Summary
Two familial and seven sporadic patients with neurofibromatosis 1—who showed dysmorphism, learning disabilities/mental retardation, and additional signs and carried deletions of the NF1 gene—were investigated by use of a two-step FISH approach to characterize the deletions. With FISH of YAC clones belonging to a 7-Mb 17q11.2 contig, we estimated the extension of all of the deletions and identified the genomic regions harboring the breakpoints. Mosaicism accounted for the mild phenotype in two patients. In subsequent FISH experiments, performed with locus-specific probes generated from the same YACs by means of a novel procedure, we identified the smallest region of overlapping (SRO), mapped the deletion breakpoints, and identified the genes that map to each deletion interval. From centromere to telomere, the ∼0.8-Mb SRO includes sequence-tagged site 64381, the SUPT6H gene (encoding a transcription factor involved in chromatin structure), and NF1. Extending telomerically from the SRO, two additional genes—BLMH, encoding a hydrolase involved in bleomycin resistance, and ACCN1, encoding an amiloride-sensitive cation channel expressed in the CNS—were located in the deleted intervals of seven and three patients, respectively. An apparently common centromeric deletion breakpoint was shared by all of the patients, whereas a different telomeric breakpoint defined a deletion interval of 0.8–3 Mb. There was no apparent correlation between the extent of the deletion and the phenotype. This characterization of gross NF1 deletions provides the premise for addressing correctly any genotype-phenotype correlation in the subset of patients with NF1 deletions.
Introduction
Neurofibromatosis type 1 (NF1) is an autosomal dominant condition (MIM 162200) mapped to chromosome 17q11.2. Following positional cloning of the NF1 gene (Cawthon et al. 1990; Viskochil et al. 1990; Wallace et al. 1990), the whole cDNA has been assembled and sequenced. However, despite advances in the definition of NF1-gene structure (Li et al. 1995), that the complete genomic sequence is not yet available is one of the factors that hamper the identification of NF1 mutations. The mutation rate of NF1 (3 × 10−5 to 1 × 10−4) is higher than that of most human genes (Stevenson and Kerr 1967; Vogel and Motulsky 1997) and accounts for the substantial fraction (>50%) of de novo patients with NF1. According to the NNFF International NF1 Genetic Analysis Consortium, 246 NF1 mutations had been characterized through February 1999, including 18 deletions of the entire gene (NF1 International Consortium 1999). Although the majority of small NF1 deletions/point mutations are of paternal origin, large NF1-gene deletions arise preferentially on the maternal chromosomes, which suggests that the molecular mechanism involved in the generation of the deletion is recombination (Lazaro et al. 1996). No genotype-phenotype correlation has so far been identified for NF1, with the exception that deletions of the entire gene have been associated with a severe phenotype that includes dysmorphism, mental retardation, and the early onset of a large number of neurofibromas (Kayes et al. 1994; Wu et al. 1995, 1997a, 1997b; Lazaro et al. 1996; Leppig et al. 1996; Riva et al. 1996; Upadhyaya et al. 1998). A study aimed at establishing whether the phenotypic traits usually associated with complete gene deletions might be predictive for the deletion subset confirmed this trend but pointed out that the presence of a deletion cannot be predicted solely on the basis of clinical phenotype (Tonsgard et al. 1997). Precise characterization of the extent of deletion is a crucial step toward addressing genotype-phenotype correlations and investigating whether common deletion breakpoints are found in different patients. FISH has proved to be useful for this purpose and also permits the detection of somatic mosaicism, which may modulate NF1 phenotypic expression (Ainsworth et al. 1997). We have recruited a group of nine patients with NF1, including seven sporadic and two familial cases, who were known either, from previous FISH characterization, to carry large deletions (Wu et al. 1995; Riva et al. 1996; Wu et al. 1997a, 1997b) or, from LOH analysis, to carry intragenic deletions. We have characterized the different deletions by FISH, using (1) YAC/PAC clones of a contig, assembled in our laboratory, that is centered on the NF1 gene and encompasses >7 Mb and (2) locus-specific probes generated from YAC clones by means of a recently described procedure (Riva et al. 1999). Using this combined FISH approach, we have been able to identify an SRO, to estimate the extent of the deletions, and to define their boundaries.
Methods
Patients
Recruitment
Recruitment of the NF1-deleted patients was performed by FISH analysis for patients 363 and 992 and by loss-of-heterozygosity (LOH) study for patients M.M., N.C., and N.R. (N.C.'s father), for all of whom no published information is available. The clinical phenotype and preliminary FISH characterization have been reported for patients 323 and 665 (Wu et al. 1995), B.L. (Riva et al. 1996), 870 (Wu et al. 1997a), and 940 and 629 (Wu et al. 1997b). The clinical phenotype and background laboratory characterization of all of the patients are given in table 1.
Table 1.
