Abstract
An adult mourning dove (Zenaidura macroura) was presented with dyspnea, poor body condition, and poor flight endurance. Nodular lesions were visible on the cere, both feet, and skin covering the pectoral region. Histopathological examination revealed epithelial hyperplasia with eosinophilic intracytoplasmic inclusion bodies consistent with Avipoxvirus infection.
Résumé
Infection à un poxvirus aviaire chez une tourterelle triste. Une tourterelle triste adulte (Zenaidura macroura) a été présentée pour dyspnée, mauvaise condition physique et faible endurance au vol. Des lésions nodulaires étaient visibles sur la cire, sur les deux pattes et sur la peau de la région pectorale. L’examen histopathologique a révélé une hyperplasie épithéliale avec des corps d’inclusion intracytoplasmique éosinophiles compatibles avec une infection à Avipoxvirus.
(Traduit par Docteur André Blouin)
An adult mourning dove (Zenaidura macroura) was presented to the Wild-life Center, Waynesboro, Virginia, in August 2004. This bird was in poor body condition with a body weight of 80 g. This bird had been found on the roadside with difficulty in flying by a local resident, who captured the bird and brought it to the wildlife center for further examination. Following clinical examination, the dove became dyspneic and unable to fly. There were 1- to 1.5-cm diameter nodular lesions on the 4th digit of both feet (Figure 1), right tibiotarsus, 1st digit of the left foot, and the left side of the cere, occluding the left naris (Figure 2). A small perforation covered with tissue debris was in the tostral end of the mandibular beak.
Figure 1.

A photograph of a large nodule on the digit of mourning dove.
Figure 2.

A photograph of mourning dove showing nodules on cere occluding left naris.
On clinical examination, nodules were moderately soft, and superficial layers of the nodules were removed without difficulty. The soft tissue beneath the superficial layers of nodules was congested and hemorrhagic. Impression smears were taken from the nodules on the left 4th digit and the right tibiotarsus. Microscopic examination revealed numerous heterophils, red blood cells, necrotic epithelial cells, and bacterial colonies, indicating inflammation, likely due to a secondary bacterial infection. Following clinical examination, a tentative diagnosis of avian pox was made, and the bird was placed in an isolation ward. A swab from the crop showed no evidence of a Trichomonas sp. infection. Supportive therapy was recommended; this involved the administration, SC, of 4 mL of lactated Ringer’s solution and 22.7 mg of enrofloxacin (Baytril; Bayer Animal Health Care, Tarrytown, New York, USA) SC.
On the following day, clinically, the bird was depressed, although the crop was full, indicating that the bird had eaten overnight. The bird was maintained in isolation with the lesions being treated as open wounds, cleaned with diluted chlorhexidine (Novalsan; Lambriat Animal Health Care, Mahaska, Kansas, USA) and silver sulfadiazine (Silvadene cream 1%; Monarch Pharmaceuticals, Bristol, Tennessee, USA) ointment. Due to the long course of the disease and the infectious nature of avian pox, it was decided to euthanize the dove on the 3rd day and perform a necropsy.
On necropsy, the digital, tibiotarsal, cere, and beak lesions were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for histopathologic examination. Two nodules on the left side of the keel and 1 on the right were firmly attached to the surrounding tissue (Figure 3). These keel lesions were soft, yellowish, 0.5 cm in diameter, and raised above the surface of the skin. Histopathological diagnosis of epidermal hyperplasia and intracytoplasmic inclusion bodies were consistent with a diagnosis of Avian pox virus infection (Figures 4 and 5).
Figure 3.
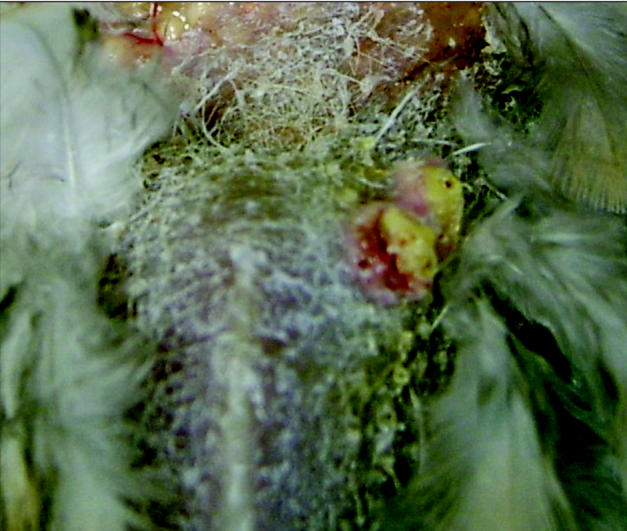
A photograph of nodules on the keel of mourning dove.
Figure 4.
