Abstract
Background
Mild cognitive impairment (MCI) was recently described as a heterogeneous group with a variety of clinical outcomes and high risk to develop Alzheimer's disease (AD). Regional cerebral blood flow (rCBF) as measured by single photon emission computed tomography (SPECT) was used to study the heterogeneity of MCI and to look for predictors of future development of AD.
Methods
rCBF was investigated in 54 MCI subjects using Tc-99m hexamethylpropyleneamine oxime (HMPAO). An automated analysis software (BRASS) was applied to analyze the relative blood flow (cerebellar ratios) of 24 cortical regions. After the baseline examination, the subjects were followed clinically for an average of two years. 17 subjects progressed to Alzheimer's disease (PMCI) and 37 subjects remained stable (SMCI). The baseline SPECT ratio values were compared between PMCI and SMCI. Receiver operating characteristic (ROC) analysis was applied for the discrimination of the two subgroups at baseline.
Results
The conversion rate of MCI to AD was 13.7% per year. PMCI had a significantly decreased rCBF in the left posterior cingulate cortex, as compared to SMCI. Left posterior cingulate rCBF ratios were entered into a logistic regression model for ROC curve calculation. The area under the ROC curve was 74%–76%, which indicates an acceptable discrimination between PMCI and SMCI at baseline.
Conclusion
A reduced relative blood flow of the posterior cingulate gyrus could be found at least two years before the patients met the clinical diagnostic criteria of AD.
Background
Mild cognitive impairment (MCI) is an operational diagnostic term developed to describe the preclinical stage of Alzheimer's disease (AD). MCI is a heterogeneous group containing preclinical stage of dementia [1]. The rate at which MCI subjects convert to AD each year is ten times more than the rate for normal subjects [2]. Identification of progressive mild cognitive impairment (PMCI) versus non-progressive mild cognitive impairment subjects (SMCI) is currently of great theoretical interest and practical importance. Early therapeutical interventions are more likely to be effective and the improvement of clinical outcome may significantly reduce the heavy economic and social burden.
The utility of functional imaging techniques, such as single photon emission computed tomography (SPECT) and positron emission computed tomography (PET) for the study of regional abnormalities in AD has been established [3,4]. There is agreement that metabolic reduction and hypoperfusion in the parietal, temporal cortex and limbic system are consistent findings in AD that often correlate with cognitive functions [3,4]. However, sensorimotor cortex, pons, and cerebellum were found to be relatively preserved [5,6].
In contrast, functional imaging findings for the preclinical stage of AD are inconsistent [7,8]. Kennedy et al. showed that there was decreased metabolism in the tempo-parietal region in patients before they met the clinical criteria of AD [7]. A marked metabolic reduction in the posterior cingulate gyrus was reported in the transitional stage of AD patients by Minoshima et al. [6]. Kogure et al.'s study used a statistical parametric mapping (SPM) technique for group comparisons and found a significant bilaterally decreased rCBF in the posterior cingulate gyrus and precunei in MCI subjects, as compared to controls at least two years before they met the clinical diagnosis of AD [8]. However, only few studies have looked at the heterogeneity of MCI. Johnson et al. demonstrated that SPECT was a promising method for the diagnosis of PMCI, in which the combination of the cingulate gyrus, hippocampal-amygdaloid complex and thalamus identified more than 80% of the subjects who would progress to AD after a 16.7 month follow-up [9].
The present study assessed baseline regional cerebral blood flow (rCBF), using an automated ROI-based analysis software, in a group of MCI subjects who were followed clinically for about two years. Specifically, we wanted to examine preclinical changes of dementia, and to assess the clinical prediction of dementia and diagnostic accuracy of SPECT for separating PMCI and SMCI at baseline.
Methods
Subjects selection
Fifty-four MCI patients were evaluated. The patients were selected from all individuals consecutively investigated for suspected dementia at the geriatric clinic, Huddinge University Hospital. Patients were primarily referred from General practitioners. The clinic serves the large Stockholm area with approximately 2 million inhabitants. All subjects with a diagnosis of MCI at the initial investigation were included in the study. Patients with other medical psychiatric diagnosis were excluded. No subject received either psychotropic medication or an acetylcholinesterase inhibitor likely to influence the results of SPECT scanning.