Clinical Features of Patients with NF1
|
Status in Patienta |
|||||||||||
| 870b | 363 | 992 | 629c | 940 | 665 | 323 | M.M. | N.C.c | N.R.b | B.L. | |
| Sex | Female | Male | Male | Male | Male | Male | Female | Female | Female | Male | Male |
| Age at diagnosis | 7 mo | 8 years 3 mo | 19 mo | 3 years | Unknown | 5 years | 2 years | 3 years 7 mo | 25 years | 47 years | 7 years |
| Age at evaluation | 7 mo | 12 years 4 mo | 19 mo | 15 years | 45 years | 15 years | 13 years | 7 years 10 mo | 25 years | 47 years | 13 years 5 mo |
| CLS | + | + | + | + | + | + | + | + | + | + | + |
| Freckling | − | + | + | + | + | + | + | + | + | + | + |
| Lisch nodules | − | + | − | + | + | − | − | + | − | + | − |
| Neurofibromas | − | + | + | + | + | + | + | − | + | + | − |
| Plexiform neurofibromas | + | − | − | + | − | − | − | + | − | − | − |
| NF1-related tumor | − | + | + | − | − | − | − | − | − | − | − |
| Macrocephaly | − | − | − | + | + | − | + | − | + | + | − |
| Large hands/feet | − | − | − | + | + | + | − | + | − | − | − |
| Facial dysmorphism | + | + | + | + | + | + | + | + | − | − | + |
| Major malformations | − | − | − | − | Mitral-valve prolapse | Pulmonic stenosis | Bilateral iris coloboma | Pulmonic stenosis | − | − | − |
| Other | Facial nerve palsy | − | Diaphysis;d flaring of rib cage | Seizures | − | Seizures | Metatarsus adductus | − | − | − | Genu valgum; abnormalities of the lower limbs and feet; skeletal anomalies |
| Mental retardatione | − | Not tested | − | Mild | − | Severe | Severe | Borderline | − | − | Severe |
+ = Presence of clinical sign; − = absence of clinical sign.
Mosaic patient.
Familial case.
Broadening diaphysis of humerus and femur.
Based on IQ test.
YAC and PAC preparation
The YAC clones 884g6, 810f5, 819d12, 909f1, 947g11, 815b11, 946h1, 747h12, 731h4, and 728f1 from the CEPH library (Chumakov et al. 1995) and PAC 1002g3 from the RPCI-5 library were obtained from the Department of Biological and Technological Research resource center at San Raffaele Hospital in Milan. The cultures from single colonies were grown in selective AHC medium and were resuspended in 100-μl LMP (Biorad) agarose plugs containing 7 × 107 cells.
The Alu 153, 154, 451, and 450 primers (Breen et al. 1992) were used for the specific amplification of YAC DNA from a total-yeast-DNA preparation. The PCR reactions contained 2 μl of the molten yeast plug as a template; 1 μM each of Alu 153, 154, 451, and 450 primers; 1 U of Taq polymerase (Bioline); 1.5 mM MgCl2; and 200 μM each of dNTP, in a total reaction volume of 50 μl. Thirty-five PCR cycles were performed at 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min.
Preparation of Locus-Specific Probes
Previously described in detail by Riva et al. (1999), the method is based on two consecutive reactions using YAC clones containing the loci of interest. In brief, using a specific primer, single-strand DNA polymerization is performed (35 cycles at 94°C for 30 s, at optimal annealing temperature for 1 min, and at 72°C for 2 min), yielding DNA fragments of 0.6–5 kb. The use of two locus-specific primers—extending the polymerization, in independent reactions, in two opposite directions—allows coverage of 10 kb around the selected locus. An aliquot (1 μl) of locus-specific single-strand product is then used as a template in four independent PCRs performed with four different Alu primers combined with the locus-specific primer (35 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min). The Alu PCR products were then collected and precipitated. The probes specific for the following 17q11.2 genes/sequence-tagged sites (STSs), listed in centromere-to-telomere order, were generated from the YACs to which they are anchored (see table 3): NOS2A, D17S1317, 64381, SUPT6H, NF1 [IVS27, IVS38, 3′ UTR], D17S145, BMLH, D17S1863, ACCN1, D17S1558, D17S798, 64384, 64382, and MCP-3. The primer pairs for all of the loci are given in Genome Database (1999). The primers specific for the novel STSs 64381, 64384, and 64382 are, respectively, 5′-gcccggccacggctaactt-3′ and 5′-gattacggaattaatgaggcc-3′, 5′-atcttgttgggtatgtaagc-3′ and 5′-ccttccgttaaatgctctg-3′, and 5′-gatggttagcagctttgctc3′ and 5′-ggttgcccatattgagtctg-3′.
Table 3.
FISH Analysis by Locus-Specific Probes
|
Finding at Locus |
||||||||||||||||
| y819d12 (1.4 Mb) |
y909f1 (.8 Mb) |
y947g11 (1.6 Mb) |
y815b11 (1.5 Mb) |
y946h1 (1.2 Mb) |
y728f1 (1.3 Mb) |
|||||||||||
| Patient | NOS2Aa | S1317b | 64381b | SUPT6Hc | int27 | int38 | 3′UTR | S145 | BLMHd | S1863 | ACCN1e,b | 1558 | S798 | 64384 | 64382 | MCP-3f |
| 870 | + | + | − | − | − | − | − | + | + | + | + | + | + | + | + | + |
| 363 | + | ND | − | − | − | − | − | − | + | + | + | + | + | + | + | + |
| 992 | + | ND | − | − | − | − | − | − | − | + | + | + | + | + | + | + |
| 665 | + | + | − | − | − | − | − | − | − | + | + | + | + | + | + | + |
| 940 | + | ND | ND | − | − | − | − | − | − | − | ND | + | + | + | + | + |
| 323 | + | ND | − | − | − | − | − | − | − | − | + | + | + | + | + | + |
| N.R. | + | + | − | − | − | − | − | − | − | − | − | + | + | + | + | + |
| M.M. | + | + | − | − | − | − | − | − | − | − | − | − | + | + | + | + |
| B.L. | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
NOS2A = nitric oxide synthase 2a.
