Summary
Choreoathetosis is a major clinical feature in only a small number of hereditary neurological disorders. We define a new X-linked syndrome with a unique clinical picture characterized by mild mental retardation, choreoathetosis, and abnormal behavior. We mapped the disease in a four-generation pedigree to chromosome Xp11 by linkage analysis and defined a candidate region containing a number of genes possibly involved in neuronal signaling, including a potassium channel gene and a neuronal G protein–coupled receptor.
Introduction
At least 178 conditions involving mental retardation have been mapped to the X chromosome. These conditions can be subdivided into syndromic and nonsyndromic forms. In addition to mental retardation, 120 syndromes (53 mapped and 22 cloned) show a characteristic pattern of biochemical, morphological, or neurological manifestations. Fifty-eight nonspecific mental retardation loci (only five cloned) show mental retardation as the only characteristic (Lubs et al. 1999). In this study we report on a family with an X-linked recessive condition that combines mental retardation with neurological symptoms. Five patients from this four-generation family show mild mental retardation in addition to choreoathetosis and abnormal behavior.
Choreoathetosis, the most distinguishing feature of these five patients, is characterized by involuntary, irregular, purposeless, nonrhythmic, abrupt, rapid movements flowing from one part of the body to another (chorea) that blend with slow, writhing, continuous movements (athetosis). It occurs in a variety of clinically and genetically diverse disorders, albeit rarely in familial forms. Only three X-linked mental retardation syndromes showing choreoathetoid movements—Lesch-Nyhan syndrome (MIM 308000), Schimke X-linked mental retardation syndrome (MIM 312840), and Pettigrew syndrome (MRXS5; MIM 304340)—have been described. Lesch-Nyhan syndrome is a rare recessive disorder of purine metabolism due to mutations in the hypoxanthine phosphoribosyl transferase gene (HPRT) (Rossiter et al. 1991) and is characterized by hypercuricemia, spasticity, choreoathetosis, dystonia, self-injurious behavior, and aggression (Nyhan et al. 1967). Childhood-onset choreoathetosis combined with postnatal microcephaly, growth and mental retardation, apparent external ophthalmoplegia, and varying degrees of deafness characterize Schimke X-linked mental retardation (Schimke et al. 1984). Manifestations of Pettigrew syndrome (Xq26) include severe mental retardation, early hypotonia with progression to spasticity, choreoathetosis, seizures, Dandy-Walker malformation, and iron accumulation in the basal ganglia (Pettigrew et al. 1991).
The clinical symptoms of the patients in the family studied do not seem compatible with any of these known disorders. Although the patients have the choreoathetoid movements in common in each of the three disorders, Lesch-Nyhan syndrome and Schimke mental retardation have, in addition, different clinical manifestations, such as the presence of uric acid renal stones and spastic cerebral palsy, and microcephaly and deafness, respectively. Pettigrew syndrome is not likely the same syndrome, since patients in our family do not show hypointense areas typical of iron accumulation in the basal ganglia. Therefore, we hypothesized that, clinically, the condition in this family is unique. We mapped the disease to Xp11 and thus defined a new X-linked syndrome.
Patients and Methods
Patients
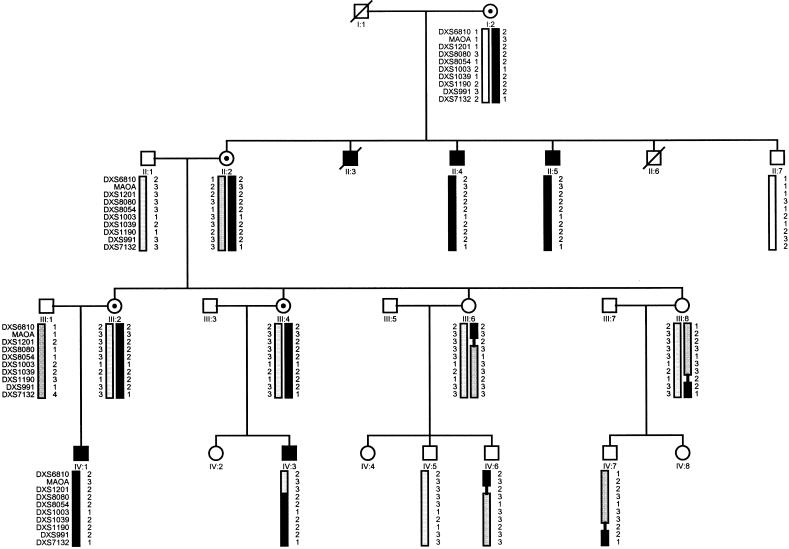
A four-generation Luxembourg family, including five male patients, was studied. The pedigree is consistent with an X-linked recessive inheritance pattern, as indicated by only female-to-male transmissions of the disease and by absence of a clinical picture in obligate carrier females (fig. 1).
