Summary
Cigarette smoke, containing reactive oxygen species, is the most important risk factor for chronic pulmonary emphysema (CPE). Heme oxygenase-1 (HO-1) plays a protective role as an antioxidant in the lung. A (GT)n dinucleotide repeat in the 5′-flanking region of human HO-1 gene shows length polymorphism and could modulate the level of gene transcription. To investigate the correlation between the length of the (GT)n repeat and susceptibility to the development of CPE, we screened the frequencies of alleles with varying numbers of (GT)n repeats in the HO-1 gene in 101 smokers with CPE and in 100 smokers without CPE. Polymorphisms of the (GT)n repeat were grouped into three classes: class S alleles (<25 repeats), class M alleles (25–29 repeats), and class L alleles (⩾30 repeats). The proportion of allele frequencies in class L, as well as the proportion of genotypic frequencies in the group with class L alleles (L/L, L/M, and L/S), was significantly higher in the smokers with CPE than in smokers without CPE. Moreover, we analyzed the promoter activities of the HO-1 gene carrying different (GT)n repeats (n=16, 20, 29, and 38), by transient-transfection assay in cultured cell lines. H2O2 exposure up-regulated the transcriptional activity of the HO-1 promoter/luciferase fusion genes with (GT)16 or (GT)20 but did not do so with (GT)29 or (GT)38. These findings suggest that the large size of a (GT)n repeat in the HO-1 gene promoter may reduce HO-1 inducibility by reactive oxygen species in cigarette smoke, thereby resulting in the development of CPE.
Introduction
Chronic obstructive pulmonary disease (COPD), which includes chronic pulmonary emphysema (CPE), chronic airway obstruction, and chronic bronchitis (Celli et al. 1995), is one of the leading causes of death worldwide, with an increasing prevalence and mortality (Thom 1989). It is generally accepted that cigarette smoke is the most common identifiable risk factor for COPD. However, only 10%–15% of smokers develop COPD (Hanrahan et al. 1996), and ∼30% of smokers with a history of >60 pack years still have FEV1:FVC ratios within the normal range (Kuperman and Riker 1973), suggesting the existence of a group susceptible to cigarette smoke.
There are two current established hypotheses in the pathogenesis of CPE. One is the endogenous protease/antiprotease theory (Janoff 1985; Snider 1992), which states that the loss of equilibrium between the level of degradative enzymes and their respective inhibitors damages the connective-tissue–matrix components of the lung (Hoidal and Niewoehner 1983). This theory could explain the mechanism of development of CPE in α1-antitrypsin deficiency (MIM 107400) (Garver et al. 1986) and in animal models with excessive expressions of elastases and collagenases (Snider 1992). The other hypothesis is the oxidant/antioxidant theory, which postulates that an excess of oxidants and free radicals in the lung directly promotes cellular and tissue damage and is the major initiator of the disease process (Farber 1994). A recent report suggests the relation between the reduced activity of an antioxidant enzyme, microsomal epoxide hydrolase, and susceptibility to CPE (Smith and Harrison 1997). Furthermore, reactive oxygen species in cigarette smoke inhibit antiproteases, by oxidation of their active sites, and promote cell and tissue proteolysis in the lung (Ogushi et al. 1991). Direct and indirect oxidative injury caused by cigarette smoke must be a crucial mechanism for the process of CPE.
Heme oxygenase (HO) oxidatively degrades heme to biliverdin, which is subsequently reduced to bilirubin by biliverdin reductase (Maines 1997). HO-1, an inducible form of HO, provides cellular protection against heme- and non-heme–mediated oxidant injury (Choi and Alam 1996; Maines 1997). Likewise, an exogenous administration of HO-1 in the rat lung by gene transfer was shown to protect against injury caused by hyperoxia (Otterbein et al. 1999). These lines of evidence support that HO-1 is an essential component for the lung to keep a delicate balance between oxidants and antioxidants.
A (GT)n repeat is the most frequent of the simple repeats scattered throughout the human genome, and many of these exhibit length polymorphism (Naylor and Clark 1990). This purine-pyrimidine alternating sequence, possessing Z-conformation potential, negatively affects transcriptional activity in the rat prolactin gene (Naylor and Clark 1990). A (GT)n repeat in the 5′-flanking region of the human HO-1 gene is indeed highly polymorphic (Kimpara et al. 1997) and may modulate gene transcription under thermal stress (Okinaga et al. 1996). Therefore, it could be hypothesized that, if the expression of the HO-1 gene alters according to the number of (GT)n repeats, the microsatellite polymorphism may be associated with the development of the oxidative stress–inducing diseases. In the present study, we focus on CPE as a paradigm for elucidation of the contribution of 5′-flanking polymorphisms in the HO-1 gene, because there is a marked association between reactive oxygen species in cigarette smoke and pathogenesis of CPE. To assess this hypothesis, we screened allelic frequencies of the (GT)n repeats in the HO-1 gene promoter from smokers with and without CPE and examined the association between the development of CPE and length of the (GT)n repeats. Moreover, we performed a transient-transfection assay with the HO-1 promoter/luciferase fusion genes carrying different (GT)n repeats (n=16, 20, 29, and 38) and compared the transcriptional activities of the fusion genes in the A549 human type II lung cell line and the Hep3B human hepatoma cell line treated with H2O2.