ND = no data.
SUPT6H = suppressor of Ty 6, homologue of S. cerevisiae.
BMLH = bleomycin hydrolase.
ACCN1 = cation-channel amiloride-sensitive neuronal 1.
MCP-3 = monocyte chemotactic protein 3.
FISH
The precipitated DNAs were resuspended in 100 μl of Tris 10 mM EDTA 1 mM, and 1 μg of each probe was labeled with digoxigenin-dUTP (Boehringer Mannheim) by use of a nick-translation kit (Boehringer Mannheim). One hundred nanograms of labeled probe were precipitated with 10 mg of salmon sperm and 5 μg of Cot-1 DNA (Boehringer Mannheim) and were resuspended in 15 μl of hybridization buffer (50% formamide, 2 × SSC, 10% dextran sulfate, 0.1% Tween 20). The probes were denatured at 72°C for 7 min and then preincubated at 37°C for 30 min. Chromosomal spreads from peripheral blood lymphocytes were denatured in 70% formamide, 2 × SSC for 2 min and then were immediately dehydrated in the ethanol series (70%, 90%, and 100%).
The FISH experiments were done according to standard procedure (Lichter and Cremer 1992). Only the first phase of the detection protocol was performed with the fluorescein-labeled detection of digoxigenin-labeled probes (Oncor). The chromosomes were counterstained with propidium iodide (0.6 μg/ml) in antifade (Oncor) and then were visualized by means of a Leitz DM-RB microscope equipped for DAPI and FITC/TRITC epifluorescence optics. The images were captured with a CCD camera (3CCD camera C5810; Hamamatsu) and visualized by Highfish software (Casti Imaging).
Results
All of the 11 patients included in this study had NF1 diagnosed by conventional clinical criteria. Six had a preliminary FISH characterization that indicated a large deletion, and two (M.M. and N.R's daughter) were found to be hemizygous by means of LOH segregation analysis at NF1 IVS27Ac28.4 and IVS38TG53.0 microsatellites. All of the patients had a severe NF1 phenotype with a number of other clinical features (table 1). Mental retardation, as assessed by IQ testing, ranges from severe (in patients 665, 323, and B.L.) to mild (629) or borderline (M.M.). It is worth noting that, of the five patients not showing mental retardation, 870 and N.R. were found by previous (Wu et al. 1997a) or present FISH characterization to be mosaics. As shown in table 1, craniofacial dysmorphisms were present in nine patients: facial photographs have been published for patients 323 and 665 (Wu et al. 1995), B.L. (Riva et al. 1996), 870 (Wu et al. 1997a), and 629 and 940 (Wu et al. 1997b). In joint evaluation of reported and novel cases, coarse facial features were displayed by 363, 992, and B.L.; down-slanting and bulbous nasal tips were shared by patients 870, 992, 629, 940, 323, M.M., and B.L.. Patient 992 also showed epicanthic folds and low-set posteriorly rotated ears, features also present in patient 323, who had prominent forehead, flame nevus, and broad neck (Wu et al. 1995). Patient M.M. had mild malar hypoplasia and mild pterygium, as did B.L., who also displayed prominent supraorbita (Riva et al. 1996). Common additional signs were large hands and feet, whereas skeletal abnormalities were present in three patients (table 1). The most severe phenotype was detected in B.L., whose characterization has been reported by Riva et al. (1996). Patients 940 and N.R. transmitted NF1 to their children, whose clinical signs are also reported in table 1. All the de novo patients, as well as the parents of the two familial cases, underwent FISH analysis on lymphocyte metaphases, by a stepwise procedure. First, mega-YACs and PACs were used to identify the clones included in the deletion interval (giving a negative FISH signal) or spanning the deletion breakpoints (giving either a decreased or a positive FISH signal). Then, locus-specific probes were generated from the YACs by means of a previously reported procedure (Riva et al. 1999), allowing the extent of the deletion and its breakpoints to be precisely defined by means of the presence of negative or positive FISH signals.