Figure 1.
Haplotype analysis on the pedigree. Blackened bars represent the chromosomal region associated with the disease.
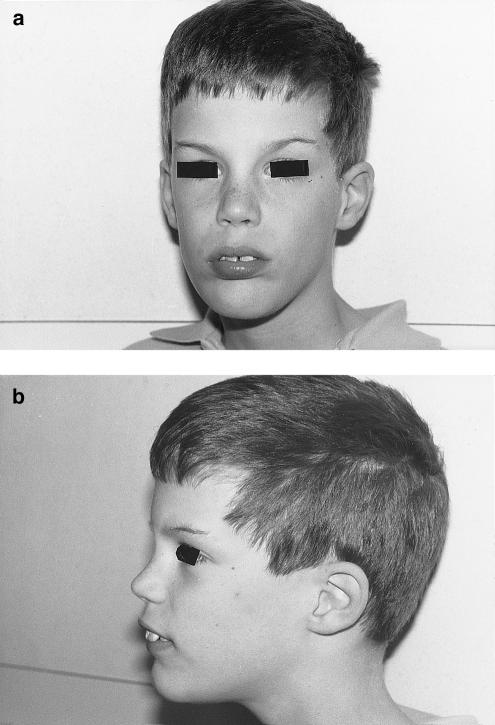
The index patient (IV-I) was born at 35 weeks' gestation and weighed 2300 grams. He showed an axial hypotonia from his first month of life and was able to walk without assistance at the age of 2 years. Involuntary choreoathetoid movements involving mainly the upper limbs and the head started at the age of 1 year and remained throughout the years. At the age of 10 years, his height and cranial circumference were at the 75th percentile and his weight was at the 25th percentile. His language was severely dysarthric, and his syntax was rudimentary, making testing for verbal IQ impossible. Nonverbal IQ was 70 (Leiter scale). A mild spastic hypertonia with hyperflexia was present in the upper and lower limbs. Gait was discretely wide based, with lumbar hyperlordosis. Behavioral problems included periods of aggression and strong agitation. Except for arachnodactily, he did not show any dysmorphic features (fig. 2). Lactate, amino acid, and organic acid profiles, very-long-chain fatty acids, uric acid, copper, ceruloplasmin, and acanthocytes counts were normal. A search for carbohydrate-deficient glycoprotein was negative. No abnormalities were noticed on cranial MRI, fundoscopy, electroretinogram, or positron emission tomography with (18-F) fluorodeoxyglucose. EEG showed a nonspecific slow dysrhythmia. The patient had a normal 46XY karyotype. His cousin (IV-III) showed choreoathetoid movements affecting all four of his limbs and his head from the age of 3 mo. His involuntary movements were more severe than those seen in the index patient. Mental retardation was less severe, affecting primarily his speech, and he showed no behavioral abnormalities. No dysmorphic features were noticed. At the age of 12 years, his weight was at the 3d percentile and his cranial circumference was between the 25th and 50th percentiles. Three uncles (two examined) of the index patient's mother showed similar clinical pictures of involuntary movements in combination with mental retardation. They were reportedly psychotic, suffering from hallucinations and automutilation. Dysmorphic features were restricted to arachnodactily (table 1).
Figure 2.
Frontal (a) and side (b) views of the index patient (IV-1), showing no characteristic dysmorphic features
Table 1.