Material and Methods
Clinical Protocol and Characteristics of Patients
We obtained blood samples from 101 male smokers with CPE and 100 male smokers without CPE, from 9 public hospitals in Miyagi Prefecture. All subjects with and without CPE were Japanese, and they represented an ethnic isolation. CPE was defined as a physical examination that demonstrated hyperresonant chest and flattened hemidiaphragms; a chest roentogenogram that demonstrated hyperinflation, flattened diaphragms, and marked loss of vascularity; a computed-tomography scan that demonstrated areas of low attenuation; and pulmonary-function testing that demonstrated decreased FEV1:FVC ratios and impaired diffusion capacity (Celli et al. 1995). Age, Brinkman's index (the number of cigarettes/day × no. of years), disorders, and pulmonary-function test results are shown in table 1. The study was approved by the Tohoku University Ethics Committee, and informed consent was obtained from each subject.
Table 1.
Physical Characteristics and Pulmonary Function Test Results
|
Patients |
|||
| Details of Patients | Control (n=100) | With CPE (n=101) | P |
| Age (years)a | 67.1 (.9) | 66.8 (.8) | .82 |
| Brinkman's indexa | 938 (44) | 1,006 (43) | .26 |
| Disorders:b | |||
| Diabetes | 19 (19.0) | 3 (3.0) | |
| Hypertension | 22 (22.0) | 15 (14.9) | |
| Cardiovascular | 38 (38.0) | 30 (29.7) | |
| Gastrointestinal | 14 (14.0) | 12 (11.9) | |
| Renal | 3 (3.0) | 2 (2.0) | |
| Dementia | 5 (5.0) | 3 (3.0) | |
| Lung cancer | 0 (.0) | 3 (3.0) | |
| None | 0 (.0) | 35 (34.7)c | |
| Pulmonary function: | |||
| FVC (% of predicted) | 98 (5) | 84 (7) | .11 |
| FEV1.0:FVC (%) | 95 (5) | 47 (2) | .01 |
| DLco (% of predicted) | NDd | 52 (5) | |
Mean (SEM).
No. (%) of patients.
No complications.
ND = not done.
Analysis of Length Variability of (GT)n Repeats in HO-1 Gene Promoter
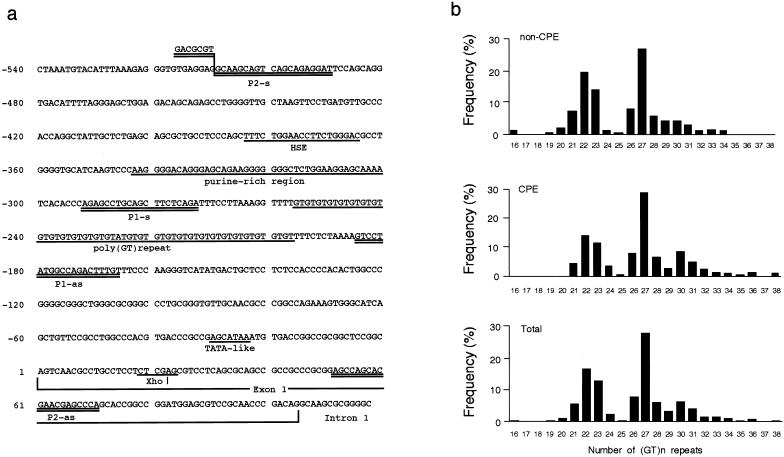
Genomic DNAs were extracted from leukocytes by conventional procedures. The 5′-flanking region containing a poly (GT)n repeat of the HO-1 gene was amplified by PCR (Okinaga et al. 1996; Kimpara et al. 1997) with a fluorescently labeled primer, p1-s (5′-AGAGCCTGCAGCTTCTCAGA-3′), and an unlabeled antisense primer, p1-as (5′-ACAAAGTCTGGCCATAGGAC-3′), which were designed according to the published sequence (fig. 1a) (Shibahara et al. 1989). The PCR was performed over 30 cycles of 20 s at 94°C, 10 s at 60°C, and 20 s at 72°C. The sizes of the PCR products were analyzed with a laser-based automated DNA sequencer (Pharmacia). In usual cases, a blood sample has two different sizes of (GT)n repeats, from different alleles. Each repeat number was calculated with Fragment Manager, version 1.2 (Pharmacia), with, as size markers, four cloned alleles that were loaded into every four lanes in the DNA sequencer. The repeat numbers of these cloned alleles used as size markers were 16, 22, 27, and 36.
Figure 1.
a, Nucleotide sequence of the 5′-flanking region and exon 1 of the human HO-1 gene (X14782) (Shibahara et al. 1989; GenBank). The fragment between P1-s and P1-as was amplified by PCR and the number of (GT)n repeats was determined by a DNA sequencer. b, Frequency distribution of the numbers of (GT)n repeats in patients without CPE (non-CPE; n=100), patients with CPE (CPE;, n=101), and total patients (Total: n=201).