All subjects underwent general medical, neurological, psychiatric and neuropsychological evaluation, as well as neuroimaging diagnostic procedures (SPECT and MRI) at the initial investigation. The subjects were clinically followed for 28.9 ± 16.3 months on average. After the follow-up period, 17 MCI subjects have progressed to AD. These MCI subjects were defined as PMCI. 37 remained MCI and did not fulfill the criteria of dementia during the observation time. These MCI subjects were defined as SMCI. The baseline PMCI and SMCI did not differ with respect to age (PMCI (years): 63.6 ± 7.3, SMCI (years): 60.3 ± 8.5), gender (PMCI (f/m): 9/8, SMCI (f/m): 24/13), follow-up time (PMCI (months) 26.6 ± 19.0, SMCI (months) 29.7 ± 16.6) and MMSE (PMCI: 26.2 ± 2.0, SMCI: 27.0 ± 2.3).
Diagnosis
Subjects who were diagnosed as MCI did not fulfill the diagnostic criteria for dementia according to DSM-IV criteria and did not have evidence of impairment in social or occupational functioning, but performed at least 1.5 SD below average for their age on at least one neuropsychological test [10]. Progressive mild cognitive impairment (PMCI) referred to the MCI subjects who converted to Alzheimer's disease according to the DSM-IV criteria during the follow up and stable mild cognitive impairment (SMCI) was defined as the subjects who still did not fulfill the criteria for dementia according to DSM-IV during the observation time.
Neuropsychological tests
All subjects were tested by experienced psychologist with five subtests (Information, Digit Span, Similarities, Block Design and Digit Symbol) from the Wechsler Adult Intelligence Scale-Revised (WAIS-R), Trail Making Test A and B, free and recognition words from the Stockholm Geriatric Research Center (SGRC) [11,12]. The general level of cognition was assessed by the Mini-Mental State Examination (MMSE) [13].
Single photon emission computed tomography (SPECT)
Each subject was injected with 1000 Mbq Tc-99m-HMPAO (Ceretec, Amersham Ltd) in a quiet surrounding with eyes closed. Acquisition started 30 minutes after injection. Data were collected in 64 projections evenly spread through 360 degrees with a single headed rotating gamma camera (Siemens Diacam) with a total acquisition time of 32 minutes. Tomographic slices were reconstructed using an iterative algorithm (Hosem, Nuclear Diagnostics AB, Sweden) with Chang attenuation correction (Attenuation coefficient: 0.12 cm-1). Data were formatted as a 3D dataset with 64 × 64 × 64 cubic voxels with 3.5 mm sides. The resolution in a tomographic slice was measured to be 10.2 mm (FWHM). The reconstructed data sets were post-filtered with a Butterworth filter, cutoff 0.7 cm-1.
SPECT registration and quantification
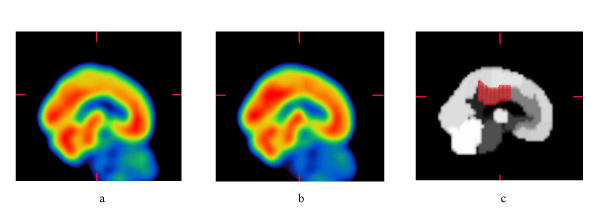
Image registration and quantification were performed with the BRASS software developed by Nuclear Diagnostics (London, England and Stockholm, Sweden) [14]. The patient datasets were iteratively registered using 9 parameter linear registration to a normal template using normalized mutual information as similarity function. The software uses a map of 46 volumes-of-interest (VOIs) that encompass the entire brain [15]. The subjects were normalized according to cerebellar cortex. Mean image of PMCI and SMCI and the region map were obtained (figure 1).
Figure 1.
Regional cerebral blood flow measured with SPECT. a) Mean image of progressive mild cognitive impairment (PMCI). b) Mean image of stable mild cognitive impairment (SMCI). c) Region map with marked posterior cingulate.
The quantification evaluations were performed in 28 cortical regions, since they are the most interesting regions in AD. The region selected were bilateral sensorimotor, occipital, parietal, anterior and posterior dorsal frontal, anterior and posterior orbital frontal, parieto-temporal, medial, lateral and posterior temporal lobe, temporal pole as well as bilateral anterior and posterior cingulate cortex. The relative regional cerebral blood flow (rCBF) in the selected regions were calculated as cerebellar ratios. (Mean value of region/mean value of bilateral cerebellar cortex.)
Statistics
The baseline VOI results of PMCI and SMCI subjects were compared using t-test, p < 0.05 was considered to the significant level, uncorrected for multiple comparisons, because of the relative small sample size. The VOIs with significantly different rCBF between groups were entered into a logistic model. Receiver operating characteristic (ROC) analysis was applied for the discrimination between PMCI and SMCI.
