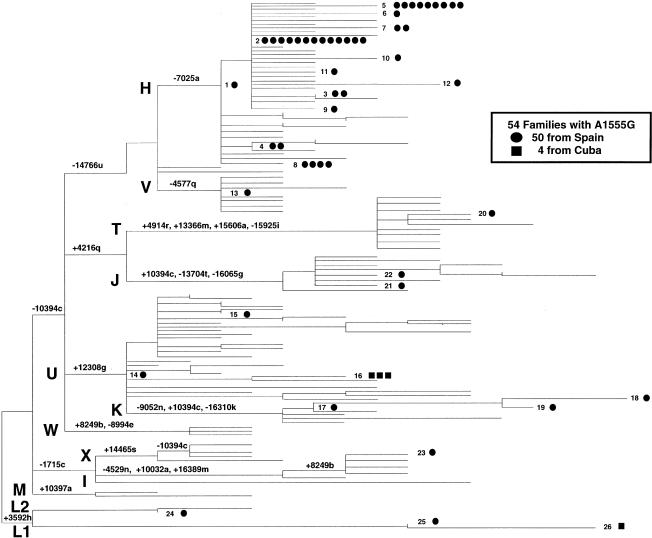
The phylogenetic relationships between the RFLP haplotypes of the 54 patients and the haplotypes previously described in 237 European controls are illustrated in figure 1. The distribution of the 54 mtDNA samples harboring the A1555G mutation in the parsimony tree appears to indicate that, at least as a first approximation, the A1555G mutation is due to 24 independent mutational events among the 50 Spanish subjects and two mutational events among the 4 Cuban patients.
Figure 1.
Phylogenetic tree of RFLP haplotypes from patients with the deafness mutation A1555G. This mid-rooting-point maximum-parsimony tree includes 24 RFLP haplotypes (1–15 and 17–25) observed in 50 apparently unrelated Spanish patients (blackened circles), 2 RFLP haplotypes (16 and 26) observed in 4 Cuban patients (blackened squares), and the haplotypes previously described in 237 unrelated European controls. The capital letters H–M and T–X indicate haplogroups, and the numbers associated with the lowercase letters indicate the sites for restriction enzymes that define the specific haplogroups. The restriction enzymes are designated by single-letter code as follows: a = AluI; b = AvaII; c = DdeI; e = HaeIII; f = HhaI; g = HinfI; h = HpaI; k = RsaI; j = MboI; i = MspI; l = TaqI; m = BamHI; n = HaeII; q = NlaIII; r = BfaI; s = AccI; t = BstoI; u = MseI. The horizontal branch lengths are proportional to the number of mutational events that separate the haplotypes, with the exception of sites 10394c, 16303k, 16310k, and 16517e. In the parsimony analysis, these sites were assigned half the weight (i.e., 1) assigned to all other sites (i.e., 2). This tree is 430 steps in length and has consistency and retention indices of .652 and .896, respectively.
Sequence Analysis of the mtDNA CR
To better define the relationships between the mtDNA samples harboring the A1555G mutation and the number of events that have generated this mutation, we sequenced the portion of the CR between nucleotide positions 16003 and 16490 that includes the entire first hypervariable segment (HVS-I). This sequence analysis was performed for all haplogroup-H mtDNA samples and for a few other mtDNA samples, including all of those which shared an identical haplotype (haplotype 16 observed in three Cubans).
This analysis revealed heterogeneity among the 12 Spanish mtDNA samples harboring haplotype 2 and indicated that they were subdivided into six subgroups (2.1–2.6) (table 1). Similarly, the two mtDNA samples harboring haplotype 4 showed different CR sequences (4.1 and 4.2). In contrast, identical CR sequences were observed among the Spanish families harboring haplotypes 3, 5, 7, and 8. Also, the three Cubans with haplotype 16 showed an identical CR.