Northern-Blot Analysis of HO-1 mRNA Expression in Cell Lines
Northern-blot analysis of HO-1 mRNA expression in cell lines was performed as described elsewhere (Okinaga et al. 1996). Human type II lung cell line A549 cells and human hepatoma cell line Hep3B cells were cultured in Dulbecco's modified Eagle medium supplemented either with 8% fetal-calf serum (DMEM/8%FCS) or with 10% fetal-calf serum (DMEM/10%FCS), respectively. Cultivation of these cells in all experiments was performed at 37°C with 5% CO2, unless stated otherwise. Total RNA was prepared from these cells, which were cultured in a six-well plate, by the addition of RNAzol (0.2 ml/106 cells; BIOTECX) according to methods as described elsewhere (Okinaga et al. 1996). Total RNA (10 μg) extracted from either A549 cells or Hep3B cells was electrophoresed in 1.1% agarose/formaldehyde gel and was transferred onto a nylon membrane (Hybond-N+; Amersham Life Science) via capillary action. The RNA blotted onto the filter was fixed by brief exposure to UV radiation and was hybridized overnight at 42°C with a 32P-labeled XhoI(position 64)-XbaI(position 923) fragment derived from human HO-1 complementary DNA, pHHO1 (Yoshida et al. 1988). The probe was labeled with α[32P]-deoxycytidine triphosphate, by use of the Random Primer DNA Labeling Kit Ver.2 (Takara). After the hybridized filter was washed, autoradiographic detection of the labeled probe was performed by exposure of the filter to Kodak Scientific Imaging film for 48–72 h at −80°C. Quantitation of autoradiographic bands was accomplished with an image analyzer (Bio Imaging Analyzer BAS-2000; Fuji Photo Film) and was expressed as the intensity of HO-1/β-actin bands.
To examine the effects of H2O2 on HO-1 mRNA expression, either A549 cells or Hep3B cells were exposed to H2O2 at concentrations of 100–1,000 μM in the culture medium. Furthermore, to examine the effects of hemin on HO-1 mRNA expression, either A549 cells or Hep3B cells were exposed to hemin at concentrations of 1–10 μM in the culture medium.
Transient-Transfection Assay
To explore the regulatory effect of a (GT)n repeat on HO-1 gene expression in either A549 cells or Hep3B cells, we constructed HO-1 promoter/luciferase fusion genes. The HO-1 promoter regions containing different numbers of (GT)n repeats (n=16, 20, 29, or 38) were amplified, by PCR, from leukocyte DNA of relevant subjects, with a sense primer, P2-s (5′-GACGCGTGCAAGCAGTCAGCAGAGGAT-3′), and an antisense primer, P2-as (5′-TGGGCTCGTTCGTGCTGGCT-3′) (fig. 1a), under the same conditions as described above. The PCR products were purified, digested by MluI and XhoI, and ligated into a luciferase reporter vector, pGL3-Basic (Promega), which does not contain any promoter sequence or enhancer. The recombinant genes with different numbers of (GT)n repeats (n=16, 20, 29, or 38) were cloned, sequenced, and used for the transient-transfection assay.
Transfection with the HO-1 promoter/ luciferase fusion gene was performed by the calcium phosphate–precipitation method, as described elsewhere (Muraosa and Shibahara 1993). A549 cells and Hep3B cells were cultured in either DMEM/8%FCS or DMEM/10%FCS, respectively. At the transfection, A549 cells or Hep3B cells (5 × 106 to 1 × 107) in six-well plates were incubated for 4 h in 1.0 ml of HBSP buffer (1.5 mM Na2HPO4, 10 mM KCl, 280 mM NaCl, 12 mM glucose, and 50 mM HEPES pH 7.1) containing 125 mM CaCl2, 5 μg of an HO-1 promoter/luciferase fusion gene, prepared as described above, and 0.5 μg of Renilla luciferase expression vector, pRL-TK (Promega), as an internal control (Takeda et al. 1994). The transfected cells were washed and cultured overnight in 2 ml of fresh medium at 37°C. Cells were then treated with either H2O2 (500 μM) or distilled water (control), at 37°C for 3 h. To measure dual luciferase activities caused by Photinus and Renilla luciferase, cellular extracts were prepared from each well, according to the method of PG-DUALTM-SP (Toyo Ink Manufacture). In brief, cells in each well were incubated with 500 μl of cell lysis buffer at 37°C for 15 min, frozen at −80°C and thawed and centrifuged at 4°C at 20,000 g for 10 min. The supernatant containing cellular extract was collected, and the intensity of light caused by Photinus and Renilla luciferase was sequentially monitored with a luminometer (Lumat LB 9507; EG & G Berthold). The valiability in transient-transfection efficiency was normalized with the internal control. The Photinus luciferase activity, which indicated the promoter activity of the HO-1 gene, was divided by Renilla luciferase activity, to calculate the relative luciferase activity, and was expressed as relative luciferase units (Kennedy et al. 1995). To examine the effects that hemin, as a standard inducer of HO-1, has on the promoter activities of the fugion genes, cells were incubated in the medium containing hemin (10 μM) (Okinaga et al. 1996).