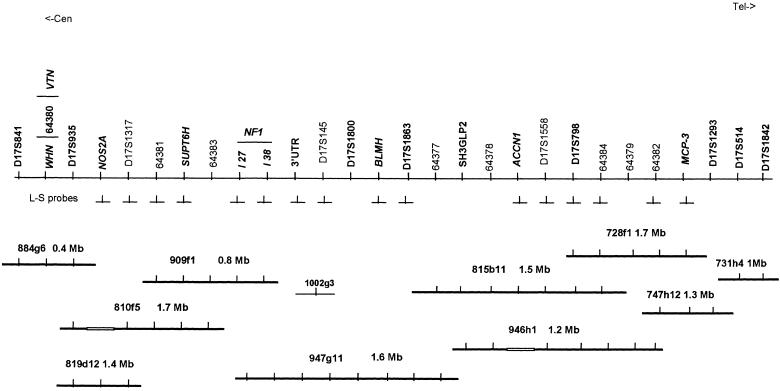
Figure 1 shows a schematic physical map of the region centered on the NF1 gene and extending from D17S841 (the most centromeric marker) to D17S1842 (the most telomeric marker). On this map, the known genes and STSs are ordered from centromere to telomere, according to their anchorage to the YACs and PACs, which are reciprocally aligned below them. The selected YACs are part of a 17q11.2 YAC/PAC contig consisting of 23 YACs and eight PACs and covering >7 Mb in a genetic interval of 9 cM (L. Corrado, P. Riva, M. Venturin, A. Bentivegna, C. Gervasini, and L. Larizza, unpublished data). For each clone, the size and assessed deletions are indicated to approximate the extent of the target region. Sixteen locus-specific probes targeting six genes (NOS2A, SUPT6H, NF1, BLMH, ACCN1, MCP-3) and eight D-loci are indicated between the corresponding loci and the YACs from which they were generated.
Figure 1.
Schematic physical map of the region centered on the NF1 gene and extending from D17S841 to D17S1842. Genes (boldface italics), STRs (boldface), and STSs (normal font) are ordered from centromere to telomere. Ten YACs (thicker lines) and one PAC (thinner line) are shown, selected from a 17q11.2 contig comprising 23 YACs and 8 PACs and covering ∼7 Mb in a genetic interval of 9 cM. Sixteen locus-specific probes (⊥) are also shown, under the corresponding loci between the map and the YACs from which they were generated.
Table 2 shows the results obtained, on the patients with NF1 deletions, by means of FISH of the selected YACs and PAC, which are entered in the table, from left to right, in centromere-to-telomere order. As can be seen, YAC 909f1 and PAC 1002g3 did not give a signal on one chromosome 17 in all of the patients, indicating the deletion of the target regions. YAC 947g11 had a decreased signal on one chromosome 17 in most patients (6/9) and did not give any signal in patients N.R., M.M., and B.L. A decreased signal on one 17 homologue was also observed for YAC 810f5 in two patients (940 and 323). A lack of signal and a decreased signal were observed for YAC 815b11 and YAC 728f1, respectively, in patient B.L., who thus bears the largest deletion.
Table 2.
FISH Analysis by YACs/PAC
|
Signal for YAC/PAC (of size)a |
||||||||
| Patient | y884g6 (.4 Mb) | y810 f5 (1.7Mb) | y909 f1 (.8Mb) | p1002g3 (60 kb) | y947 g11 (1.7 Mb) | y815 b11 (1.5 Mb) | y728f1 (1.7 Mb) | y747h12 (1.3 Mb) |
| 870 | + | + | − | − | +/− | + | + | + |
| 363 | + | + | − | − | +/− | + | + | + |
| 992 | + | + | − | − | +/− | + | + | + |
| 665 | + | + | − | − | +/− | + | + | + |
| 940 | + | +/− | − | − | +/− | + | + | + |
| 323 | + | +/− | − | − | + | + | + | + |
| N.R. | + | + | − | − | − | + | + | + |
| M.M. | + | + | − | − | − | + | + | + |
| B.L. | + | + | − | − | − | − | +/− | + |
+ = Positive finding on both chromosomes 17; − = negative finding on one chromosome 17; +/− = signal decreased as compared with that on the homologue.
To refine the deletion breakpoints and better estimate the size of the deletions, a further set of FISH experiments was performed, with use of the locus-specific probes diagrammed in figure 1. From centromere to telomere along the contig YACs used in the initial FISH characterization, FISH probes specific for NOS2A and D17S1317 were generated from y819d12, to which they are anchored. SUPT6H- and 64381-specific probes were produced from y909f1. The probes targeted on anonymous DNA fragments such as D17S145 and D17S1863, intragenic NF1 markers, and the BLMH gene were developed from y947g11. The two STSs D17S1558 and D17S798 and the ACCN1 gene were generated from y815b11, STSs 64384 and 64382 were obtained from y946h1, and an MCP-3 specific probe was generated from y728f1. As outlined in table 3, these second-round FISH experiments allowed us to map the distal deletion breakpoints for all of the patients except patient 940. From centromere to telomere along the interval that includes the 3′ breakpoints, D17S145 is the telomeric boundary of the deletion in patient 870. The distal deletion limit could be identified at the BLMH gene in patient 363, at D17S1863 in patients 992 and 665, at ACCN1 in patient 323, at D17S1558 in N.R., and at D17S798 in M.M. Patient B.L. was found to have deletions at STSs 64384 and 64382. MCP-3, anchored to y728f1 at the telomeric portion of the contig, is present in all patients, including B.L., whose distal breakpoint is fixed to this gene. With regard to the proximal deletion breakpoint, D17S1317 appears to be the centromeric boundary in most patients. As indicated in table 3, patients 363, 992, 940, and 323 could not be fully investigated, because of shortage of material.
