Introduction
Embryogenesis is regulated by a number of complex signaling cascades, which are critical for normal development. One such pathway begins with a secreted protein called “SONIC HEDGEHOG” (SHH [MIM 600725]), which sets off a chain of events in target cells, leading to the activation and repression of target genes by transcription factors in the Gli family. Dysregulation of the Sonic hedgehog–Patched–Gli (Shh-Ptch-Gli) pathway leads to several human diseases, including birth defects and cancers.
Elements of the Shh-Ptch-Gli pathway are highly conserved, indicating its essential role in development. However, Shh signaling is also apparently susceptible to evolutionary pressures. Thus, Shh signaling has been adapted to serve tremendously diverse functions in a wide array of animal models, in both embryonic and adult life. Shh and the downstream molecules involved in signal transduction vary, to some extent, between animals, as well. Although a great deal can be learned from the study of animal models, it is essential to not assume that what is true in one model system is necessarily true in another. Thus, this review focuses specifically on our current understanding of the Shh-Ptch-Gli pathway and its clinical significance in humans.
The Human SHH-PTCH-GLI Signaling Pathway
During the past few years, a great deal of information about the mechanisms and significance of human SHH-PTCH-GLI signaling has emerged. SHH induces cell proliferation in a tissue-specific manner during embryogenesis, and inherited or sporadic mutations in SHH-pathway genes have been implicated in a number of human birth defects. If the SHH-PTCH-GLI pathway is aberrantly activated during adult life, the resulting cellular proliferation manifests as cancer. Thus, studying the human SHH-PTCH-GLI pathway is essential in the understanding of the mechanisms of human birth defects and cancer and, ultimately, in the identification therapeutic targets in SHH-pathway diseases.
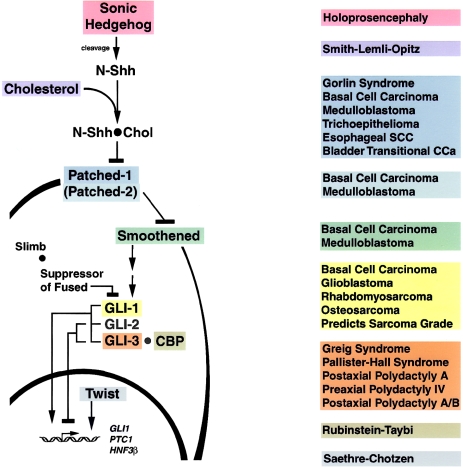
Our current understanding of the human SHH-PTCH-GLI pathway and its role in human disease is outlined in figure 1. Since the Shh-pathway components, their tissue specificity, and their functions vary between species (Lee et al. 1997; Sasaki et al. 1997; Brewster et al. 1998; Keys et al. 1999; King and Brown 1999), only information from human genes and proteins is presented here. Interestingly, defects in any of several steps in the SHH-PTCH-GLI pathway lead to similar clinical phenotypes, presumably through functionally equivalent effects on downstream target genes.
Figure 1 .
Human SHH-PTCH-GLI pathway (left) and its links to human diseases (right). The association of the human disease with malfunction of a given element in the pathway is indicated by the matching colors of the boxes. The placement of the elements of the pathway, as well as their role as a repressor (bars) or as an activator (arrows), has been demonstrated in humans. The exceptions are that interaction between human GLI3 and CBP was demonstrated using mouse CBP (Dai et al. 1999), human GLI genes were tested in frog and mouse cells (Ruiz i Altaba 1998; Dai et al. 1999), and mouse gli2 protein function is shown for completeness, although human GLI2 has not been fully characterized (Tanimura et al. 1998; Sasaki et al. 1999).
Sonic Hedgehog
During development of the human embryo, SONIC HEDGEHOG (SHH) is expressed in the notochord, the floorplate of the neural tube, the brain, the zone of polarizing activity in the developing limbs, and the gut (Odent et al. 1999). The tissue-specific expression of other SHH-pathway members has not yet been determined in human embryogenesis, although GLI1 (MIM 165220), GLI2 (MIM 165230), and GLI3 (MIM 165240) RNA expression is known to persist in some adult human tissues (Ruppert et al. 1988). Sporadic and inherited mutations in the human SHH gene have been shown to cause holoprosencephaly (HPE [MIM 236100]), a severe midline defect that includes cleft lip and palate, single maxillary incisor, impaired CNS septation, and phenotypes ranging in severity from hypotelorism to a single cyclopic eye (Belloni et al. 1996; Roessler et al. 1996, 1997; Nanni et al. 1999). HPE is a frequent cause of prenatal death in humans; it is detected in 1/250 induced abortions but in only 1/16,000 live births (Cohen 1989).