Clinical Features of the Patients
|
Patient |
||||
| Phenotype | IV:1 | IV:3 | II:4 | II:5 |
| Cognitive ability: | ||||
| MR | Mild | Borderline | Mild | Mild |
| Language | + /- | - | Unknown | Unknown |
| Movement: | ||||
| Choreoathetosis | 1 | +++ | ++ | 5 |
| Spastic hypertonia | 0 | ++ | + | 0 |
| Behavior: | ||||
| Aggressiveness | +++ | - | - | - |
| Agitation | +++ | - | - | - |
| Hallucination | - | - | + | 0 |
| Automutilation | - | - | + | 0 |
| Dysmorphology: | ||||
| Arachnodactily | 0 | - | + | 0 |
DNA and Linkage Analysis
Blood samples were obtained, with informed consent, from the index patient, his cousin, two affected uncles (a third affected uncle has died), obligate carriers, two spouses, and four unaffected males. Genomic DNA was extracted from lymphocytes by standard procedures.
To cover the whole X chromosome, we selected markers from two sets of fluorescent-labeled polymorphic markers. One set contained fluorescent-labeled markers described by Reed et al. 1994 (pter-DXS996-DXS999-DXS451-DMD-DXS538-DXS1068-DXS991-DXS984-qter); a second set contained microsatellite markers from The Cooperative Human Linkage Center fluorescent-labeled human screening set (Weber version 6a) (pter-DXS6807-DXS987-DXS989-DXS6810-DXS7132-DXS6800-DXS6789-DXS6799-DXS6797-DXS6804-DXS1001-DXS1193-qter). All polymorphic markers were genotyped with an ABI 377 sequencer (Applied Biosystems) and analyzed with the aid of GENESCAN version 2.1 and GENOTYPER version 2.0 software. Additional markers of the Xp11 region were analyzed by means of standard autoradiographic techniques.
We calculated two-point LOD scores between the disease and each individual marker, using MLINK of the linkage package 5.1 (Lathrop and Lalouel 1984). The frequency of the disease was estimated at .00001, and equal allele frequencies of .2 were used for each marker.
Results
To define the molecular etiology of the disease in the family studied, we typed a series of polymorphic markers along the X chromosome on all persons from whom blood samples were obtained, except for individual IV-6 who was not available at the time. Linkage of the disease with the X chromosome was excluded except for the region between the markers DXS451 and DXS6789 (table 2). Additional markers from within this region (pter-MAOA-DXS1201-DXS8080-DXS8054-DXS1003-DXS1039-DXS1190-cen) were typed on all family members, including individual IV-6. A maximal two-point LOD score of 2.14 at recombination fraction θ=0 was obtained with markers DXS8080 and DXS8054 (table 2), indicating linkage of the disease with the chromosomal region Xp11. Haplotype analysis of this chromosomal region was performed to identify recombination events and to define a critical disease region. A distal recombination event occurred between markers DXS1201 and DXS8080 (identified in individuals IV-3 and IV-6), and a proximal recombination event occurred between markers DXS1039 and DXS991 (identified in individual IV-7) (fig. 1). Thus, the disease gene could be positioned in a 20-cM interval between markers DXS1201 and DXS991 on The Marshfield Medical Research Foundation X-chromosome map.
Table 2.
