Summary
The increasing number of diagnosed cases of inherited thrombocytopenias, owing to the routine practice of including platelet counts in blood tests, suggests that this condition is not so rare as expected. In the majority of cases, the molecular basis of the disease is unknown, although the defect is likely to affect thrombocytopoiesis and regulation of the normal platelet count. Here we report a genomewide search in a large Italian family affected by autosomal dominant thrombocytopenia. Patients showed a moderate thrombocytopenia with minimal symptoms characterized by normocellular bone marrow, normal medium platelet volume, and positive aggregation tests. Microsatellite analysis demonstrated that the disease locus (THC2) is linked to chromosome 10p11.1-12, within a candidate region of 6 cM between markers D10S586 and D19S1639. A maximum LOD score of 8.12 at recombination fraction .00 was obtained with the microsatellite D10S588. These data localized the first locus of an autosomal dominant thrombocytopenia, and the subsequent identification of the gene will provide new insight into the basic mechanism of megakaryocytopoiesis disorders.
Introduction
Inherited thrombocytopenias represent a heterogeneous group of disorders (Dowton et al. 1985). Different criteria have been suggested to classify the different forms, such as platelet volume, number and morphology of megakaryocytes, or inheritance pattern. X-linked, autosomal dominant, and recessive thrombocytopenias have been reported. In addition to the clinical syndromes in which thrombocytopenia is associated with other abnormalities, such as Fanconi anemia, Wiskott-Aldrich syndrome, and thrombocytopenia with absent-radius syndrome (Derry et al. 1994; Villa et al. 1995; Savoia et al. 1996; Strippoli et al. 1998), thrombocytopenia can be the only clinical finding in many families. In these cases, some thrombocytopenias are characterized by a hypomegakaryocytic bone marrow with mono- or multilineage involvement, whereas others have normal megakaryocyte count. On the basis of the medium platelet volume (MPV), nonsyndromic forms could further be classified as macro-, micro-, and normothrombocytopenia (Najean and Lecompte 1995; Bellucci 1997).
The prevalence of nonsyndromic thrombocytopenias is unknown. However, the incidence might be high, because of the existence of clinically asymptomatic individuals. Indeed, the recent widespread use of automated cell counters has led to an increase in the diagnosis of thrombocytopenia. In some patients, thrombocytopenia is not discovered until adulthood, and a mistaken diagnosis is often made, resulting in an inappropriate treatment and splenectomy (Majado et al. 1992).
Moderate thrombocytopenia with minimal symptoms, normal platelet morphology, and normal marrow megakaryocytes are characteristics in most patients with autosomal dominant traits (MIM 188000 [Bithell et al. 1965; Najean and Lecompte 1990; Fabris et al. 1997]). We have recently described a large family in which 17 patients had similar features. Linkage analysis excluded thrombopoietin (TPO) and its receptor c-mpl as candidate genes, as well as another two candidate loci on chromosome 11 and 21 (Iolascon et al., in press). The results of a genomewide search performed in this family showed that the thrombocytopenia locus of this family (THC2) is located on chromosome 10p11.2-12 in an interval between microsatellites D10S586 and D10S1639.
Material and Methods
Genotyping
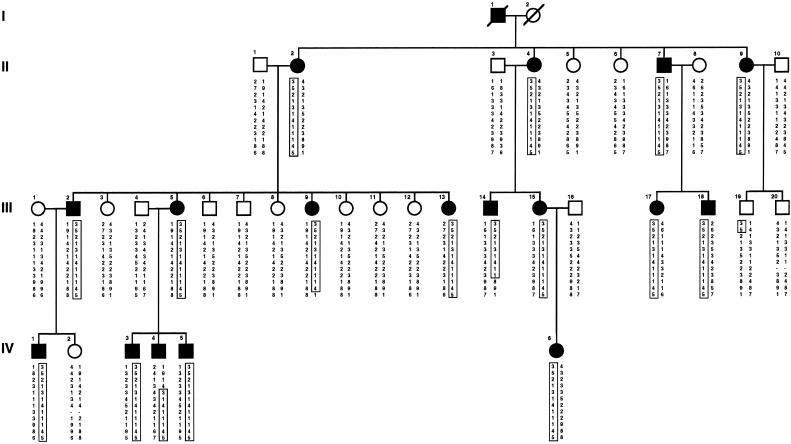
Genomic DNA from peripheral-blood lymphocytes of 36 family members, including 17 affected individuals, 12 unaffected bloodline relatives, and 7 spouses, was used for genetic analysis (fig. 1). Clinical data on the patients have been described by Iolascon et al. (in press). Genotyping was performed by a DNA sequencer apparatus (ABI 377A) and the ABI PRISM Linkage Mapping Set, version 2 (PE Biosystems). This set comprises 400 fluorescently labeled markers that define a 10-cM-resolution map in humans. PCR conditions were the same as those specified in the manufacturer's instructions. DNA fragment–sizing analysis was performed by GENESCAN and GENOTYPER software.