The SHH protein needs to be processed to an active form in order to function in developmental patterning. SHH is cleaved to an active N-terminal form, which is then modified by the addition of a cholesterol moiety (Pepinsky et al. 1998; Williams et al. 1999). Defects in cholesterol biosynthesis lead to the autosomal recessive Smith-Lemli-Opitz (SLOS [MIM 270400]) syndrome in humans, which shares some features with SHH-pathway diseases. This suggests that some or all of the clinical features of SLOS may be due to defective SHH signaling (Donnai et al. 1987; Tint et al. 1994; Kelley et al. 1996). Patients with SLOS generally have syndactyly, anteverted nares, ptosis, cryptorchidism, CNS hypoplasia, failure to thrive, occasional polydactyly and HPE, and, in a subset of patients, death within the 1st year (Kelley et al. 1996; Jones 1997).
PATCHED-1, PATCHED-2, and SMOOTHENED
SHH functions by binding its 12-span transmembrane receptor proteins, PATCHED-1 (PTCH-1 [MIM 601309]) and PATCHED-2 (PTCH-2 [MIM 603673]), in target cells (Stone et al. 1996; Smyth et al. 1999). PATCHED-1 and, possibly, PATCHED-2, normally inhibit downstream signaling through a physical interaction with a 7-span transmembrane protein, SMOOTHENED (SMOH [MIM 601500]). In model systems in which Shh is expressed nearby, it relieves the tonic inhibition of Smoothened by Patched and allows Shh-Ptch-Gli signaling to progress (Murone et al. 1999).
Like all of the other components of the SHH-PTCH-GLI pathway, the PATCHED and SMOOTHENED genes are also clinically relevant. The PTCH gene functions as a tumor suppressor, and activating SMOH mutants have been shown to function as oncogenes (Stone et al. 1996; Xie et al. 1998). Mutations of human PTCH-1, PTCH-2, and SMOH have all been detected in basal cell carcinomas and in medulloblastomas (MIM 155255) and result in dysregulated GLI signaling. Sporadic inactivating PTCH-1 mutations have also been reported in trichoepitheliomas (Vorechovsky et al. 1997), esophageal squamous cell carcinomas (Maesawa et al. 1998 [MIM 133239]), and transitional cell carcinomas of the bladder (McGarvey et al. 1998 [MIM 109800]). Furthermore, PTCH-1–inactivating mutations cause the genetic disease nevoid basal cell carcinoma syndrome (NBCCS), also called “Gorlin syndrome” or “basal cell nevus syndrome” (BCNS [MIM 109400]), which is an autosomal dominant disorder characterized by basal cell nevi with a high rate of malignant transformation, an increased risk of medulloblastomas and rhabdomyosarcomas, generalized overgrowth, and developmental defects including short metacarpals, rib defects, broad facies, and dental abnormalities (Bale et al. 1991; Gorlin 1995; Jones 1997).
The GLI Family
The GLI1 gene was originally identified as an amplified gene in a malignant glioma (Kinzler et al. 1987). It was the first member described in the human GLI gene family. During the past decade, four human genes have been designated “GLI1”–“GLI4”, and these genes have been mapped to separate loci by FISH (Arheden et al. 1989; Ruppert et al. 1990; Kas et al. 1996; Matsumoto et al. 1996).
GLI1, GLI2, and GLI3 encode transcription factors that share five highly conserved tandem C2-H2 zinc fingers and a consensus histidine-cysteine linker sequence between zinc fingers (Ruppert et al. 1988). These features distinguish between the similar Gli and Krüppel protein families (Kinzler et al. 1988). The GLI1 and GLI3 proteins recognize a conserved GACCACCCA sequence in the promoters of target genes (Kinzler and Vogelstein 1990; Ruppert et al. 1990; Pavletich and Pabo 1993; Vortkamp et al. 1995), and GLI2 recognizes a nearly identical GAACCACCCA motif (Tanimura et al. 1998). GLI4, which is also known as “HKR4” (human Krüppel-related gene 4), is actually most similar to the Drosophila Krüppel family of zinc-finger proteins (Ruppert et al. 1988) and was misclassified as a member of the human GLI gene family (GLI4 [MIM 165280]) (Kas et al. 1996). This nomenclature issue has caused confusion in the Gli literature during recent years. Throughout the present report, the human GLI family of genes refers to GLI1–GLI3. The chromosomal locations of human GLI1–GLI4 are summarized in table 1.