Two-Point LOD Scores between the Disease Locus and Markers on the X Chromosome[Note]
|
Two-Point LOD Score at θ = |
|||||||||
| CytogeneticLocationa | Distanceb(cM) | Locus | 0 | .01 | .05 | .1 | .2 | .3 | .4 |
| Xp22.3 | 4.4 | DXS6807 | −∞ | −4.26 | −2.22 | −1.40 | −.66 | −.30 | −.10 |
| Xp22.33 | 8.8 | DXS996 | −∞ | −5.96 | −3.21 | −2.08 | −1.03 | −.49 | −.17 |
| Xp22.2 | 22.4 | DXS987 | −∞ | −2.80 | −1.44 | −.89 | −.39 | −.15 | −.04 |
| Xp22.13 | 24.2 | DXS999 | −∞ | −7.06 | −3.66 | −2.27 | −1.01 | −.42 | −.11 |
| Xp22.11 | 37.5 | DXS989 | −∞ | −3.96 | −1.94 | −1.13 | −.42 | −.12 | .00 |
| Xp22.11 |
Unknown |
DXS451c |
−∞ | −1.11 | −.46 | −.23 | −.06 | −.01 | .00 |
| Xp21.1 | Unknown | DMDc | .9 | .89 | .81 | .72 | .52 | .3 | .09 |
| Xp11 | Unknown | DXS538c | −∞ | .02 | .56 | .65 | .53 | .29 | .07 |
| Xp11.4 | 52.6 | DXS1068 | −∞ | .32 | .87 | .97 | .86 | .59 | .27 |
| Xp11.4 | 63.6 | DXS6810 | 1.15 | 1.13 | 1.02 | .88 | .58 | .28 | .06 |
| Xp11.4 | Unknown | MAOAc | 1.23 | 1.21 | 1.14 | 1.03 | .78 | .49 | .2 |
| Xp11.4 | 63.6 | DXS1201 | −∞ | −.78 | −.14 | .08 | .19 | .15 | .04 |
| Xp11.3 | 67.9 | DXS8080 | 2.14 | 2.09 | 1.93 | 1.71 | 1.25 | .75 | .28 |
| Xp11.3 | 69.9 | DXS8054 | 2.14 | 2.09 | 1.91 | 1.69 | 1.21 | .71 | .25 |
| Xp11.23 | 72.4 | DXS1003 | 1.83 | 1.8 | 1.66 | 1.48 | 1.1 | .7 | .3 |
| Xp11.23 | 83.3 | DXS1039 | 1.83 | 1.8 | 1.66 | 1.48 | 1.1 | .7 | .3 |
| Xp11.21 | 83.3 | DXS1190 | .33 | .33 | .33 | .32 | .28 | .22 | .12 |
| Xp11.21 | 83.3 | DXS991 | −∞ | .32 | .86 | .95 | .08 | .51 | .21 |
| Xq11.11 | 83.3 | DXS7132 | −∞ | 7 | 1.23 | 1.32 | 1.15 | .81 | .39 |
| Xq13.2 |
93.2 |
DXS6800 |
.9 | .89 | .81 | .72 | .52 | .3 | .09 |
| Xq21.33 | 103.6 | DXS6789 | −∞ | −1.97 | −.66 | −.18 | .16 | .22 | .15 |
| Xq21.33 | 107.4 | DXS6799 | −∞ | −3.37 | −1.39 | −.64 | −.06 | .12 | .12 |
| Xq23 | 112.9 | DXS6797 | −∞ | −3.37 | −1.39 | −.64 | −.06 | .12 | .12 |
| Xq23 | 116.2 | DXS6804 | −∞ | −4.26 | −2.22 | −1.39 | −.64 | −.29 | −.10 |
| Xq24 | 130.4 | DXS1001 | −∞ | −4.85 | −2.77 | −1.89 | −1.04 | −.56 | −.23 |
| Xq26.3 | 150.2 | DXS984 | −∞ | −7.36 | −3.95 | −2.55 | −1.26 | −.61 | −.23 |
| Xq28 | 175.3 | DXS1193 | −∞ | −4.85 | −2.77 | −1.90 | −1.05 | −.58 | −.25 |
Note.— The excluded regions are boxed; the critical region is underlined.
Inferred cytogenetic location, according to The Genetic Location Database..
From locus to pter, according to The Marshfield Medical Research Foundation genetic maps.
Positioned according to Reed et al. (1994).
In some X-linked conditions, evidence for skewed X inactivation in carrier females has been described; therefore, we analyzed the methylation of HhaI site near the human androgen-receptor gene in the four female carriers of the family. However, no evidence for skewed X inactivation was found (data not shown).