Results
Conversion rate
The conversion rate of MCI to AD was 13.7% per year.
Brain perfusion of PMCI and SMCI
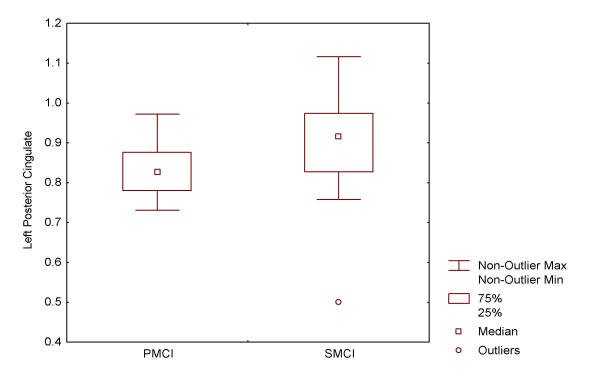
The mean and standard deviation of each relative rCBF value was shown in Table 1. The box-and-whisker plots of Left Posterior Cingulate ratio value were shown in figure 2. There was one outlier in SMCI group. T-test was performed within the whole group (W) and after excluding the outlier (O), separately. PMCI group had a significantly decreased rCBF in the left posterior cingulate cortex (0.84 ± 0.07) compared to SMCI (W: 0.91 ± 0.11, t = -2.3529, p = 0.0224, O: 0.92 ± 0.09, t = -3.2602, p = 0.0020). The blood flow of the left parieto-temporal lobe had a tendency to decrease in PMCI (0.88 ± 0.07) compared to SMCI (W: 0.91 ± 0.05, t = -1.8326, p = 0.0726, O: 0.91 ± 0.05, t = -1.9911, p = 0.0518).
Table 1.
Means and standard deviations of relative blood flow in PMCI and SMCI
| Regions | Mean (SD) | |
| PMCI | SMCI | |
| L sensorimotor | 0.90 (0.05) | 0.91 (0.05) |
| R sensorimotor | 0.89 (0.04) | 0.90 (0.05) |
| L occipital lobe | 0.90 (0.06) | 0.92 (0.05) |
| R occipital lobe | 0.89 (0.06) | 0.91 (0.05) |
| L superior parietal lobe | 0.87 (0.06) | 0.90 (0.06) |
| R superior parietal lobe | 0.87(0.06) | 0.89 (0.07) |
| L anterior dorsal frontal lobe | 0.84 (0.04) | 0.83 (0.06) |
| R anterior dorsal frontal lobe | 0.85 (0.04) | 0.85 (0.07) |
| L posterior dorsal frontal lobe | 0.89 (0.04) | 0.89 (0.06) |
| R posterior dorsal frontal lobe | 0.90 (0.04) | 0.90 (0.06) |
| L anterior orbital frontal lobe | 0.84 (0.04) | 0.84 (0.06) |
| R anterior orbital frontal lobe | 0.85 (0.05) | 0.84 (0.07) |
| L posterior orbital frontal lobe | 0.88 (0.04) | 0.87 (0.06) |
| R posterior orbital frontal lobe | 0.86 (0.05) | 0.86 (0.06) |
| L parieto-temporal lobe | 0.88 (0.07) | 0.91 (0.05) |
| R parieto-temporal lobe | 0.89 (0.06) | 0.91 (0.06) |
| L medial temporal lobe | 0.82 (0.05) | 0.83 (0.07) |
| R medial temporal lobe | 0.82 (0.04) | 0.81 (0.06) |
| L lateral temporal lobe | 0.91 (0.06) | 0.91 (0.07) |
| R lateral temporal lobe | 0.90 (0.05) | 0.90 (0.06) |
| L posterior temporal lobe | 0.94 (0.06) | 0.95 (0.06) |
| R posterior temporal lobe | 0.93 (0.06) | 0.94 (0.06) |
| L temporal pole | 0.78 (0.06) | 0.80 (0.07) |
| R temporal pole | 0.78 (0.05) | 0.78 (0.06) |
| L anterior cingulate cortex | 0.88 (0.06) | 0.89 (0.08) |
| R anterior cingulate cortex | 0.88 (0.06) | 0.89 (0.08) |
| L posterior cingulate cortex | 0.84 (0.07) | 0.91 (0.11) |
| R posterior cingulate cortex | 0.80 (0.08) | 0.85 (0.129 |
L: left, R: right
Figure 2.