Figure 1.
Family pedigree with haplotype reconstruction for informative markers on 10p11.2-12. The at-risk haplotype is boxed.
Linkage Analysis
Classic two-point LOD-score analysis was done in this pedigree, under the assumption of autosomal dominant inheritance and complete genetic penetrance. All of the individuals with a platelet value <1.00×1011/liter were classified as affected. The MLINK program included in the LINKAGE package was used to perform linkage analysis (Lathrop et al. 1984).
Haplotype Reconstruction
To better characterize the possible meiotic recombinants and to define the critical region of the disease locus, we performed haplotype analysis. Five microsatellites (D10S595, D10S1660, D10S586, D10S572, and D10S1775, from Généthon), which are known to map between D10S548 and D10S197 of the ABI PRISM Linkage Mapping Set, and another four microsatellites (D10S1771, D10S588, D10S1639, and D10S213, from Généthon), which span the interval between D10S197 and D10S208 and which also are in the ABI PRISM Linkage Mapping Set, were synthesized by a DNA synthesizer (Applied Biosystems). All the forward primers were labeled with 6-FAM or HEX fluorescent amidites and were purified with OPC columns (ABI/PE Biosystems). PCR was performed under standard conditions, and amplification products were analyzed in an ABI 377A automated sequencer.
Results
Genomewide Search
We previously have described the clinical, biological, and molecular findings of an autosomal dominant thrombocytopenia in a large Italian family (Iolascon et al., in press). After excluding a linkage to candidate loci (Iolascon et al., in press), we considered this pedigree for genomewide linkage mapping. A prescreening was done only with the 17 affected DNA samples, by use of the ABI PRISM Linkage Mapping Set markers. Microsatellite mapping excluded most of the genome and identified two loci, on chromosomes 10 and 11, because of the fact that all the patients shared the same allele. These markers were then tested in all pedigree members, and the candidate locus on chromosome 11 was clearly excluded from linkage (data not shown). Under the assumption of complete genetic penetrance, marker D10S197 on chromosome 10p gave a maximum two-point LOD score of 3.91 at recombination fraction (θ) .00 (table 1). The two flanking microsatellites, D10S548 and D10S208, showed linkage to the thrombocytopenia locus, with maximum LOD score of 4.96 and 3.57 at θ=.07 and .09, respectively (table 1).
Table 1.
Two-Point LOD Scores between Chromosome 10p Markers and Disease Locus TCH2
|
LOD Score at θ = |
|||||||||
| Locus | .00 | .01 | .05 | .10 | .20 | .30 | .40 | Maximum LOD Score | Maximum θ |
| D10S548 | −∞ | 4.00 | 4.92 | 4.89 | 4.11 | 2.90 | 1.37 | 4.96 | .07 |
| D10S595 | −∞ | 4.00 | 4.92 | 4.89 | 4.11 | 2.90 | 1.37 | 4.96 | .07 |
| D10S1660 | −∞ | 1.25 | 1.74 | 1.76 | 1.45 | .97 | .43 | 1.78 | .08 |
| D10S586 | −∞ | .96 | 1.46 | 1.51 | 1.27 | .86 | .40 | 1.51 | .09 |
| D10S572 | 2.70 | 2.66 | 2.46 | 2.21 | 1.68 | 1.10 | .51 | 2.70 | .00 |
| D10S1775 | 6.62 | 6.51 | 6.08 | 5.52 | 4.29 | 2.90 | 1.33 | 6.62 | .00 |
| D10S197 | 3.91 | 3.84 | 3.57 | 3.22 | 2.45 | 1.59 | .62 | 3.91 | .00 |
| D10S1771 | 7.52 | 7.40 | 6.92 | 6.29 | 4.90 | 3.34 | 1.57 | 7.52 | .00 |
| D10S588 | 8.12 | 8.00 | 7.48 | 6.80 | 5.31 | 3.63 | 1.73 | 8.12 | .00 |
| D10S1639 | −∞ | 4.52 | 4.80 | 4.57 | 3.69 | 2.53 | 1.15 | 4.81 | .04 |
| D10S213 | −∞ | 6.00 | 6.20 | 5.84 | 4.71 | 3.27 | 1.55 | 6.23 | .03 |
| D10S208 | −∞ | 2.41 | 3.44 | 3.56 | 3.13 | 2.29 | 1.16 | 3.57 | .90 |