Discussion
By linkage analysis, we have mapped an X-linked neurological disorder to the short arm of chromosome X in a 20-cM genetic interval between markers DXS1201 and DXS991. Except for mental retardation, patients show a unique clinical picture, including choreoathetosis and abnormal behavior. The genetic interval shows overlap with regions defined for other X-linked neuromuscular syndromes, including Allan-Herndon-Dudley syndrome (MIM 309600), Norrie disease (MIM 310600), optic atrophy 2 (MIM 311050), Wieacker-Wolff syndrome (MIM 314580), and Hamel BCD (Lubs et al. 1999). It also encompasses genes or genetic intervals defined for a metabolic disease (MAOA [MIM 309850]) and for a number of defined mental retardation syndromes, namely Aarskog-Scott syndrome (MIM 305400), Renpenning syndrome 1 (MIM 309500), Prieto syndrome (MRXS2; MIM 309610), Sutherland-Haan syndrome (MRXS3; MIM 309470), Miles-Carpenter syndrome (MRXS4; MIM 309605), Wilson-Turner syndrome (MRXS6; MIM 309585), and Ahmad syndrome (MRXS7) (Lubs et al. 1999). The clinical pictures of these syndromes and of the patients in our family do not overlap except for the Sutherland-Haan syndrome (MRXS3) and Hamel BCD. The Sutherland-Haan syndrome is characterized by mental retardation and spasticity as well as by microcephaly, short stature, and small testes (Sutherland et al. 1988; Martinez et al. 1998). Hamel BCD is characterized by mental retardation with spasticity as well as by blindness, convulsions, hypomethylation, and early death (Lubs et al. 1999). Since our patients do not show signs of microcephaly, short stature, small testes, blindness, convulsions, or early death, it is unlikely that this new syndrome is allelic to these described syndromes, and, therefore, we conclude that the mental retardation/choreoathetosis syndrome in this family is clinically and genetically unique.
Of the genes mapped to the defined region, the genes involved in neural signaling and neurotransmitting are considered potential candidate genes. For instance, point mutations in cationic channel genes are observed in a number of autosomal paroxysmal neurological disorders. Mutations in the potassium channel gene KCNA1 cause episodic ataxia with myokymia (MIM 160120) (Browne et al. 1994); mutations in the sodium channel gene SCN4A cause hyperkalemic periodic paralysis (MIM 170500) and paralysis periodica paramyotonia (MIM 168300) (Rojas et al. 1991); whereas mutations in the calcium channel genes CACNLIA3 and CACNA1A cause hypokalemic periodic paralysis (MIM 170400) (Jurkat-Rott et al. 1994) and episodic ataxia, type 2 (MIM 108500) (Denier et al. 1999), respectively. Three ion channel genes—a calcium channel gene (CACNA1F), a chloride channel gene (CLCN5), and a potassium channel gene (KCND1)—have been mapped to the candidate region. The first two are not likely candidate genes for the syndromic features of our family, since loss of function mutations in CACNA1F cause night blindness, congenital stationary, type 2 (MIM 300071) (Strom et al. 1998), and the expression level of this gene in the brain is low (Fisher et al. 1997). Mutations in the chloride channel gene CLCN5 are found in different renal tubular disorders complicated by nephrolithiasis (Lloyd et al. 1996). The KCND1 gene is a more likely candidate. It encodes a Shal-type potassium channel gene, and the transmembane domain S7 of this gene shows high homology with the corresponding S6 transmebrane domain of the Shaker-type potassium channel KCNA1 mutated in episodic ataxia/myokymia (Browne et al. 1994).
Two presynaptic proteins potentially involved in neurotransmitter release are mapped to the region of interest, synapsin (Yang-Feng et al. 1986) and synaptophysin (Ozcelik et al. 1990). Synapsin I has a possible role in regulation of axonogenesis and synaptogenesis (Li et al. 1995), whereas synaptophysin is one of the major integral membrane proteins of the small electron-translucent transmitter-containing vesicles (Ozcelik et al. 1990). No disease has yet been associated with mutations in one of these genes. However, gene-deficient mice lacking either synapsin (Li et al. 1995) or synaptophysin (Eshkind and Leube 1995) have been constructed and studied, and no mention of choreoathetosis or spasticity in either of the two knockout mice was made. This does not formally exclude these two genes as candidate genes: knockout mice deficient in HPRT synthesis, a mouse model for Lesch-Nyhan syndrome, do not show any evidence of the symptoms characteristic for the human disease, including choreoathetosis, spasticity, or self-mutilation (Engle et al. 1996).
Other potential candidate genes in the region, such as the monoamine oxidases A (MAOA) and B (MAOB), involved in the degradation of biogenic amines serotonin and norepinephrine, and phenylethylamine and dopamine, respectively, map outside the candidate region.