Statistical Analysis
Associations between disease groups and specific classes of allele, as well as those between disease groups and genotype groups, were analyzed for significance, by the two-tailed χ2 test. Probability values were corrected by Bonferroni's method. Odds ratios and 95% confidence intervals (CIs) were calculated, to assess the relative disease risk conferred by a particular allele and genotype. Ages of subjects with and without CPE were matched in the present study (table 1). All subjects, with and without CPE, were male. Furthermore, all subjects were Japanese, and they represented an ethnic isolation. Therefore, statistical artifacts caused by population stratification could be ruled out, as described by Pritchard and Rosenberg (1999). The values are reported as mean ± SEM. Statistical analysis of age, Brinkman's index, and pulmonary-function test results was performed by unpaired t-test. In the transient-transfection assay and Northern-blot analysis, statistical analysis was performed by use of a two-way analysis of variance (ANOVA), followed by Bonferroni's method. Significance was accepted at P<.05.
Results
Allele Frequencies at the Polymorphic Locus
The numbers of (GT)n repeats in the human HO-1 gene showed a distribution of 16–38 in the individuals studied (fig. 1b). The distribution of the numbers of (GT)n repeats was trimodal, with one peak located at 22 GT repeats and the other two peaks located close together, at 27 and 30 GT repeats. Therefore, we divided the alleles into three subclasses, according to the numbers of (GT)n repeats, as described in the analysis, by McGinnis et al. (1995), of variable numbers of tandem-repeat loci adjacent to the 5′ end of the insulin gene. The lower component, with <25 GT repeats, was designated as “class S”; the middle component, with GT repeats ⩾25 and <30, was designated as “class M”; and the upper component, with ⩾30 GT repeats, was designated as “class L.” When all subjects—with and without CPE—were considered, distributions of 402 alleles were 159 (40%) for class S, 181 (45%) for class M, and 62 (15%) for class L (table 2).
Table 2.
Allele Frequencies at the Polymorphic Locus
|
No. (%) of Patients |
Odds Ratio (95%CI) versus Allele class |
|||||
| Allele Class | Without CPE (n=200) | With CPE (n=202) | All Other Classes | S | M | L |
| L | 20 (10) | 42 (21) | 2.4 (1.3–4.1)a | 2.9 (1.6–5.3)b | 2.0 (1.1–3.6)c | 1.0 |
| M | 88 (44) | 93 (46) | 1.1 (.7–1.6) | 1.5 (.9–2.2) | 1.0 | |
| S | 92 (46) | 67 (33) | 0.6 (.4–.9)d | 1.0 | ||
P<.004.
P<.001.
P<.03.
P<.02.
In the patients without CPE, distributions of 200 alleles were 92 (46%) for class S, 88 (44%) for class M and 20 (10%) for class L (table 2). The proportion of allelic frequencies in class L was significantly higher in patients with CPE than in those without CPE (42 [21%] with CPE vs. 20 [10%] without CPE; P<.004). In contrast, the proportion of allelic frequencies in class S was significantly lower in patients with CPE than in those without CPE (67 [33%] with CPE vs. 92 [46%] without CPE; P<.02). For patients with CPE, the odds ratio for class L versus all other classes was 2.4 (95% CI 1.3–4.1), and that for class S versus all other classes was 0.6 (95% CI 0.4–0.9) (table 2).
Genotypic Frequencies in Control and CPE Groups
Six genotypes (L/L, L/M, L/S, M/M, M/S, and S/S) of (GT)n repeats in the human HO-1 gene promoter were divided into two subgroups, according to their allele subclasses: group I with a class L allele (L/L, L/M, and L/S), and group II without a class L allele (M/M, M/S, and S/S) (tables 3 and 4). The proportion of genotypic frequencies in group I was significantly higher in patients with CPE than in those without CPE (38 [38%] with CPE vs. 20 [20%] without CPE; P<.008). The odds ratio for group I was 2.4 (95% CI 1.3–5.7) for patients with CPE (table 4). Since the class S allele had a reduced risk for CPE, the proportion of the genotype subgroup containing L/L and L/M, a part of group I, was significantly higher in the patients with CPE than in those without CPE (21 [21%] with CPE vs. 6 [6%] without CPE; P<.004) with an odds ratio of 4.1 (95% CI 1.7–10.1) for patients with CPE. Furthermore, all subjects homozygous for the class L allele (L/L) suffered from CPE.
Table 3.
Distribution of Genotypes
|
No. of Patients with Genotype |
|||||||
| Status | L/L | L/M | L/S | M/M | M/S | S/S | Total |
| Without CPE | 0 | 6 | 14 | 22 | 38 | 20 | 100 |
| With CPE | 4 | 17 | 17 | 21 | 34 | 8 | 101 |
Table 4.