Linkage Analysis
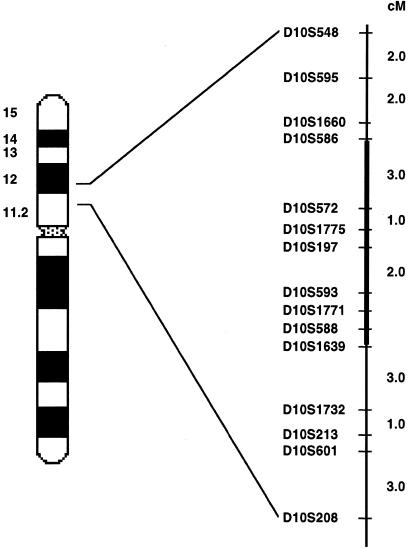
D10S548 and D10S208 defined the THC2 locus in a region of ∼17 cM (fig. 2). The marker density was further increased, with nine additional microsatellites that were tested on all pedigree members. Markers D10S588 and D10S1771 yielded the maximum two-point LOD scores: 8.12 and 7.52 (θ=0), respectively (table 1). Haplotype analysis was performed on the basis of the microsatellite order tel-D10S548-D10S595-D10S1660-D10S586-D10S572-D10S1775-D10S197-D10S1771-D10S588-D10S1639-D10S213-D10S208-cen, deduced from data from the Whitehead Institute/MIT Genome Sequencing Project (figs. 1 and 2). A centromeric recombination was evident between D10S588 and D10S1639, in individual III-14. In IV-4, a crossing-over between D10S586 and D10S572 established the distal limit of the disease region, at D10S586. Another recombinational event was observed between markers D10S595 and D10S1775 in individual III-19. In this case, however, microsatellites D10S1660, D10S586, and D10S572 were not informative, since the patient's mother was homozygous at all three loci. Taken together, the recombinations defined the candidate region at an interval of ∼6 cM flanked by microsatellites D10S586 and D10S1639 (fig. 2).
Figure 2.
Schematic representation of a partial linkage map of 12 microsatellite markers located on 10p11.2-12. The mapping order and genetic distances (in cM) were obtained from the Whitehead Institute/MIT Genome Sequencing Project. The boldface segment denotes the linked interval determined by haplotype analysis.
Discussion
Inherited thrombocytopenias are heterogeneous syndromes. In many cases, the only finding is an increased platelet volume, inherited as an autosomal dominant trait, without other hematologic abnormalities. We have recently described a large pedigree carrying an autosomal dominant thrombocytopenia characterized by normal MPV (Iolascon et al., in press). The megakaryocytes were morphologically normal and had not increased in number. Platelets were functionally active, suggesting that the basic mechanism of this form could be an abnormal platelet release from bone marrow. The family was sufficiently large to allow a genomewide search and localization of the THC2 locus on the short arm of chromosome 10 (10p11.2-12), between microsatellites D10S586 and D10S1639.
Another two different forms of thrombocytopenia have been mapped, to chromosomes 11q23.3-qter and 21q22.1-22.2 (Breton-Gorius et al. 1995; Ho et al. 1996). Microsatellites in these candidate regions were not initially found in linkage with the THC2 locus (Iolascon et al., in press). The exclusion was consistent with the clinical and hematologic features of patients in each family, compared with those of thrombocytopenic individuals reported in the present study. The first form represented a new entity of congenital thrombocytopenia, associated with a partial deletion of the long arm of chromosome 11, the presence of giant α-granules in a subpopulation of platelets, an increased number of bone-marrow megakaryocytes, including numerous micromegakaryocytes, and a normal platelet life span (Breton-Gorius et al. 1995). The other was an autosomal dominant platelet disorder with a striking propensity toward development of hematologic malignancies (Ho et al. 1996). All patients had laboratory abnormalities, including shortened platelet life span, large platelets, increased number of bone-marrow megakaryocytes, neutrophil nuclear hyperpigmentation, and eosinophilia.