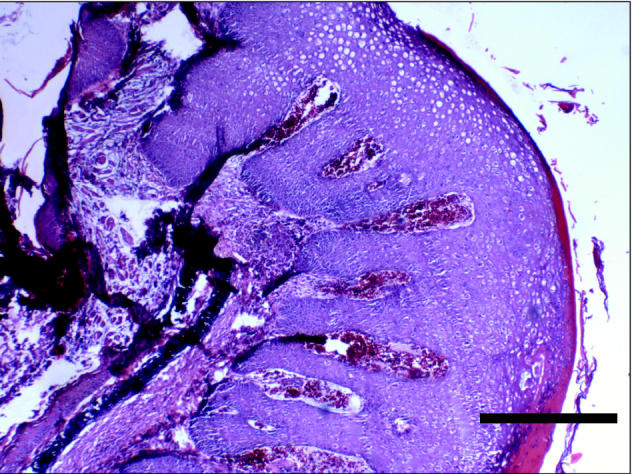
A low power photomicrograph of section through a nodule showing epithelial hyperplasia. Bar = 1 mm
Figure 5.
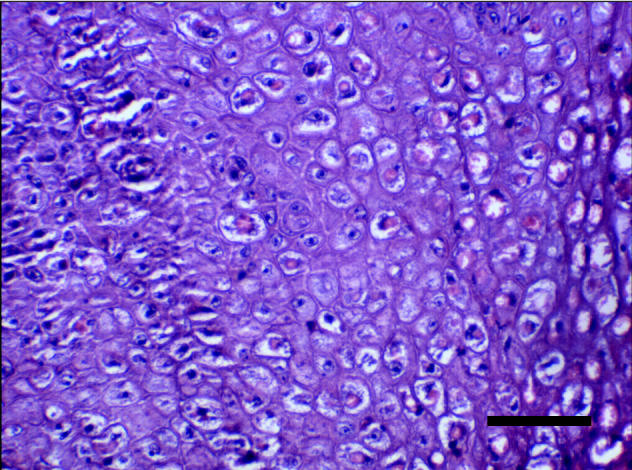
A high power photomicrograph of a nodule showing intracytoplasmic eosinophilic inclusion bodies in keratinocytes. Bar = 100 μm
Avian pox virus is a member of the family Poxviridae and genus Avipoxvirus. Poxviridae viruses are large, double-stranded DNA viruses (3). Ten species (canary, fowl, junco, mynah, pigeon, psittacine, quail, sparrow, starling, turkey) and 2 tentative species (peacock and penguin) are recognized (5). Two forms, cutaneous and diphtheritic, are generally seen, although a third, systemic, is occasionally observed. About 232 species of birds in 23 orders are known to have been naturally infected with Avipoxvirus (1).
Avian pox has been described worldwide, although it is considered a fairly new disease in North America (6). The case report frequency has recently increased and involves newly affected bird species, indicating this virus is an emerging disease (2). The virus is typically slow spreading via direct and indirect contact. Indirect spread is through contact with contaminated feed, water, feeders, and perches, and inhalation of contaminated dander. Direct spread is through ingestion of infected carcasses or physical contact with an infected bird. The virus enters through a break in the skin or mucous membrane. Dry scabs can harbor the virus for months, if not years (2,7).
Mosquitoes are considered to be an important vector for the spread of Avian pox virus. Mosquitoes feed on a viremic bird or contaminated lesion and then feed on a healthy bird, transmitting the virus. It is believed that mosquitoes can harbor the virus for a month or more (7). An increase in avian pox cases has been seen to match the mosquito season (2,7). Stable flies have been shown to transmit the virus experimentally (6,7). Blowfly larvae have been found in lesions, indicating it is possible that these insects may also play a role in transmission (4). Blowflies could lay eggs in necrotic lesions, become contaminated, and then contact broken skin of a noninfected bird (4).
Incubation is 7 to 14 d, after which small vesicles appear (7). Cutaneous or dry pox consists of nodular lesions on areas free of feathers, such as legs, cere, and eyelids (2,7,10). The diphtheritic or wet form affects the digestive and upper respiratory tracts (2,7,10). The viremic or systemic form is rarely seen, although mortality is high in affected birds (2,10). Birds can be affected with both forms of the disease at one time. Mortality is generally low, particularly with the cutaneous form, if eating and respiration are not severely affected. Small vesicles form first. Once these break, nodules form and eventually progress to scabs. Scarring is minimal, if lesions are not traumatized during healing (7). Secondary infections can also lead to severe scarring (7). Infections are typically self-limiting; however, secondary infections do occur. Cell-mediated immunity is thought to be most important in clearing the virus (7). Latency of Avian pox virus infection is suspected, although further studies are required (2,3).
There is some variation in forms and severity in different bird species. Symptoms seen with the systemic form include wart-like masses on the skin, depression, cyanosis, and anorexia. This form is most common in canaries and finches (2,7). These birds may die acutely before microscopic changes are seen. Raptors are typically affected by the dry form of the virus, but mortality is higher in them than in other birds (2). There are few reported cases in waterfowl (2).