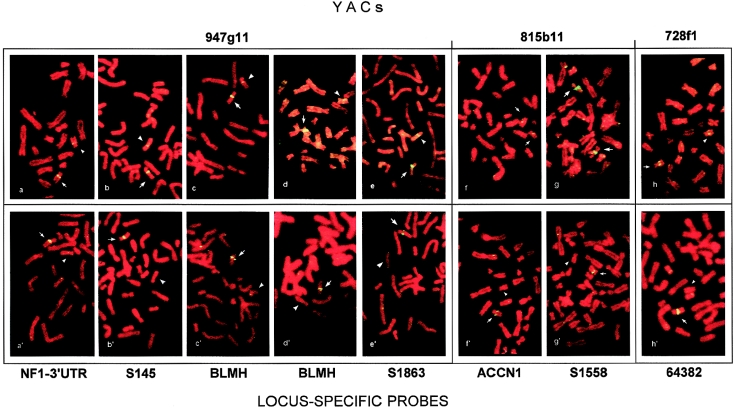
In figure 2, representative examples of the higher resolution of locus-specific probes are shown, in comparison with whole-YAC probes, to define the telomeric boundaries of the deletions of eight patients. For each patient, the upper panel shows the results of YAC FISH, and the lower panel shows the results of FISH using the most telomeric locus-specific probe included in the deletion. As can be seen, FISH of y947g11 gave a decreased hybridization signal in the patients whose deletions could be differentiated after FISH with NF1 3′ UTR–, S145-, BLMH-, and S1863-specific probes (table 3 and fig. 2a–e and a′–e′). A shift from YAC 815b11 to ACCN1- and S1558-specific probes made it possible to differentiate the apparently similar deletions in patients N.R. and M.M. (table 3 and fig. 2f–g and f′–g′). Probe 64382 targeted the last telomeric sequence deleted in B.L., allowing us to resolve the 1.7-Mb y728f1 that gave a decreased signal in the same patient (table 3 and fig. 2h and h′).
Figure 2.
Top, FISH performed with YACs mapped at the telomeric deletion boundary of patients 870, 363, 992, 665, 323, N.R., M.M., and B.L. (a–h, respectively). Bottom, FISH with the most telomeric locus-specific probes included in patients' deletions (a′–h′, respectively). On metaphases from patients (a–e), FISH with YAC 947g11 gives a decreased signal on one chromosome 17 (arrowhead) in respect to the homologue (arrow): each patient's deletion can be differentiated by locus-specific–probe FISH, the most telomeric deleted probe being NF1-3′ UTR for patient 870 (a′), S145 for patient 363 (b′), BLMH for patients 992 and 665 (c′ and d′, respectively) and S183 for patient 323. FISH with YAC 815b11 gives a signal of similar intensity on both chromosomes 17 of patients N.R. and M.M. (f and g, respectively), whose deletions can be differentiated by probes ACCN1 and S1558 respectively (f′ and g′, respectively). Patient B.L. (h) shows a decreased signal on one chromosome 17 with YAC 728f1 (arrowhead) but is deleted up to the 64382 locus-specific probe (h′).
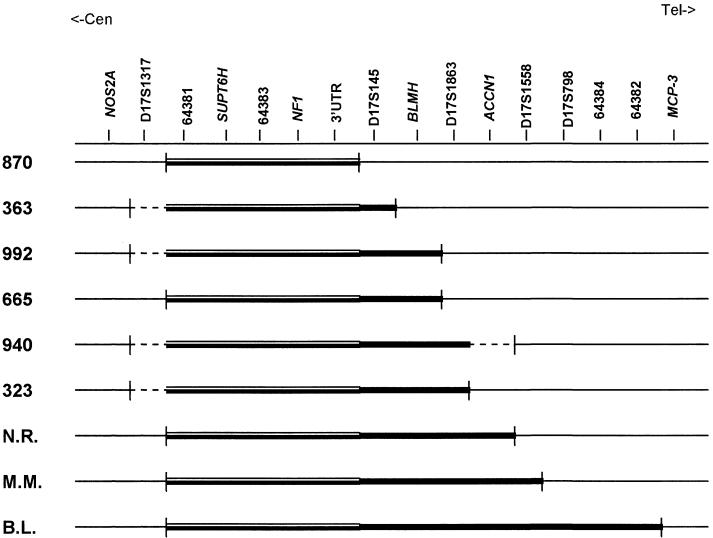
Figure 3 compares the deletions carried by the nine patients, by aligning each one along the genomic region extending from NOS2A to MCP-3. As can be seen, an SRO including the SUPT6H gene 5′ to NF1 could be identified. On the basis of the size of the covering YAC 909f1, the length of the SRO was calculated as being ∼0.8 Mb. All of the patients who could be characterized by locus-specific probes generated from the most centromeric y819d12 were found to share D17S1317 as the proximal boundary, whereas the telomeric boundaries of the deletions were very different, from that of patient 870, whose deletion overlaps the SRO (0.8 Mb), to that of patient B.L., whose deletion can be estimated as ∼3 Mb. The deletion in patient 665 could be sized as being 2 Mb, that of patients N.R. and M.M. as being 2.3 Mb. Because of the low-resolution mapping of the centromeric breakpoint, the extent of the deletion was estimated as 0.8–1.2 Mb in patient 363 and as 1–1.5 Mb in patients 992 and 323. The size of the deletion in patient 940 was very approximately estimated as 1.8–2.8 Mb, because of the poor definition of both breakpoints.