To further study the origin of the A1555G mutation in the families harboring haplotype 5, we sequenced the CR between nucleotide positions 40 and 460, which includes the entire second hypervariable segment (HVS-II) of two haplotype-5 mtDNA samples (samples 14 and 21). These were both defined by the motif 93G-95C-263G and by the presence of an additional C in the stretch of C's located between nucleotide positions 311 and 315. In addition, sample 21 was found to be heteroplasmic for the presence of an extra C in the hypervariable stretch of C's between nucleotide positions 303 and 309.
Haplogroup Distribution of mtDNA from Spanish Patients and Spanish Controls
Table 2 illustrates the haplogroup distribution of the mtDNA observed in the 50 Spanish subjects harboring the A1555G mutation. To determine whether this distribution corresponded to that of the general Spanish population, 182 control mtDNA samples from different regions of Spain were screened for the marker mutations that distinguish European haplogroups (Torroni et al. 1996; Macaulay et al. 1999).
Table 2.
Haplogroup Distribution of mtDNA from Spanish Subjects with the Deafness Mutation A1555G and from Spanish Controls
|
Frequency of mtDNA, from Spanish Subjects, in Haplogroup(%) |
||||||||||||
| Group | Ha | I | J | K | T | U | V | W | X | L | M | Other |
| Deaf individuals (n=50) | 76.0 | 2.0 | 4.0 | 6.0 | 2.0 | 4.0 | 2.0 | … | … | 4.0 | … | … |
| Controls (n=182) | 45.1 | 1.1 | 4.4 | 3.3 | 9.9 | 14.3 | 5.5 | 1.1 | 1.6 | 3.3 | .5 | 9.9 |
The difference between patients and controls is highly significant (two-sided Fisher's exact test; P=.0001).
This comparison showed that haplogroup H encompassed ∼76% of the patients' mtDNA samples but only ∼45% of the Spanish control mtDNA samples, a difference that is highly significant (two-sided Fisher's exact test; P=.0001). In contrast, observed frequencies of other haplogroups in patients were all compatible with a random distribution of the A1555G mutation.
Discussion
Multiple Origins of the A1555G Mutation in Spain
High-resolution RFLP analysis of 50 Spanish subjects affected by sensorineural deafness has shown that the A1555G mutation is associated with 24 RFLP haplotypes and that those haplotypes are members of a wide range of haplogroups (H–K, T–V, L1, and L2). The presence of the mutation on different haplogroups confirms that it has occurred multiple times in the Spanish population (Estivill et al. 1998). However, RFLP analysis also allows a quantitation of the number of independent occurrences of the A1555G mutation. A previous study has estimated that, with the set of restriction enzymes used in this analysis, one restriction-site change (other than 16517 HaeIII, which is hypervariable) from a founder haplotype requires 24,420 years, on average (Torroni et al. 1998). Thus, unless the possibility is accepted that the A1555G mutation has been transmitted by descent for tens of thousands of years to mtDNA that, in the meantime, has diverged into different haplotypes, we have to believe that each of the 24 different haplotypes, including the 12 haplotypes belonging to haplogroup H, represents at least one independent occurrence of the A1555G mutation. This scenario gets support from the observation that most of these haplotypes also have been previously detected in population controls from either Spain or other European countries (Torroni et al. 1994, 1996). For instance, haplogroup-H haplotypes 1–4 and 10 all have been observed in a sample of only 25 haplogroup-H mtDNA samples from the Basque population (Torroni et al. 1998). Two notable exceptions are represented by haplotypes 5 and 8, which encompass 18% and 8%, respectively, of the Spanish subjects with the A1555G mutation. The former haplotype is characterized by the presence of the sites HaeIII at nucleotide position 93 and RsaI at nucleotide position 8255, and the latter haplotype is characterized by the absence of the HaeIII site at nucleotide position 8838 (table 1). Neither of these haplotypes had been observed previously in European populations. To obtain further information on the distribution of haplotypes 5 and 8, we screened 82 haplogroup-H control mtDNA samples from Spain, to determine their distinguishing RFLP mutations; none of the controls was found to harbor these haplotypes.