Genotype Frequencies at the Polymorphic Locus
|
No. (%) of Patients |
|||
| Genotype Subgroup | Without CPE (n=100) | With CPE (n=101) | Odds Ratio (95%CI) |
| I | 20 (20) | 38 (38) | 2.4 (1.3–5.7)a |
| II | 80 (80) | 63 (62) | |
P<.008.
Functional Significance of the Polymorphic (GT)n Repeats for H2O2-Induced HO-1 Induction in Human Cell Lines
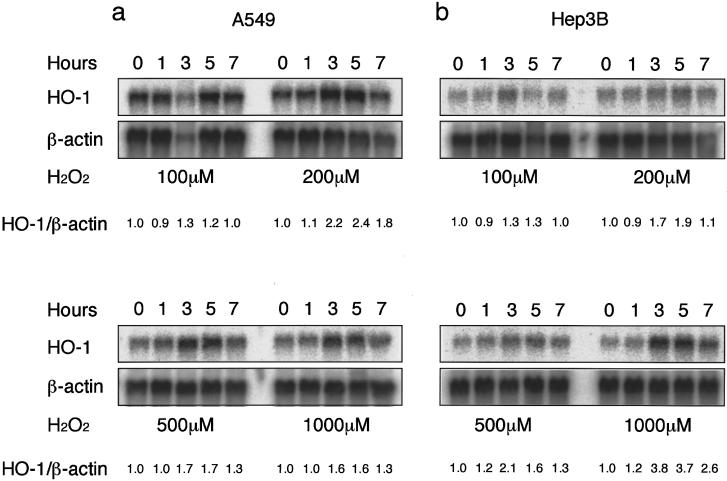
To examine the induction of human HO-1 by oxidants in cigarette smoke, we chose H2O2 as an oxidant and examined its effect on the expression of human HO-1 mRNA in A549 cells and Hep3B cells. Both A549 cells and Hep3B cells constitutively express HO-1 mRNA. Exposure of A549 cells and Hep3B cells to H2O2 caused increases in HO-1 mRNA, compared with values at the control (time 0). HO-1 mRNA expression in A549 cells and Hep3B induced by H2O2 was concentration dependent and time dependent (fig. 2). The treatment with H2O2 at 500 μM for 3 h was optimal for HO-1 mRNA induction in both A549 cells and Hep3B cells (fig. 2).
Figure 2.
Northern-blot analysis demonstrating increases in HO-1 mRNA levels in A549 cells (a) and Hep3B cells (b), before (0 h) and 1, 3, 5, and 7 h after treatment with either 100, 200, 500, or 1,000 μM of H2O2. β-Actin was used as an internal control. HO-1 mRNA was normalized with a constitutive expression of β-actin mRNA. The ratio of each normalized value to that of the 0-time control is shown as a relative expression level of HO-1 mRNA (HO-1/β-actin). Data are representative of three different experiments.
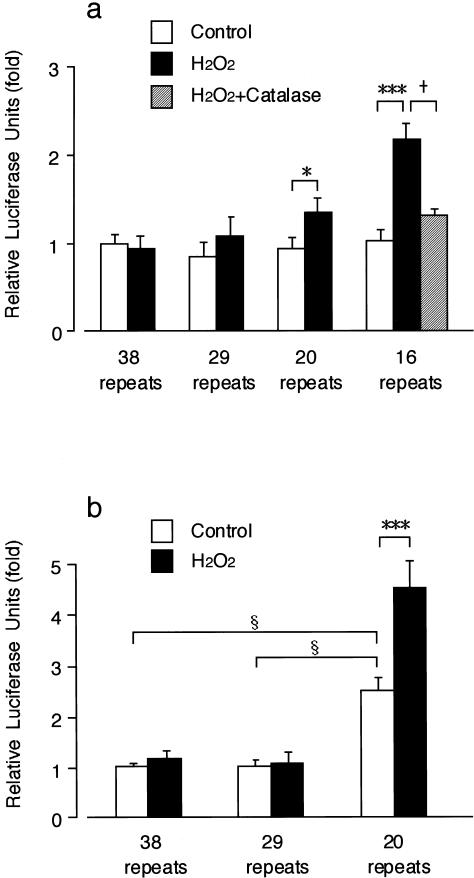
To explore the correlation between the risk of CPE and size of (GT)n repeats, we examined the regulatory effects of (GT)n repeats on HO-1 gene induction by oxidant stress. Transient-transfection assay using the recombinant fusion genes was performed in A549 cells and Hep3B cells under the optimized conditions (H2O2 at 500 μM for 3 h). Exposure to H2O2 increased relative luciferase activity in the A549 cells transfected with the gene carrying a (GT)16 or (GT)20 repeat, but it did not increase relative luciferase activity in the A549 cells transfected with either (GT)29 or (GT)38 (fig. 3a). Furthermore, pretreatment with catalase reversed the H2O2-induced increase in relative luciferase activity in the A549 cells with (GT)16 (fig. 3a). In the Hep3B cells, the basal luciferase activity was significantly higher in cells transfected with a (GT)20 repeat than in those with either (GT)29 or (GT)38 (fig. 3b). Exposure of H2O2 further increased the relative luciferase activity in the Hep3B cells transfected with a (GT)20 repeat (fig. 3b).