The chromosomal region 10p11.2-12 between markers D10S586 and D10S1639 is physically covered by a YAC contig (WC10.1; Whitehead Institute/MIT Genome Sequencing Project). On the basis of this map, the size of this interval between markers D10S586 and D10S1639 was estimated to be 6 cM. This interval contains only two known genes (e3B1 [for an eps8-binding protein] and GAD2 [for glutamate decarboxylase 2]) but several expressed sequence tags, some of which, on the basis of their homologies and expression, could represent candidate genes (National Center for Biotechnology Information).
The localization of the THC2 locus represents an important step in the understanding of the genetic basis of inherited thrombocytopenias. New families will be tested for linkage to the chromosome 10p. Pedigrees that do not show linkage to this locus could then be used for a further genomewide search. The search for the gene through the candidate-gene approach could add clues to the understanding, at the functional level, of the molecular defects causing platelet reduction in affected individuals.
Acknowledgment
This study was supported by the Italian Ministry of Health.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Généthon, http://www.genethon.fr (for microsatellite markers)
- Online Mendelian inheritance in man (OMIM); http://www.ncbi.nlm.nih.gov/Omim (for THC2 [MIM 188000])
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov (for genes and expressed sequence tags physically mapped between markers D10S586 and D10S1639)
- Whitehead Institute/MIT Genome Sequencing Project, http://carbon.wi.mit.edu (for YAC contig, mapping order, and genetic distances of microsatellite markers)
References
- Bellucci S (1997) Megakaryocytes and inherited thrombocytopenias. Bailliere Clin Haematol 10:149–162 [DOI] [PubMed]
- Bithell TC, Didisheim P, Cartwright GE, Wintrobe MM (1965) Thrombocytopenia inherited as an autosomal dominant trait. Blood 25:231–239 [PubMed] [Google Scholar]
- Breton-Gorius J, Favier R, Guichard J, Cherif D, Berger R, Debili N, Vainchenker W, et al (1995) A new congenital dysmegakaryocytopoietic thrombocytopenia (Pris-Trousseau) associated with giant platelet alpha-granules and chromosome 11 deletion at 11q23. Blood 85:1805–1813 [PubMed]
- Derry JMJ, Ochs HD, Francke U (1994) Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 78:635–644 [DOI] [PubMed]
- Dowton SB, Beardsley D, Jamison D, Blattner S, Li FP (1985) Studies of a familial platelet disorder. Blood 65:557–563 [PubMed]
- Fabris F, Cordiano I, Salvan F, Ramon R, Valente M, Luzzatto G, Girolami A (1997) Chronic isolated macrothrombocytopenia with autosomal dominant transmission: a morphological and qualitative platelet disorder. Eur J Haematol 58:40–45 [DOI] [PubMed]
- Ho YC, Otterud B, Legare RD, Varvil T, Saxena R, DeHart DB, Kohler SE, et al (1996) Linkage of a familial platelet disorder with propensity to develop myeloid malignancies to human chromosome 21q22.1–22.2. Blood 87:5218–5224 [PubMed]
- Iolascon A, Perrotta S, Amendola G, Altomare M, Bagnara GP, Del Vecchio ME, Savoia A (1999) Familial dominant thrombocytopenia: clinical, biological and molecular studies. Pediatr Res (in press) [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed]
- Majado MJ, Gonzalez C, Tamayo M, Sanchez A, Moreno M (1992) Effective splenectomy in familial isolated thrombocytopenia. Am J Hematol 39:70 [DOI] [PubMed]
- Najean Y, Lecompte T (1990) Genetic thrombocytopenia with autosomal dominant transmission: a review of 54 cases. Br J Haematol 74:203- 208 [DOI] [PubMed]
- ——— (1995) Hereditary thrombocytopenias in childhood. Semin Thromb Hemost 21:294–304 [DOI] [PubMed]
- Savoia A, Zatterale A, Del Principe D, Joenje H (1996) Fanconi anaemia in Italy: high prevalence of complementation group A in two geographic clusters. Hum Genet 97:599–603 [DOI] [PubMed]
- Strippoli P, Savoia A, Iolascon A, Tonelli R, Savino M, Giordano P, D'Avanzo M, et al (1998) Mutational screening of thrombopoietin receptor gene (c-mpl) in patients with congenital thrombocytopaenia and absent radii (TAR). Br J Haematol 103:311–314 [DOI] [PubMed]
- Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, Brugnoni D, Strina D, et al (1995) X-linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nat Genet 9:414–417 [DOI] [PubMed]