Haplotype 2 is considered to be the founder haplotype of haplogroup H, is the most common haplotype in European populations, and encompasses more than one-third of haplogroup-H mtDNA samples (Torroni et al. 1998). Therefore, it was not unexpected that 13 of the 38 haplogroup-H mtDNA samples with the A1555G mutation were associated with this haplotype. In addition, because of the high frequency of haplotype 2 in the general population, it is also likely that the A1555G mutation has occurred more than once, on different haplotype-2 mtDNA samples. Thus, to obtain a better estimation of the number of mutational events that had produced the A1555G mutation in our Spanish sample, we performed CR sequencing of all haplogroup-H mtDNA samples.
This analysis revealed that six different CR motifs (2.1–2.6) were associated with RFLP haplotype 2. With the exception of CR motif 2.2, all of these had been reported previously in European populations. Haplotype 2.3 is identical to the reference sequence and is the most common haplogroup-H CR motif. Haplotypes 2.1 and 2.6 have been described in several populations, including some from Spain (Côrte-Real et al. 1996; Salas et al. 1998); haplotype 2.4 has been described in the British (Piercy et al. 1993) and Italians (Torroni A, unpublished data); and haplotype 2.5 has been reported in Germans (Richards et al. 1996). Also, the two haplotype-4 mtDNA samples were found to harbor different CR sequences (4.1 and 4.2 in table 1), both of which had been reported previously in numerous European populations. Thus, it appears that the A1555G mutation has occurred at least six times on haplotype-2 mtDNA and twice on haplotype-4 mtDNA and that, overall, this mutation was originated by at least 30 independent mutational events in the 50 Spanish families.
Origin of the A1555G Mutation in Cuban Subjects
One of the Cuban mtDNA samples was found to belong to the African haplogroup L2 and obviously represents one mutational event of the A1555G mutation. In contrast, the other three Cuban mtDNA samples were found to harbor the same RFLP haplotype (haplotype 16). These three subjects were not maternally related in the preceding four generations, but they were all from the same area (the town of Guines). Sequencing of the CR confirmed the homogeneity of the three samples and showed that all of them harbored the CR motif 16163-16172-16219-16311. This motif is characteristic of a particular subset of subhaplogroup U6 (Macaulay et al. 1999), and it is restricted to the Canary Islands (Rando et al. 1998). Thus it appears that haplotype 16 observed in the Cubans most likely arrived from the Canary Islands. This origin is further supported by the fact that the town of Guines historically has been a settlement place for immigrants from the Canary Islands.
Founder Events of the A1555G Mutation
The analysis of the haplogroup distribution of the Spanish mtDNA samples harboring the A1555G mutation has shown a strong excess of haplogroup-H mtDNA. A priori, two alternative genetic phenomena could explain this observation. One is that haplogroup H—or a specific subset of this haplogroup—plays a role in deafness expression and increases the penetrance of the A1555G mutation. For instance, this hypothetical increase of penetrance could be due to the presence of a predisposing mutation that exacerbates the effect of the A1555G mutation. In this scenario, the excess of haplogroup H—or of specific subsets of H—would be caused by a sampling bias due to the fact that affected individuals or families with a more extreme phenotype are more likely to attract the attention of clinicians and thus are more likely to be sampled. A role of mtDNA background in disease expression has been postulated previously for Leber hereditary optic neuropathy (LHON). Among patients with LHON, a strong excess of haplogroup-J mtDNA has been observed, and it has been proposed that a number of mutations specific to this haplogroup increase the penetrance of the LHON mutations 11778 and 14484 (Howell et al. 1995; Brown et al. 1997; Hofmann et al. 1997; Lamminen et al. 1997; Torroni et al. 1997). Alternatively, the excess of haplogroup-H mtDNA samples harboring the A1555G mutation could be due to founder events. In other words, the A1555G mutation observed in different families would not be due to independent mutational events but would have been transmitted by descent to families that are indeed maternally related.