Box-and-whisker plots of relative regional blood flow in Left Posterior Cingulate. PMCI: progressive mild cognitive impairment. SMCI: stable mild cognitive impairment.
Logistic regression
The rCBF of left posterior cingulate cortex was entered into a logistic regression model for the differential diagnosis between PMCI and SMCI at baseline within the whole group (Estimate: -7.17 (-13.89, -0.45), Standard Error 3.43, p = 0.037). ROC analysis was performed with 74% of the area under the curve. An alternative logistic regression was performed after we excluded the outlier (Estimate: -12.41 (-21.10, -3.73), Standard Error 4.43, p = 0.005) and the area under the ROC curve was 76%.
Discussion
The present study showed that the conversion rate of MCI to AD was 13.7% per year, which is comparable with other cohorts of MCI-patients [2]. Using a recently developed automated analysis program for rCBF analysis in individual patients, we found posterior cingulate hypoperfusion to be the earliest deficit in the transitional stage of AD. A logistic regression applied for the ROC curve calculation gave an area under the curve of 74%–76% indicating an acceptable discrimination of the posterior cingulate cortex between PMCI and SMCI at baseline.
It was reported in Fox et al's study, using compression mapping of serial magnetic resonance images, that genetically at risks subjects had posterior cingulate atrophy years before the onset of dementia [16]. Our SPECT study was not corrected by MRI. The results could be influenced by partial volume effect. However, several studies found that posterior cingulate had reduced metabolism in preclinical dementia, even after the correction of atrophy [17]. MRI will be performed in our following SPECT study. For the clinical purpose, the current findings might be valuable for the prediction of dementia in the heterogeneous group of subjects presenting with MCI.
Posterior cingulate cortex was found to participate in cognitive functions such as memory and spatial orientation [18]. The associations of the posterior cingulate cortex with medial temporal lobe structures have been demonstrated in previous studies. This might imply a major role in memory-related functions of the cingulate cortex. Area 23a and 29/30 of posterior cingulate afferents terminate in the entorhinal cortex, subiculum has projections to area 29/30 and parahippocampus also have wide associations with the posterior cingulate region [19,20]. Several investigators showed that the posterior cingulate cortex contributes to the spatial orientation and spatial working memory which might rest on the association of parietal area 7 and parahippocampal gyrus with the spatially selective firing in layer II of entorhinal cortex [18].
Preclinical AD has been reported having the neuropathological features of mild AD, including neurofibrillary tangles and neuritic plagues in the medial temporal lobe [21]. Some studies demonstrated that the initial neuronal lesions develop in the entorhinal cortex [22]. Gomez-Isla et al. reported that the predromal phase of AD patients had a 60% of neuron loss in layer II of the entorhinal cortex and 40% loss in layer IV, as compared to controls [23]. However, no pathological changes have been reported in the posterior cingulate. Based on the above findings, we hypothesize that in the preclinical stage of AD, isolation of the posterior cingulate cortex from the input and output of the medial temporal lobe structures is probably an important mechanism for the deficits of cognitive function seen in PMCI subjects. Such cortico-cortical disconnection might subsequently result in the decreased rCBF found in the posterior cingulate cortex. It was also reported that the antero-dorsal nucleus of the thalamus had pathological changes in preclinical AD [22]. A study of rhesus monkey showed that the antero-dorsal nucleus associates with area 29 of the posterior cingulate gyrus [24]. According to the disconnection hypothesis, a lesion of the antero-dorsal nucleus in transitional AD might also contribute to the subsequent hypoperfusion showed in the posterior cingulate cortex.
A metabolic reduction of the posterior cingulate was also found in lewy body disease, indicating that the involvement of the posterior cingulate might be a common pathophysiological process in neurodegenerative disease [25]. In addition, our study showed that the parieto-temporal association cortex had a relatively mild hypoperfusion in PMCI at baseline. However, previous studies have indicated that hypoperfusion of the parietal-temporal cortex was a typical and consistent finding in AD, which suggests that the parieto-temporal association cortex might be only slightly affected at the preclinical stage of dementia, but significantly develops as the disease spreads [8].
Conclusion
Topographical analysis of rCBF in preclinical AD using SPECT and an automated VOI-based analysis could show a reduced relative blood flow in the posterior cingulate cortex at least two years before the subjects with MCI met the clinical diagnostic criteria of AD. Cingulate hypoperfusion is a promising marker for the early detection of AD in the heterogeneous group of subjects presenting with mild cognitive impairment.