Figure 3.
Deletions carried by the nine patients, compared in terms of their alignment along the 17q11.2 region extending from NOS2A to MCP-3. The locus-specific probes are ordered from centromere to telomere. The SRO that includes the SUPT6H gene 5′ to NF1 is represented by a double line and the overall deleted region is represented by a thicker line. All of the patients who could be characterized by locus-specific probes generated from the most centromeric y819d12 were found to share D17S1317 as a proximal boundary, but all of the deletions are differentiated at the telomeric boundary. The dashed lines point to regions that YAC FISH showed as spanning the deletion breakpoints but that may not be included in the deletion interval.
Discussion
We have investigated 11 patients with NF1 who had a complex phenotype and gross deletions and who represent the largest subgroup of such unusual patients so far investigated by FISH analysis. Our aims were to identify the SRO and extent of the deletion and to assess possible common deletion breakpoints. We used a stepwise FISH procedure consisting of YAC FISH followed by the high-resolution FISH technique using locus-specific probes (Riva et al. 1999). Associations between deletions spanning the NF1 gene and the presentation of complex NF1 have been described and commented on in several studies (Kayes et al. 1994; Wu et al.1995, 1997a, 1997b; Leppig et al. 1996; Riva et al. 1996; Upadhyaya et al. 1996, 1998). One study reviewing 16 patients with NF1 who carried large deletions (12 from the literature and 4 of novel description) has raised the question as to whether NF1-gene deletions lead to a characteristic phenotype (Tonsgard et al. 1997). The authors of that report concluded that, although large NF1 deletions are relatively frequent in patients with facial dysmorphism, mental retardation/learning disabilities, and an early onset of multiple neurofibromas, the presence of the deletion cannot be predicted solely on the basis of the clinical phenotype.
As shown in table 1, most of our patients (9/11) presented dysmorphism, although no single dysmorphic trait was shared by all of them. Nine of the patients developed neurofibromas, but the onset did not appear to be any earlier than that in classical NF1. According to IQ tests, 4 (36%) of our 11 patients were mentally retarded, a finding consistent with the higher incidence of mental retardation in the subgroup of NF1-deletion patients (Tonsgard et al. 1997) than in patients with classic NF1 (8%–10%) (Ferner 1994). Cardiovascular anomalies, observed in 2% of patients with NF1 (Friedman and Birch 1997) and in 3 of our 11 patients, may also have a higher incidence in NF1-deletion patients. In agreement with previous findings (Tonsgard et al. 1997), our patients generally have a severe phenotype, but this did not lead to an early clinical diagnosis of NF1. Our study also confirms the conclusion of Tonsgard et al. that the phenotype may not always predict an extensive gene deletion, as exemplified by our patient N.C. Interestingly, the father of this patient (N.R.), who was included in the study following the detection of LOH at intragenic NF1 markers, proved, on FISH characterization, to be a mosaic, and the same was true for the mother of the familial case reported in the review cited above (Tonsgard et al. 1997). Previous studies have indicated that familial NF1 may contribute to the subgroup of NF1-deletion patients (Tonsgard et al. 1997; Wu et al. 1997b), and this is confirmed by our study. Therefore, patients with both sporadic and familial NF1 who have a severe phenotype should be taken into account in the search for gross deletions. Furthermore, the relatively mild phenotype of patients with mosaic NF1, which is only detectable by FISH methods, may contribute to the underdetection of NF1 deletions.
The characterization of the few NF1-deletion patients reported in the literature has mainly been based on FISH analysis with YAC and cosmid clones mapped to the NF1 gene and its flanking regions. However, because of the lack of a refined physical map of 17q11.2, this characterization could not be made at high resolution. For the current FISH analysis, we used ordered clones belonging to a >7-Mb YAC/PAC contig that we have recently constructed (Corrado et al. 1999; L. Corrado, P. Riva, M. Venturin, A. Bentivegna, C. Gervasini, and L. Larizza, unpublished data). This contig is centered on the NF1 gene and is distally linked to the chemokine contig that has also been mapped at 17q11.2 (Naruse et al. 1996). Using this resource made it possible to cover nine different deletions in their entirety (table 2). Three deletion subsets were identified, differentiated mainly at the telomeric boundary. A further refinement was then achieved with a new tool, consisting of the “ad hoc” generation of locus-specific probes from YAC clones encompassing the deletion boundaries, that resolved apparently similar deletions and showed that they were differentiated at the telomeric boundary (fig. 2 and table 3). The SRO could also be precisely identified (fig. 3), and its gene content could be established on the basis of the gene mapping of the YAC/PAC contig. The SUPT6H gene encoding a transcription factor involved in chromatin structure (Chiang et al. 1996) is the only extra-NF1 gene lying within the SRO (fig. 3). It is still unknown whether SUPT6H is a dosage-sensitive gene, haploinsufficiency of which contributes to the complex NF1 phenotype.