The combined RFLP-CR analysis permits us to discriminate between these two alternative scenarios. Eighteen of the 30 mutational events observed in the 50 Spanish families have occurred on haplogroup-H mtDNA samples. This proportion is not significantly different from the random occurrence expectation of 13.5 of 30 mutations (Yates's χ2=2.1; P>.10) when the fact that haplogroup H represents ∼45% of the general Spanish population is taken into account. This finding indicates that independent mutational events on a haplogroup-H background are not more likely to be identified by clinicians because of increased penetrance. Therefore, the possibility of a role of haplogroup-H background in disease expression appears to be unlikely. In contrast, the possibility that the increased frequency of haplogroup H among Spanish patients is due to founder events appears to be substantiated by two pieces of evidence. First, since haplogroup H is by far the most common haplogroup in Spain, it is most likely that random founder events would involve this haplogroup. Second, the combined RFLP-CR haplotype analysis revealed that several haplotypes—2.1, 2.3, 3.1, 5.1, 7.1, and 8.1—are shared by different families. Among these haplotypes, 2.1 (three individuals), 5.1 (nine individuals), and 8.1 (four individuals) were not observed among our Spanish controls and have not been reported previously in extensive population studies conducted in Spain and other European countries. Thus, it is beyond doubt that affected families with haplotypes 2.1, 5.1, and 8.1 have acquired the A1555G mutation by descent from common maternal ancestors. Since our families have been unrelated for the preceding four generations, these common female ancestors in whom the mutational event occurred are not very recent. This raises the possibility that the degree of selection against the A1555G mutation is much lower than that of most other pathological mtDNA mutations—and that this mutation can be transmitted for numerous generations, at least in some particular demographic situations. However, a confirmation of this would require accurate and extensive genealogical studies that, thus far, we have not been able to conduct. Similarly, it is also most likely that the three Cuban families harboring haplotype 16.1 all have acquired the disease mutation from the same woman. Extensive genealogical studies of the Cuban families and identification of affected families living in the Canary Islands could indicate whether this mutation has occurred in the Canary Islands, or alternatively, in a Cuban woman of Canarian ancestry and could define the age of this particular mutational event.
In conclusion, here we have reported the most extensive study yet of mtDNA variation in individuals affected by sensorineural deafness and harboring the A1555G mutation in the 12S RNA gene. This mutation can be attributed to ⩾30 independent mutational events among 50 affected Spanish families. This indicates that the relatively high detection rate of this mutation in Spain (Estivill et al. 1998; Sarduy et al. 1998) is not due to either sampling biases or a single major founder event. Moreover, the distribution of these mutational events on different haplogroups is compatible with a random occurrence of the A1555G mutation and supports the general conclusion that mtDNA backgrounds do not play a significant role in the expression of the mutation. As would be expected when numerous families affected by a relatively uncommon disease are sampled, some founder events also were detected. The fact that all of these families were not related in the preceding four generations raises the possibility that the A1555G mutation and the associated phenotype are not under a strong selective pressure and can be transmitted for numerous generations.