Recently, a novel orphan G protein–coupled receptor gene (GPR34), with high expression in the brain, has been discovered and assigned to the candidate region (Xp11.3) as well as to a nonhomologous chromosome (4p12) by fluorescence in situ hybridization (Marchese et al. 1999). G protein–coupled receptors (GPCRs) mediate signals to the interior of the cell by activation of G proteins. These G proteins, in turn, activate a number of effector proteins and possibly regulate Ras-like GTPase pathways (Bokoch 1996). It has been demonstrated that there is an association between cognitive impairment and a defective signaling pathway depending on Ras-like GTPase in three different types of nonsyndromic mental retardation (Bienvenu et al. 1998; Billuart et al. 1998; D'Adamo et al. 1998), and it has been postulated that GPCRs play a role in behavior and neuropsychiatric disorders (Blum and Noble 1997). Therefore, mutations in GPR34 (Xp11.3) could theoretically explain the phenotype of the patients in our family.
In conclusion, we have defined a thus far unknown neurological syndrome that maps to Xp11. The region contains several interesting candidate genes involved in neuronal signaling. Mutation analysis of these genes may in the future elucidate the molecular defect of these patients. In addition, knowledge of the defective gene may help us to understand more about cognitive function and movement disorders.
Acknowledgments
Part of this study was supported by a grant from the Fund for Scientific Research–Flanders and by a concerted action of the University of Antwerp.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- The Cooperative Human Linkage Center, http://lpg.nci.nih.gov/CHLC (for the fluorescent linkage markers)
- The Genetic Location Database, http://cedar.genetics.soton.ac.uk/public_html/ (for the inferred cytogenetic location of the X-chromosome loci markers)
- The Marshfield Medical Research Foundation, http://www.marshmed.org/genetics (for the genetic map of the X chromosome)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for Lesch-Nyhan syndrome [MIM 308000], Schimke X-linked mental retardation syndrome [MIM 312840], MRXS5 Pettigrew syndrome [MIM 304340], Allan-Herndon-Dudley syndrome [MIM 309600], Norrie disease [MIM 310600], optic atrophy 2 [MIM 311050], Wieacker-Wolff syndrome [MIM 314580], MAOA [MIM 309850], Aarskog-Scott syndrome [MIM 305400], Renpenning syndrome 1 [MIM 309500], Prieto syndrome [MRXS2; MIM 309610], Sutherland-Haan syndrome [MRXS3; MIM 309470], Miles-Carpenter syndrome [MRXS4; MIM 309605], Wilson-Turner syndrome [MRXS6; MIM 309585], episodic ataxia with myokymia [MIM 160120], hyperkalemic periodic paralysis [MIM 170500], paralysis periodica paramyotonia [MIM 168300], hypokalemic periodic paralysis [MIM 170400], episodic ataxia, type 2 [MIM 108500], and night blindness, congenital stationary, type 2 [MIM 300071])
References
- Bienvenu T, des Portes V, Saint Martin A, McDonell N, Billuart P, Carrie A, Vinet MC, et al (1998) Non-specific X-linked semidominant mental retardation by mutations in a Rab GDP-dissociation inhibitor. Hum Mol Genet 7:1311–1315 [DOI] [PubMed]
- Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, Crollius HR, et al (1998) Oligophrenin 1 encodes a rho-GAP protein involved in X-linked mental retardation. Nature 392:923–926 [PubMed]
- Blum K, Noble EP (1997) Handbook of psychiatric genetics. CRC Press, New York [Google Scholar]
- Bokoch GM (1996) Interplay between Ras-related and heterotrimeric GTP binding proteins: lifestyles of the BIG and little. FASEB J 10:1290–1295 [DOI] [PubMed]
- Browne DL, Gancher ST, Nutt JG, Brunt ER, Smith EA, Kramer P, Litt M (1994) Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet 8:136–140 [DOI] [PubMed]
- D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, et al (1998) Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat Genet 19:134–139 [DOI] [PubMed]