Centromerically, from the SRO, the first mapped gene is NOS2A, which was found, in all nine patients, not to be deleted (fig. 3). Two additional genes, BLMH and ACCN1, were found to be located in the deleted interval of seven and three patients, respectively (fig. 3). BLMH encodes a hydrolase involved in bleomycin resistance (Lazo and Humphreys 1983), which has been associated with an increased risk of Alzheimer disease (Montoya et al. 1998), whereas ACCN1 encodes an amiloride-sensitive cation channel expressed in the CNS (Garcia-Anoveros et al. 1997). A common centromeric breakpoint appears to characterize all of the patients whose deletions do not include the NOS2A gene and the nearby marker D17S1317 (table 3 and fig. 3). More-precise mapping of the centromeric deletion breakpoints could not be pursued in four cases.
The telomeric breakpoints were all different, except in patients 992 and 665. The deletion boundary in patient 940 was fixed to D17S1558, but the 5′ ACCN1 was not tested, because of inadequate material. The largest deletion, with a breakpoint between 64382 and MCP-3, was found in patient B.L.; the mapping of his telomeric breakpoint coincides with the 17q breakpoint underlying the constitutional translocation t(1;17) found in a neuroblastoma patient (Van Roy et al. 1997). More generally, the 17q12-21 region clusters the 17q breakpoints of t(1;17), a cytogenetic marker associated with the development of neuroblastoma and found in a number of neuroblastoma tumors and cell lines (Van Roy et al. 1997). The involvement of this chromosomal region in constitutional and cancer breakpoints suggests the presence of DNA-sequence motifs that might predispose to rearrangement.
The finding of a common centromeric breakpoint in all of our patients may be caused by the structural features of the involved regions. Over the past few years, many microdeletion syndromes have been defined at the molecular level, and, in some of these syndromes, the commonly deleted region has been found to be flanked by low-copy-repeat sequences (Lupsky 1998). The sharing of a common centromeric breakpoint by a number of patients with NF1 deletions and the coincidence of the telomeric breakpoint in a patient with a neuroblastoma-specific rearrangement suggest that the NF1-microdeletion syndrome may be viewed as a genomic disorder (Lupsky 1998).
In conclusion, our study demonstrates that the refined FISH characterization of gross NF1 deletions is feasible with suitable resources and technologies. Precise definition of gene/STS content and of the extent and boundaries of deletions is a prerequisite for study of the complex problem of genotype-phenotype correlations. This is beyond the scope of the present study, which should be seen as a pilot study concentrating on a new method, the extensive application of which may make it possible to investigate genotype-phenotype correlations correctly.
Acknowledgments
The authors thank Patrizia Colapietro (Department of Biology and Genetics, Milan) for expert technical assistance and Dr. M. Mancini (YAC Screening Center HSR-DIBIT, Milan) for providing YAC and PAC clones. This work was supported by Fondazione Téléthon-Italy grant E.780 (to L.L. and P.R.) and by the Associazione Emma ed Ernesto Rulfo per la Genetica Medica (support to R.T. and M.C.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Genome Database, http://www.gdb.org/
- NNFF International NF1 Consortium, http://www.nf.org/nf1gene/nf1gene.home.html
- Online Mendelian Inheritance in Man (Omim), http://www.ncbi.nlm.nih.gov/Omim/
- Technical Tips Online, http://tto.trends.com (for article T01618: Riva P, Corrado L, Colapietro P, Larizza L (1999) A rapid and simple method of generating locus-specific probes for FISH analysis)
References
- Ainsworth PJ, Chakraborty PK, Weksberg R (1997) Example of somatic mosaicism in a series of the novo neurofibromatosis type 1 cases due to a maternally derived deletion case of sporadic neurofibromatosis. Hum Mutat 9:452–457 [DOI] [PubMed]
- Breen M, Arveiler B, Murray I, Gosden JR, Porteus D (1992) YAC mapping by FISH using Alu-PCR-generated probes. Genomics 13:726–730 [DOI] [PubMed]
- Cawthon RM, Weiss R, Xu G, Viskochil D, Culver M, Stevens J, Robertson M, et al (1990) A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutation. Cell 62:193–201 [DOI] [PubMed]
- Chiang PW, Wang S, Smithivas P, Song WJ, Ramamoorthy S, Hillman J, Puett S, et al (1996) Identification and analysis of the human murine putative chromatin structure regulator SUPT6H and Supt6h. Genomics 34:328–333 [DOI] [PubMed]
- Chumakov IM, Rigault P, Le Gall I, Bellanne-Chantelot C, Billault A, Guillou S, Soularue P, et al (1995) A YAC contig map of the human genome. Nature 377:175–297 [DOI] [PubMed]
- Corrado L, Colapietro P, Larizza L, Riva P (1999) Mapping of human WHN gene in a 17q11.2 YAC contig and identification of an intragenic STR. Mol Cell Probes 13:199–202 [DOI] [PubMed]