Overall, these results raise a major question. If the A1555G in Spain is due to numerous independent mutational events occurring randomly on all mtDNA backgrounds, and if mtDNA backgrounds do not affect its penetrance, why has this mutation been so rarely detected/reported in other European populations? One theoretical possibility is that the Spanish population is much more exposed to exacerbating environmental factors, including aminoglycoside treatment, than are other European populations. During the 1950s and '60s, penicillin associated with streptomycin was commonly given to Spanish patients with tonsillitis. Also, streptomycin was used in the treatment of tuberculosis, which was observed to have a high prevalence in Spain after the civil war. However, there is no evidence of any other major difference—in diet, living conditions, or medical treatments—between Spain and most other western European countries. In addition, even if we were to hypothesize that our conclusion about the lack of a role of mtDNA background in disease expression was wrong, no major differences would be expected between the Spanish and other Europeans, since haplogroup H is by far the most common mtDNA haplogroup and has similar frequencies in all western European populations. Thus, there are only two explanations left. One explanation is that in the Spanish there is, relative to other European populations, a higher incidence of unidentified nuclear genetic variants that facilitate the expression of the A1555G mutation. However, this explanation also appears to be unlikely. For instance, the large body of genetic studies summarized by Cavalli-Sforza et al. (1994) does not reveal major genetic differences in the gene pool of the general Spanish population, relative to the gene pools of other western European countries. Alternatively, the rare detection of the A1555G mutation in other European populations could be due to inadequacy in patient identification and/or in pedigree analysis and to a bias, on the part of most research groups that investigate the genetic basis of deafness, toward the ascertainment of congenital cases.
Acknowledgments
We are indebted to Drs. E. Perelló and C. Ayuso for providing DNA samples and to the patients and the clinicians who have collaborated in the study. The work was supported by European Community grant PL951324 and Fondo Investigaciones Sanitarias grant FIS-96/1556 (both to F.M.), Fundació La Marató de TV grant 3/981710 (to X.E. and N.L-B.), Fondo Investigaciones Sanitarias grant FIS-99/0917 (to X.E. and R.R.), and Progetto Finalizzato Beni Culturali contract 97.00702.PF36 (to R.S.); by the Grandi Progetti Ateneo (Italian Ministry of Universities, 60%) (to R.S.), the Faculty of Science, University of Urbino (to A.T.), and the Italian Ministry of Universities Progetti Ricerca Interesse Nazionale 1997 (to R.S.); and by Italian Consiglio Nazionale delle Ricerche grant 98.00524.CT04 and Telethon-Italy grants 921 and E.0890 (all to A.T.).
Electronic-Database Information
Accession numbers and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/omim (for nonsyndromic sensorineural deafness [MIM 561000 and MIM 580000])
References
- Abe S, Usami S, Shinkawa H, Weston MD, Overbeck LD, Hoover DM, Kenyon JB (1998) Phylogenetic analysis of mitochondrial DNA in Japanese pedigrees of sensorineural hearing loss associated with the A1555G mutation. Eur J Hum Genet 6:563–569 [DOI] [PubMed]
- Anderson S, Bankier AT, Barrell BG, De Bruijn MHL, Coulson AR, Drouin J, Eperon IC, et al (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465 [DOI] [PubMed]