- Denier C, Ducros A, Vahedi K, Joutel A, Thierry P, Ritz A, Castelnovo G, et al (1999) High prevalance of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology 52:1816–1821 [DOI] [PubMed]
- Engle SJ, Womer DE, Davies PM, Boivin G, Sahota A, Simmonds HA, Stambrook PJ, et al (1996) HPRT-APRT-deficient mice are not a model for Lesch-Nyhan syndrome. Hum Mol Genet 5:1607–1610 [DOI] [PubMed]
- Eshkind LG, Leube RE (1995) Mice lacking synaptophysin reproduce and form typical synaptic vesicles. Cell Tissue Res 282:423–433 [DOI] [PubMed]
- Fisher EF, Ciccodicola A, Tanaka K, Curci A, Desicato S, D'Urso M, Craig IW (1997) Sequence-based exon prediction around the synaptophysin locus reveals a gene-rich area containing novel genes in human proximal Xp. Genomics 45:340–347 [DOI] [PubMed]
- Jurkat-Rott K, Lehmann-Horn F, Elbaz A, Heine R, Gregg RG, Hogan K, Powers PA, et al (1994) A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet 3:1415–1419 [DOI] [PubMed]
- Lathrop GM, Lalouel JM (1984) Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, et al (1995) Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA 92:9235–9239 [DOI] [PMC free article] [PubMed]
- Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, et al (1996) A common molecular basis for three inherited kidney stone diseases. Nature 379:445–449 [DOI] [PubMed]
- Lubs HA, Chiurazzi P, Arena JF, Schwartz C, Tranebjaerg L, Neri G (1999) XLMR genes: update 1998. Am J Med Genet 83:237–247 [PubMed]
- Marchese A, Sawzdargo M, Nguyen T, Cheng R, Heng HH, Nowak T, Im DS, et al (1999) Discovery of three novel orphan G-protein-coupled receptors. Genomics 56:12–21 [DOI] [PubMed]
- Martinez F, Tomas M, Millan JM, Fernandez A, Palau F, Prieto F (1998) Genetic localisation of mental retardation with spastic diplegia to the pericentromeric region of the X chromosome: X inactivation in female carriers. J Med Genet 35:284–287 [DOI] [PMC free article] [PubMed]
- Nyhan W, Olivier W, Lesch M (1967) A familial disorder of uric acid metabolism and central nervous system function. J Pediatr 1:5–13 [DOI] [PubMed] [Google Scholar]
- Ozcelik T, Lafreniere RG, Archer BT 3d, Johnston PA, Willard HF, Francke U, Sudhof TC (1990) Synaptophysin: structure of the human gene and assignment to the X chromosome in man and mouse. Am J Hum Genet 47:551–561 [PMC free article] [PubMed]
- Pettigrew AL, Jackson LG, Ledbetter DH (1991) New X-linked mental retardation disorder with Dandy-Walker malformation, basal ganglia disease, and seizures. Am J Med Genet 38:200–207 [DOI] [PubMed]
- Reed PW, Davies JL, Copeman JB, Bennett ST, Palmer SM, Pritchard LE, Gough SC, et al (1994) Chromosome-specific microsatellite sets for fluorescence-based, semi-automated genome mapping. Nat Genet 7:390–395 [DOI] [PubMed]
- Rojas CV, Wang JZ, Schwartz LS, Hoffman EP, Powell BR, Brown RHJ (1991) A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature 354:387–389 [DOI] [PubMed]
- Rossiter BJF, Edwards A, Caskey CT (1991) HPRT mutation and the Lesch-Nyhan syndrome. In: Brosius J, Freneau R (eds) Molecular genetic approaches to neuropsychiatric disease. Academic Press, New York [Google Scholar]
- Schimke RN, Horton WA, Collins DL, Therou L (1984) A new X-linked syndrome comprising progressive basal ganglion dysfunction, mental and growth retardation, external ophthalmoplegia, postnatal microcephaly and deafness. Am J Med Genet 17:323–332 [DOI] [PubMed]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, et al (1998) An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet 19:260–263 [DOI] [PubMed]
- Sutherland GR, Gedeon AK, Haan EA, Woodroffe P, Mulley JC (1988) Linkage studies with the gene for an X-linked syndrome of mental retardation, microcephaly and spastic diplegia (MRX2). Am J Med Genet 30:493–508 [DOI] [PubMed]
- Yang-Feng TL, DeGennaro LJ, Francke U (1986) Genes for synapsin I, a neuronal phosphoprotein, map to conserved regions of human and murine X chromosomes. Proc Natl Acad Sci USA 83:8679–8683 [DOI] [PMC free article] [PubMed]