Figure 3.
Promoter activities of 5′-flanking regions of the HO-1 gene in either A549 cells (a) or Hep3B cells (b) transfected with the recombinant gene carrying either (GT)16, (GT)20, (GT)29, or (GT)38 repeat after treatment with either H2O2 (500 μM for 3 h) or distilled water (control), and the effects of catalase (2,000 U/ml for 15 min) on the increase in relative luciferase activity induced by H2O2 in the A549 cells transfected with the gene carrying (GT)16. Each luciferase activity was normalized to Renilla luciferase activity and was expressed as a ratio to the control value of the cells with (GT)38. Results are reported as mean ± SEM from nine samples. Significant increases by H2O2 are indicated by * (P<.05) and *** (P<.001), respectively. Significant decrease by catalase is indicated by + (P<.05). Significant differences in basal promoter activity are indicated by § (P<.05).
We also performed the same experiments by using hemin as a standard inducer of HO-1 (Okinaga et al. 1996). Exposure of A549 cells and Hep3B cells to hemin increased HO-1 mRNA in a concentration- and time-dependent manner. However, in the transient-transfection assay, hemin (10 μM for 5 h) did not increase relative luciferase activity in either A549 cells or Hep3B cells (data not shown).
Discussion
The present study has shown that the 5′-flanking polymorphism in the HO-1 gene is associated with CPE in Japanese smokers. This association may be a result of the promoter activity being modulated by the length variability of the (GT)n repeats. Taken together with the notion that antioxidant activity of HO-1 covers wide varieties of stimulating stresses (Choi and Alam 1996), these results suggest that HO-1 may play important roles in lung protection from exposure to reactive oxygen species.
The distribution of the numbers of (GT)n repeats in smokers without CPE in the present study is consistent with that in the previous report, by Kimpara et al. (1997), of patients with either Alzheimer disease or Parkinson disease and of normal patients. In contrast, the proportion of allelic frequencies and genotypes with ⩾30 GT repeats was significantly larger in CPE patients than in smokers without CPE. Because the distribution of the numbers of (GT)n repeats was trimodal, with one peak located at 22 GT repeats and the other two peaks located close together, at 27 and 30 GT repeats, we classified the alleles into three classes, according to the numbers of the (GT)n repeats. Using the χ2 test, we observed significantly higher frequencies of class L alleles and of genotypes containing class L (L/L, L/M, and L/S), in smokers with CPE compared with smokers without CPE. Conversely, the frequency of the class S allele was significantly lower in smokers with CPE than in smokers without CPE. These results suggest that the large size of (GT)n repeats is associated withCPE susceptibility induced by cigarette smoke. Furthermore, in the present study, all patients of L/L genotype are smokers with CPE. Therefore, the smokers carrying the class L allele (i.e., those with genotypes L/L, L/M, and L/S) may have a higher risk for CPE. On the other hand, the class S allele seemed to act as a negative risk factor. In fact, the frequency of the L/S genotype in CPE patients did not differ significantly from that in smokers without CPE.
We then attempted to detect the influence of the number of (GT)n repeats on the inducibility of the HO-1 gene promoter under oxidative stimulus, by transient-transfection assay. Significant induction of the HO-1 promoter/luciferase fusion gene by H2O2 was observed only when the number of GT repeats in the fusion gene was ⩽20 (categorized as a class S allele), whereas the expression of the fusion gene carrying ⩾29 GT repeats (categorized as a class L allele) remained at a control level during H2O2 exposure. Furthermore, basal expression of the fusion gene with 20 GT repeats was 2.5 times higher than that of the gene with ⩾29 GT repeats, in the case of transfection into Hep3B cells. These data are consistent with the idea that the lungs of patients with the group II genotype (alleles M/M, M/S, and S/S) are capable of utilizing more of the antioxidant activity of HO-1 than are those of patients with the group I genotype (alleles L/L, L/M, and L/S) when the lungs are exposed to reactive oxygen species in cigarette smoke.