- Arnaiz-Villena A, Martinez-Laso J, Gomez-Casado E, Diaz-Campos N, Santos P, Martinho A, Breda-Coimbra H (1997) Relatedness among Basques, Portuguese, Spaniards, and Algerians studied by HLA allelic frequencies and haplotypes. Immunogenetics 47:37–43 [DOI] [PubMed]
- Brown MD, Sun F, Wallace DC (1997) Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 and 14484 mtDNA mutations on an mtDNA lineage. Am J Hum Genet 60:381–387 [PMC free article] [PubMed]
- Casano RA, Bykhovskaya Y, Johnson DF, Hamon M, Torricelli F, Bigozzi M, Fischel-Ghodsian N (1998) Hearing loss due to the mitochondrial A1555G mutation in Italian families. Am J Med Genet 12:388–391 [PubMed]
- Cavalli-Sforza LL, Menozzi P, Piazza A (1994) The history and geography of human genes. Princeton University Press, Princeton, NJ [Google Scholar]
- Chen Y-S, Torroni A, Excoffier L, Santachiara-Benerecetti AS, Wallace DC (1995) Analysis of mtDNA variation in African populations reveals the most ancient of all human continent-specific haplogroups. Am J Hum Genet 57:133–149 [PMC free article] [PubMed]
- Côrte-Real HBSM, Macaulay VA, Richards MB, Hariti G, Issad MS, Cambon-Thomsen A, Papiha S, et al (1996) Genetic diversity in the Iberian peninsula determined from mitochondrial sequence analysis. Ann Hum Genet 60:331–350 [DOI] [PubMed]
- El-Schahawi M, López de Munain A, Sarrazin AM, Shanske AL, Basirico M, Shanske S, DiMauro S (1997) Two large Spanish pedigrees with non-syndromic sensorineural deafness and the mtDNA mutation at nt 1555 in the 12S rRNA gene: evidence of heteroplasmy. Neurology 48:453–456 [DOI] [PubMed]
- Estivill X, Govea N, Barceló A, Perelló E, Badenas C, Romero E, Moral L, et al (1998) Familial progressive Sensorinerural deafness is mainly due to the mtDNA A1555G mutation and is enhanced by treatment with aminoglycosides. Am J Hum Genet 62:27–35 [DOI] [PMC free article] [PubMed]
- Fischel-Ghodsian N (1998) Mitochondrial mutations and hearing loss: paradigm for mitochondrial genetics. Am J Hum Genet 62:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischel-Ghodsian N (1999) Mitochondrial deafness mutations reviewed. Hum Mutat 13:261–270 [DOI] [PubMed]
- Fischel-Ghodsian N, Prezant TR, Bu X, Oztas S (1993) Mitochondrial ribosomal RNA gene mutation in a patient with sporadic aminoglycoside ototoxicity. Am J Otolaryngol 14:399–403 [DOI] [PubMed]
- Fischel-Ghodsian N, Prezant TR, Chaltraw WE, Wendt KA, Nelson RA, Arnos KS, Falk RE (1997) Mitochondrial gene mutation is a significant predisposing factor in aminoglycoside ototoxicity. Am J Otolaryngol 18:173–178 [DOI] [PubMed]
- Gardner JC, Goliath R, Viljoen D, Sellars S, Cortopassi G, Hutchin T, Greenberg J, et al (1997) Familial streptomycin ototoxicity in a South African family: a mitochondrial disorder. J Med Genet 34:904–906 [DOI] [PMC free article] [PubMed]
- Hofmann S, Jaksch M, Bezold R, Mertens S, Aholt S, Paprotta A, Gerbitz KD (1997) Population genetics and disease susceptibility: characterization of central European haplogroups by mtDNA gene mutations, correlations with D loop variants and association with disease. Hum Mol Genet 6:1835–1846 [DOI] [PubMed]
- Howell N, Kubacka I, Halvorson S, Howell B, McCullough DA, Mackey D (1995) Phylogenetic analysis of mitochondrial genomes from Leber hereditary optic neuropathy pedigrees. Genetics 140:285–302 [DOI] [PMC free article] [PubMed]
- Hutchin T (1999) Sensorineural hearing loss and the A1555G mitochondrial DNA mutation. Acta Otolaryngol (Stockh) 119:48–52 [DOI] [PubMed]
- Hutchin T, Cortopassi G (1997) Multiple origins of a mitochondrial mutation conferring deafness. Genetics 145:771–776 [DOI] [PMC free article] [PubMed]
- Hutchin T, Haworth I, Higashi K, Fischel-Ghodsian N, Stoneking M, Saha N, Arnos C, et al (1993) A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Res 21:4174–4179 [DOI] [PMC free article] [PubMed]