Diagnosis is generally made by the clinical presentation and the lesions; however, a differential diagnosis that should be considered for the dry form is bumblefoot or staphylococcal infection (9). Avian pox can be confirmed by histological identification of eosinophilic intracytoplasm inclusion bodies (Bollinger bodies) (2–4,7,9). Smears and tissues should be prepared with Wright’s, Giemsa’s, or similar stains (1). Hematoxylin and eosin stain is also typically used. Degenerating keratinocytes will be enlarged or ballooned with a pale vacuolated cytoplasm (3,9). Virus isolation can be attempted on the chorioallantoic membrane of embryonated chicken eggs or in duck embryo fibroblast cells (4). Under electron microscopic examination, the virions are seen to be enveloped, pleomorphic, and brick-shaped, with a diameter of 200 nm (3).
No successful treatment is available at present. Antibiotics can be administered to control secondary bacterial infections, particularly in birds with respiratory and gastrointestinal lesions (7). For falcons, lesions can be typically extensive and treatment can be attempted to stop development of nodules. Thermocautery or electrocautery followed by debridement of the upper epithelial layers and dressing with solvent-based tinctures or antibiotic or corticosteroid ointments will assist in minimizing scab formation (8). If scabs have already formed, surgical debridement and dressing with a hydrocolloid paste to promote granulation tissue has been attempted (8).
Modified live vaccines against Pigeonpox and Fowlpox virus are available for poultry (2,11). Control for wild bird species includes elimination of standing water to control the mosquito population and decontamination of bird feeders and perches regularly with a 10% bleach solution (6).
Avian pox may play a larger role in wild bird mortality than previously believed. Avian pox is suspected of having oncogenic properties (1,7). Columbiformes often develop skin tumors after dry pox infection, whereas finches commonly develop lung tumors following wet pox infection (7). Furthermore, the infection may not be the direct cause of mortality; poor flight ability and endurance, ataxia, and weakness will increase the risk of an infected bird for predation (10). Avian pox is a well-documented disease historically, but further studies are needed to determine the role of insect vectors in the spread of the virus and the possible oncogenic role the virus plays in infected birds. Although it is commonly seen in species such as the mourning dove, its species range appears to be broadening.
Mourning doves are commonly encountered birds in central Virginia. They are usually seen in small groups in backyard feeders. This behavior increases their contact with other birds, thereby increasing the risk of acquiring infectious diseases from other birds, such as avian pox.
Acknowledgments
The author acknowledges Dr. Carey Adams of The Wildlife Center, Waynesboro, Virginia, for clinical guidance; Dr. John Spahr of Blue Ridge Pathologists for supplying the histologic photographs; and Dr. Susantha Gomis of the Western College of Veterinary Medicine for his advice. CVJ
Footnotes
Dr. Pledger’s current address is 330 Northridge Road, Ruckersville, Virginia 22968, USA.
Dr. Pledger will receive 50 free reprints of her article, courtesy of The Canadian Veterinary Journal.
References
- 1.Bolte AL, Meurer J, Kaleta EF. Avian host spectrum of avipoxviruses. Avian Pathol. 1999;28:415–432. doi: 10.1080/03079459994434. [DOI] [PubMed] [Google Scholar]
- 2.Ciganovich EA, ed. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. Washington DC: United States Geological Survey, 1999:163–170.
- 3.Deem SL, Heard DJ, Fox JH. Avian pox in eastern screech owls and barred owls from Florida. J Wildl Dis. 1997;33:323–327. doi: 10.7589/0090-3558-33.2.323. [DOI] [PubMed] [Google Scholar]
- 4.Docherty DE, Long RIR, Flickinger EL, Locke LN. Isolation of poxvirus from debilitating cutaneous lesions on four immature grackles (Quiscalus sp) Avian Dis. 1991;35:244–247. [PubMed] [Google Scholar]
- 5.DsDna Viruses [database on the internet]: The International Committee on Taxonomy of Viruses c1998. Available from http://www.ncbi.nlm.nih.gov/ICTV/overview/dsdna.html Last accessed October 13, 2004.
- 6.Michigan Department of Natural Resources [page on the Internet]: Michigan Wildlife Disease Manual: Avian Pox, c2001. Available from http://www.michigan.gov/dnr/0,1607,7-153-10370_12150_12220-26362—,00.html Last accessed January 16, 2004
- 7.Ritchie BW. Avian viruses: Function and control. Lake Worth, Florida: Winger’s Publ, 1995:285–311.
- 8.Samour JH, Naldo JL. Medical management of avian pox lesions on the cere in captive falcons. Exotic DVM. 2001;2:14–15. [Google Scholar]
- 9.Schoemaker NJ, Dorrestein GM, Lumeij JT. An avipoxvirus infection in a goshawk (Accipiter gentiles) Avian Pathol. 1998;27:103–106. doi: 10.1080/03079459808419282. [DOI] [PubMed] [Google Scholar]
- 10.Tangredi BP. Avian pox in a mourning dove. Vet Med Small Anim Clin. 1974;69:700–701. [PubMed] [Google Scholar]
- 11.Winterfield RW, Reed W. Avian pox: Infection and immunity with quail, psittacine, fowl, and pigeon pox viruses. Poult Sci. 1985;64:65–70. doi: 10.3382/ps.0640065. [DOI] [PubMed] [Google Scholar]