In the present study, we have constructed the HO-1 promoter/luciferase fusion genes by ligating the proximal promoter region of the HO-1 gene (Shibahara et al. 1989) that includes a (GT)n repeat to a luciferase reporter basic vector, which originally does not have a promoter sequence or enhancer. We transiently transfected A549 cells and Hep3B cells with the fusion genes and monitored the inducibility of the promoter region containing the (GT)n repeats triggered by H2O2 or hemin. H2O2 induced luciferase gene expression in the cells transfected with fusion genes containing small numbers of (GT)n repeat, but hemin could not do so. These results suggest that the human HO-1 promoter region used in the present experiment contains a responsive element for H2O2, but not for hemin as has been reported elsewhere (Shibahara et al. 1989; Takeda et al. 1994; Okinaga et al. 1996). HO-1 expression is induced by many stimuli, but its regulation differs in cell types, tissue types, and animal species. It was reported that H2O2 induces the HO-1 gene in mouse fibroblast (Alam et al. 1995) but fails to induce the expression of human HO-1 reporter constructs in transfected avian liver-cell cultures (Lu et al. 1997). Likewise, hemin up-regulates HO-1 mRNA in a human erythroblastic cell line and in human macrophage (Yoshida et al. 1988; Okinaga et al. 1996). Although various regulatory elements have been identified within the 5′-flanking regions of the mouse, rat, and human HO-1 genes, an H2O2-responsive element has not been identified. Moreover, a (GT)n repeat has been identified only in the promoter region of the human HO-1 gene. Further study is needed to identify the H2O2-responsive element and the induction mechanisms of human HO-1 gene expression.
The results of transient-transfection assay also suggest that the (GT)n repeat in the human HO-1 gene promoter per se functions as a novel cis-acting element modulating the transcription under basal and H2O2-stimulated conditions. A (GT)n repeat has a structure of Z-potential DNA sequence, and its negative effect on the transcription of the rat prolactin gene has been reported (Naylor and Clark 1990). In fact, the deletion of the segment encompassing both the (GT)n repeat and purine-rich sequences in the HO-1 5′-flanking region restored the function of the heat-shock element in the transient-transfection assay (Okinaga et al. 1996). In this context, we observed that, as reported elsewhere (Okinaga et al. 1996), heat shock does not increase relative luciferase activity in our fusion gene (data not shown), which contains a (GT)n repeat and purine-rich sequences. A (GT)n repeat in the HO-1 gene may be a part of the machinery to regulate HO-1 expression to a proper level under oxidative stimulus.
The initial degradation of heme by microsomal HO involves the liberation of iron and carbon monoxide and the formation of biliverdin, which is subsequently reduced to bilirubin by cytosolic biliverdin reductase (Tenhunen et al. 1969, 1970). Therefore, higher intracellular HO-1 activity results in an increased content of bilirubin, which is an efficient scavenger of reactive oxygen species (Stocker et al. 1987).
Recently, the first human case of HO-1 deficiency was reported (MIM 141250) (Yachie et al. 1999). The patient was a 6-year-old boy with growth retardation, anemia, iron deposition, and vulnerability to stressful injury, all of which were characteristics observed in HO-1–targeted mice (Poss and Tonegawa 1997a). Epstein-Barr virus–transformed lymphoblastoid cell lines established from the patient revealed a reduced survival rate against oxidative stress of hemin, compared with that in control cell lines (Yachie et al. 1999). Likewise, cultured embryonic fibroblasts from the HO-1–targeted mice were more sensitive to exposure to hemin, H2O2, paraquat, or cadmium than were wild-type cells, and they showed hypersensitivity to cytotoxicity caused by H2O2 (Poss and Tonegawa 1997b). In addition, the induction of endogenous HO-1 suppressed inflammatory pleural exudate caused by carrageenin in vivo (Willis et al. 1996). Therefore, it is possible that the anti-inflammatory effect of HO-1 prevents the development of CPE.
The present study demonstrates that the 5′-flanking polymorphism in the HO-1 gene is associated with CPE susceptibility caused by cigarette smoke in Japanese patients with CPE. However, CPE is a multifactorial disorder and cannot be defined by a single-gene polymorphism. Therefore, further studies are needed that will examine a larger population or sample from different ethnic origins.
Acknowledgments
We thank Drs. Munehiko Ishii, Masaharu Sugiyama, Ryo Kikuchi, Hideki Nakazawa, Kiyoshi Zayasu, Kazuhiko Satoh, Shigeru Itabashi, Akio Kanda, Mizue Monma, Mitsutoshi Shinkawa, and Hidenori Takahashi for samples, and we thank Mr. G. Crittenden for English corrections. This work was supported in part by a Grant-in-Aid for Exploratory Research (to M. Y.) and a Grant-in-Aid for Scientific Research on Priority Areas (to S. S.), both from the Japanese Ministry of Education, Science, Sports, and Culture.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/index.html (for the 5′-flanking region and exon 1 of the human HO-1 gene [X14782]) [Google Scholar]
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for α1-antitrypsin deficiency [MIM 107400] and HO-1 deficiency [MIM 141250])