- Lamminen T, Huoponen K, Sistonen P, Juvonen V, Lahermo P, Aula P, Nikoskelainen E, et al (1997) mtDNA haplotype analysis in Finnish families with Leber hereditary optic neuropathy (LHON). Eur J Hum Genet 5:271–279 [PubMed]
- Macaulay V, Richards M, Hickey E, Vega E, Cruciani F, Guida V, Scozzari R, et al (1999) The emerging tree of West Eurasian mtDNAs: a synthesis of control-region sequences and RFLPs. Am J Hum Genet 64:232–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijs G, Claes S, Longo-Mbenza B, Cassiman J-J (1996) Non-syndromic deafness associated with a mutation and a polymorphism in the mitochondrial 12S ribosomal RNA gene in a large Zairean pedigree. Eur J Hum Genet 4:46–51 [DOI] [PubMed]
- Pandya A, Xia X, Radnaabazar J, Batsuuri J, Dangaansuren B, Fischel-Ghodsian N, Nance WE (1997) Mutation in the mitochondrial 12S rRNA gene in two families from Mongolia with matrilineal aminoglycoside ototoxicity. J Med Genet 34:169–172 [DOI] [PMC free article] [PubMed]
- Piercy R, Sullivan KM, Benson N, Gill P (1993) The application of mitochondrial DNA typing to the study of white Caucasian genetic identification. Int J Legal Med 106:85–90 [DOI] [PubMed]
- Prezant TR, Agapian JV, Bohlman MC, Bohlman MC, Bu X, Öztas S, Qiu W-Q, et al (1993) Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet 4:289–294 [DOI] [PubMed]
- Rando JC, Pinto F, Gonzales AM, Hernandez M, Larruga JM, Cabrera VM, Bandelt HJ (1998) Mitochondrial DNA analysis of Northwest African populations reveals genetic exchanges with European, Near-Eastern, and sub-Saharan populations. Ann Hum Genet 62:531–550 [DOI] [PubMed]
- Richards M, Côrte-Real H, Forster P, Macaulay V, Wilkinson-Herbots H, Demaine A, Papiha S, et al (1996) Paleolithic and Neolithic lineages in the European mitochondrial gene pool. Am J Hum Genet 59:185–203 [PMC free article] [PubMed]
- Salas A, Comas D, Lareu MV, Bertranpetit J, Carracedo A (1998) mtDNA analysis of the Galician population: a genetic edge of European variation. Eur J Hum Genet 6:365–375 [DOI] [PubMed]
- Sarduy M, del Castillo I, Villamar M, Romero L, Herraiz C, Hernández FJ, Tapia MC, et al (1998) Genetic study of mitochondrially inherited sensorineural hearing impairment in eight large families from Spain and Cuba. In: Stephens D, Read A, Martini A (eds) Developments in genetic hearing impairment. Whurr Publishers, London, pp 121–125 [Google Scholar]
- Swofford D (1993) Phylogenetic analysis using parsimony (PAUP), version 3.1.1. Illinois Natural History Survey, Champaign [Google Scholar]
- Torroni A, Bandelt H-J, D'Urbano L, Lahermo P, Moral P, Sellitto D, Rengo C, et al (1998) mtDNA analysis reveals a major late Paleolithic population expansion from southwestern to northeastern Europe. Am J Hum Genet 62:1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, Obinu D, et al (1996) Classification of European mtDNAs from an analysis of three European populations. Genetics 144:1835–1850 [DOI] [PMC free article] [PubMed]
- Torroni A, Lott MT, Cabell MF, Chen Y-S, Lavergne L, Wallace DC (1994) mtDNA and the origin of Caucasians: identification of ancient Caucasian-specific haplogroups, one of which is prone to a recurrent somatic duplication in the D-loop region. Am J Hum Genet 55:760–776 [PMC free article] [PubMed]
- Torroni A, Petrozzi M, D'Urbano L, Sellitto D, Zeviani M, Carrara F, Carducci C, et al (1997) Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet 60:1107–1121 [PMC free article] [PubMed]
- Usami S, Abe S, Kasai M, Shinkawa H, Moeller B, Kenyon JB, Kimberling WJ (1997) Genetic and clinical features of sensorineural hearing loss associated with the 1555 mitochondrial mutation. Laryngoscope 107:483–490 [DOI] [PubMed]