- Ferner RE (1994) Intellect in neurofibromatosis 1. In: Huson SM, Hughes RAC (eds) The neurofibromatoses, a pathogenetic and clinical overview. Chapman Hall, London, p 233–252 [Google Scholar]
- Friedman JM, Birch PH (1997) Type 1 neurofibromatosis: a descriptive analysis of the disorder in 1,728 patients. Am J Med Genet 70:138–143 [DOI] [PubMed]
- Garcia-Anoveros J, Derfler B, Neville-Golden, Bradley TH, Corey DP (1997) BNaC1 and BnaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA 94:1459–1464 [DOI] [PMC free article] [PubMed]
- Kayes LM, Burke W, Riccardi V, Bennet R, Ehrlich P, Rubenstein A, Stephens K (1994) Deletions spanning the neurofibromatosis I gene: identification and phenotype of five patients. Am J Hum Genet 54:424–436 [PMC free article] [PubMed]
- Lazaro C, Gaona A, Ainsworth P, Tenconi R, Vidaud D, Kruyer H, Ars E, et al (1996) Sex differences in mutational rate and mutational mechanism in the NF1 gene in neurofibromatosis 1 patients. Hum Genet 98:696–699 [DOI] [PubMed]
- Lazo JS, Humphreys CJ (1983) Lack of metabolism as the biochemical basis of bleomycin-induced pulmonary toxicity. Proc Natl Acad Sci USA 80:3064–3068 [DOI] [PMC free article] [PubMed]
- Leppig KA, Viskhochil D, Neil S, Rubestein A, Johnson VP, Zhu XL, Brothman AR, et al (1996) The detection of contiguous gene deletions at the neurofibromatosis 1 locus with fluorescence in situ hybridization. Cytogenet Cell Genet 72:95–98 [DOI] [PubMed]
- Li Y, O'Connell P, Breidenbach HH, Cawthon R, Stevens J, Xu G, Neil S, et al (1995) Genomic organization of the neurofibromatosis 1 gene (NF1). Genomics 25:9–18 [DOI] [PubMed]
- Lichter P, Cremer T (1992) A practical approach. In: Rooney DE, Czipolkowski BH (eds) Human cytogenetics. IRL Press at Oxford University Press, Oxford, pp 157–192 [Google Scholar]
- Lupsky JR (1998) Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet 14:417–422 [DOI] [PubMed]
- Montoya SE, Aston CE, DeKosky ST, Kamboh MI, Lazo JS, Ferrel RE (1998) Bleiomycin hydrolase is associated with risk of sporadic Alzheimer's disease. Nat Genet 18:211–212 [DOI] [PubMed]
- Naruse K, Ueno M, Satoh T, Nomiyama H, Tei H, Takeda M, Ledbetter DH, et al (1996) A YAC contig of the human CC chemokine genes clustered on chromosome 17q11.2. Genomics 34:236–240 [DOI] [PubMed]
- Riva P, Castorina P, Manoukian S, Dalpra L, Doneda L, Marini G, den Dunnen J, et al (1996) Characterization of a cytogenetic 17q11.2 deletion in an NF1 patient with a contiguous gene syndrome. Hum Genet 98:646–650 [DOI] [PubMed]
- Stevenson AC, Kerr CB (1967) On the distribution of frequencies of mutation to genes determining harmful traits in man. Mutat Res 4:339–352 [DOI] [PubMed]
- Tonsgard JH, Yelavarthi KK, Cushner S, Short MP, Lindgren V (1997) Do NF1 gene deletions result in a characteristic phenotype? Am J Med Genet 73:80–86 [DOI] [PubMed]
- Upadhyaya M, Roberts SH, Maynard J, Sorour E, Thompson PW, Vaughan M, Wilkie AOM, et al (1996) A cytogenetic deletion, del(17)(q11.2q21.1), in a patient with sporadic neurofibromatosis type 1 (NF1) associated with dysmorphism and developmental delay. J Med Genet 33:148–152 [DOI] [PMC free article] [PubMed]
- Upadhyaya M, Ruggeri M, Maynard J, Osborn M, Hartog C, Mudd S, Penttinen M, et al (1998) Gross deletions of the neurofibromatosis type 1 (NF1) gene are predominantly of maternal origin and commonly associated with a learning disability, dysmorphic features and developmental delay. Hum Genet 102:591–597 [DOI] [PubMed]
- Van Roy N, Laureys G, Van Gele M, Opdenakker G, Miura R, Van der Drift P, Chan A, et al (1997) Analysis of 1;17 translocation breakpoints in neuroblastoma: implications for mapping of neuroblastoma genes. Eur J Cancer 33:1974–1978 [DOI] [PubMed]
- Viskochil DH, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolfer CM, Carey J, et al (1990) Deletion and translocation interrupt a cloned gene at neurofibromatosis 1 locus. Cell 62:187–192 [DOI] [PubMed]
- Vogel F, Motulsky AG (eds) (1997) Human genetics: problems and approaches. Springer Verlag, Berlin, Heidelberg, New York [Google Scholar]
- Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, et al (1990) Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 249:181–186 [DOI] [PubMed]
- Wu BL, Austin MA, Schneider GH, Boles RG, Korf BR (1995) Deletion of the entire NF1 gene detected by FISH: four deletion patients associated with severe manifestations. Am J Med Genet 59:528–535 [DOI] [PubMed]
- Wu BL, Boles RG, Yaari H, Weremowicz S, Schneider GH, Korf B (1997a) Somatic mosaicism for deletion of the entire NF1 gene identified by FISH. Hum Genet 99:209–213 [DOI] [PubMed]
- Wu BL, Schneider GH, Korf B (1997b) Deletion of the entire NF1 gene causing distinct manifestation in a family. Am J Med Genet 69:98–101 [PubMed]