Competing interests
None declared.
Authors' Contributions
CH, participated in the design of the study, carried out the SPECT analysis, statistical analysis and drafted the manuscript. L-OW, participated in the design and coordination, was responsible for the evaluation of clinical diagnosis and in the general supervision of the project. LS, was responsible for SPECT examinations, SPECT data basing and participated in the SPECT image analysis. BW, participated in the design and coordination of the study. PJ, conceived of the study and participated in the design, coordination and supervised the SPECT image and statistical analysis. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgments
This work was supported by the Swedish Medical Research Council, the Swedish Council for Social Research, the Gamla Tjänarinnor Foundation, the Greta Lindenau-Hansell's Foundation, the Karolinska Institute and the Swedish Society of Medicine. Perry Radau and Nuclear Diagnostics Ltd are gratefully acknowledged for the generous support and cooperation regarding the BRASS program.
Contributor Information
Chaorui Huang, Email: chaorui.huang@neurotec.ki.se.
Lars-Olof Wahlund, Email: lars-olof.wahlund@neurotec.ki.se.
Leif Svensson, Email: Leif@asf.hs.sll.se.
Bengt Winblad, Email: bengt.winblad@neurotec.ki.se.
Per Julin, Email: per.julin@neurotec.ki.se.
References
- Almkvist O, Basun H, Backman L, Herlitz A, Lannfelt L, Small B, Viitanen M, Wahlund LO, Winblad B. Mild cognitive impairment – an early stage of Alzheimer's disease? J Neural Transm Suppl. 1998;54:21–29. doi: 10.1007/978-3-7091-7508-8_3. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Holman BL, Johnson KA, Gerada B, Carvalho PA, Satlin A. The scintigraphic appearance of Alzheimer's disease: a prospective study using technetium-99m-HMPAO SPECT. J Nucl Med. 1992;33:181–185. [PubMed] [Google Scholar]
- Syed GM, Eagger S, O'Brien J, Barrett JJ, Levy R. Patterns of regional cerebral blood flow in Alzheimer's disease. Nucl Med Commun. 1992;13:656–663. doi: 10.1097/00006231-199209000-00004. [DOI] [PubMed] [Google Scholar]
- Benson DF, Kuhl DE, Hawkins RA, Phelps ME, Cummings JL, Tsai SY. The fluorodeoxyglucose 18F scan in Alzheimer's disease and multi-infarct dementia. Arch Neurol. 1983;40:711–714. doi: 10.1001/archneur.1983.04050110029003. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Kennedy AM, Frackowiak RS, Newman SK, Bloomfield PM, Seaward J, Roques P, Lewington G, Cunningham VJ, Rossor MN. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer's disease. Neurosci Lett. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, Nakano S, Takasaki M. Longitudinal evaluation of early Alzheimer's disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–1162. [PubMed] [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (4th ed.). Washington: American Psychiatric Association. 1994.
- Wechsler D. Wechsler Adult Intelligence Scale-Revised Manual. San Antonio: Psychological Corp. 1981.
- Backman L, Forsell Y. Episodic memory functioning in a community-based sample of old adults with major depression: utilization of cognitive support. J Abnorm Psychol. 1994;103:361–370. doi: 10.1037//0021-843X.103.2.361. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Radau PE, Slomka PJ, Julin P, Svensson L, Wahlund L-O. Automated segmentation and registration technique for HMPAO-SPECT imaging of Alzheimer's patients. Medical imaging 2000: Image processing, Kenneth M Hansson, Ed Proceedings of SPIE. 2000;3979:372–384. doi: 10.1117/12.387699. [DOI] [Google Scholar]
- Radau PE, Slomka PJ, Julin P, Svensson L, Wahlund L-O. Evaluation of linear registration algorithms for brain SPECT and the errors due to hypoperfusion lesions. J Med Phys. 2001;28:1660–1668. doi: 10.1118/1.1388894. [DOI] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bierer LM, Perl DP, Delacourte A, Buee L, Bouras C, Morrison JH. Evidence for early vulnerability of the medial and inferior aspects of the temporal lobe in an 82-year-old patient with preclinical signs of dementia. Regional and laminar distribution of neurofibrillary tangles and senile plaques. Arch Neurol. 1992;49:946–953. doi: 10.1001/archneur.1992.00530330070019. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Albin RL, Minoshima S, D'Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47:462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]