References
- Alam J, Camhi S, Choi AMK (1995) Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer. J Biol Chem 270:11977–11984 [DOI] [PubMed]
- Celli BR, Snider GL, Heffner J, Tiep B, Ziment I, Make B, Braman S, et al (1995) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med Suppl 152:S77–S120 [PubMed]
- Choi AMK, Alam J (1996) Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 15:9–19 [DOI] [PubMed]
- Farber JL (1994) Mechanisms of cell injury by activated oxygen species. Environ Health Perspect 102:17–24 [DOI] [PMC free article] [PubMed]
- Garver RI Jr, Mornex J-F, Nukiwa T, Brantly M, Courtney M, LeCoco J-P, Crystal RG (1986) Alpha 1-antitrypsin deficiency and emphysema caused by homozygous inheritance of non-expressing alpha 1-antitrypsin genes. N Engl J Med 314:762–766 [DOI] [PubMed]
- Hanrahan JP, Sherman CB, Bresnitz EA, Emmons KM, Mannino DM (1996) Cigarette smoking and health. Am J Respir Crit Care Med 153:861–865 [DOI] [PubMed]
- Hoidal JR, Niewoehner DE (1983) Pathogenesis of emphysema. Chest 83:679–685 [DOI] [PubMed]
- Janoff A (1985) Elastases and emphysema: current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis 132:417–433 [DOI] [PubMed]
- Kennedy GC, German MS, Rutter WJ (1995) The minisatellite in the diabetes susceptibility locus IDDM2 regulates insulin transcription. Nat Genet 9:293–298 [DOI] [PubMed]
- Kimpara T, Takeda A, Watanabe K, Itoyama Y, Ikawa S, Watanabe M, Arai H, et al (1997) Microsatellite polymorphism in the human heme oxygenase-1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet 100:145–147 [DOI] [PubMed]
- Kuperman AS, Riker JB (1973) The variable effect of smoking on pulmonary function. Chest 63:655–660 [DOI] [PubMed]
- Lu T-H, Pepe JA, Gildemeister OS, Tyrrell RM, Bonkovsky HL (1997) Regulation of expression of the human heme oxygenase-1 gene in transfected chick embryo liver cell cultures. Biochim Biophys Acta 1352:293–302 [DOI] [PubMed]
- Maines MD (1997) The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37:517–554 [DOI] [PubMed]
- McGinnis RE, Spielman RS (1995) Insulin gene 5′ flanking polymorphism: length of class 1 alleles in number of repeat units. Diabetes 44:1296–1302 [DOI] [PubMed]
- Muraosa Y, Shibahara S (1993) Identification of a cis-regulatory element and putative trans-acting factors responsible for 12-O-tetradecanoylphorbol-13-acetate (TPA)-mediated induction of heme oxygenase expression in myelomonocytic cell lines. Mol Cell Biol 13:7881–7891 [DOI] [PMC free article] [PubMed]
- Naylor LH, Clark EM (1990) d(TG)n·d(CA)n sequences upstream of the rat prolactin gene form Z-DNA and inhibit gene transcription. Nucleic Acids Res 18:1595–1601 [DOI] [PMC free article] [PubMed]
- Ogushi F, Hubbard RC, Vogelmeier C, Fells GA, Crystal RG (1991) Risk factors for emphysema: cigarette smoking is associated with a reduction in the association rate constant of lung α1-antitrypsin for neutrophil elastase. J Clin Invest 87:1060–1065 [DOI] [PMC free article] [PubMed]
- Okinaga S, Takahashi K, Takeda K, Yoshizawa M, Fujita H, Sasaki H, Shibahara S (1996) Regulation of human heme oxygenase-1 gene expression under thermal stress. Blood 87:5074–5084 [PubMed]
- Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AMK (1999) Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest 103:1047–1054 [DOI] [PMC free article] [PubMed]
- Poss KD, Tonegawa S (1997a) Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 94:10919–10924 [DOI] [PMC free article] [PubMed]
- ——— (1997b) Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA 94:10925–10930 [DOI] [PMC free article] [PubMed]
- Pritchard JK, Rosenberg NA (1999) Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet 65:220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S, Sato M, Muller RM, Yoshida T (1989) Structural organization of the human heme oxygenase gene and the function of its promoter. Eur J Biochem 179:557–563 [DOI] [PubMed]
- Smith CAD, Harrison DJ (1997) Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. Lancet 350:630–633 [DOI] [PubMed]
- Snider GL (1992) Emphysema: the first two centuries—and beyond: a historical overview, with suggestions for future research. Part 2. Am Rev Respir Dis 146:1615–1622 [DOI] [PubMed]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological importance. Science 235:1043–1046 [DOI] [PubMed]
- Takeda K, Ishizawa S, Sato M, Yoshida T, Shibahara S (1994) Identification of a cis-acting element that is responsible for cadmium-mediated induction of the human heme oxygenase gene. J Biol Chem 269:22858–22867 [PubMed]
- Tenhunen R, Marver HS, Schmid R (1969) Microsomal heme oxygenase: characterization of the enzyme. J Biol Chem 244:6388–6394 [PubMed]
- Tenhunen R, Ross ME, Marver HS, Schmid R (1970) Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 9:298–303 [DOI] [PubMed]
- Thom TJ (1989) International comparisons in COPD mortality. Am Rev Respir Dis Suppl 140:S27–S34 [DOI] [PubMed]
- Willis D, Moore AR, Frederick R, Willoughby DA (1996) Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med 2:87–90 [DOI] [PubMed]
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, et al (1999) Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103:129–135 [DOI] [PMC free article] [PubMed]
- Yoshida T, Biro P, Cohen T, Müller RM, Shibahara S (1988) Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem 171:457–461 [DOI] [PubMed]