Table 1.
Human GLI Genes and Associated Diseases
| Gene (Locus) | Associated Human Disease(s) | Presumed Mechanism(s) |
| GLI1 (12q13) | Glioblastoma | Amplification |
| Rhabdomyosarcoma | Amplification | |
| Osteosarcoma | Amplification | |
| B cell lymphoma | Amplification | |
| Basal cell carcinoma | Misexpression | |
| Predicts adult sarcoma grade | Misexpression | |
| GLI2 (2q14) | Unknown | Unknown |
| GLI3 (7p13) | Greig cephalopolysyndactyly syndrome | Deletion, point mutation, or translocation |
| Pallister-Hall syndrome | Frameshift or nonsense mutation | |
| Postaxial polydactyly type A | Frameshift | |
| Postaxial polydactyly type A/B | Frameshift, nonsense, or missense mutation | |
| Preaxial polydactyly type-IV | Frameshift | |
| GLI4 (HKR4)a (8q24.3 ) | Unknown | Unknown |
Gene encodes a Krüppel-like protein and was named “GLI4” by the Human Gene Mapping Workshops.
GLI1
In addition to its amplification in glioblastomas (Kinzler et al. 1987), GLI1 has also been detected as an amplified gene in some osteosarcomas, rhabdomyosarcomas, and B cell lymphomas (Roberts et al. 1989; Werner et al. 1997), as shown in table 1. The clinical significance of GLI1 amplification in tumors is unknown, since the MDM2 (MIM 164785) and CDK4 (MIM 123829) genes are usually coamplified. GLI1 is also overexpressed in basal cell carcinomas, compared with that in normal human skin (Dahmane et al. 1997; Ghali et al. 1999). The degree of GLI1 overexpression (whether by amplification or mutation) correlates with tumor grade in adult bone and soft-tissue sarcomas (Stein et al. 1999). GLI1 has been classified as an oncogene on the basis of its ability to transform cells in cooperation with adenovirus E1A (Ruppert et al. 1991).
Expression of human GLI1 in transgenic mice results in a range of developmental defects, including failure to thrive and Hirschsprung-like dilatation of the gastrointestinal (GI) tract (Yang et al. 1997). The GI-tract phenotype is consistent with endogenous mouse gli1 expression patterns (Walterhouse et al. 1993) and with recent evidence that, in the mouse, Shh is responsible for radial patterning of the intestine (Sukegawa et al. 2000).
GLI2
Although no defects in GLI2 have been correlated, to date, with human diseases, studies in mice suggest that associations with basal cell carcinomas, skeletal defects, and other disorders are likely (Sasaki et al. 1999; Grachtchouk et al. 2000; Park et al. 2000).
GLI3
Mutations in human GLI3 have been implicated in several types of birth defects. Translocations, deletions, and point mutations throughout the GLI3 gene cause Greig cephalopolysyndactyly syndrome (GCPS [MIM 175700]), which is characterized by syndactyly, predominantly preaxial polydactyly, broad thumbs and first toes, and facial anomalies such as hypertelorism and frontal bossing (Vortkamp et al. 1991; Jones 1997; Wild et al. 1997; Kalff-Suske et al. 1999). GLI3 frameshift and nonsense mutations have also been linked to Pallister-Hall syndrome (PHS [MIM 146510]), an autosomal dominant disease involving hypothalamic hamartoma, central or postaxial polydactyly, syndactyly, imperforate anus, anteverted nares and other facial abnormalities, and, occasionally, associated HPE and malformations of the axial skeleton (Jones 1997; Kang et al. 1997). Recently, frameshift, nonsense, and missense GLI3 mutations were detected in the human polydactyly syndromes postaxial polydactyly type A (PAPA [MIM 174200]), autosomal dominant preaxial polydactyly type IV (MIM 174700), and postaxial polydactyly type A/B (Radhakrishna et al. 1997, 1999). Contrary to early reports, the type and location of GLI3 mutations do not appear to correlate with clinical disease phenotype (Kalff-Suske et al. 1999). Table 1 summarizes the GLI family’s associations with human birth defects and cancers.
Suppressor of Fused
Little is known, in human cells, about how information gets from the cell surface to the human GLI proteins, which are downstream effectors of SHH via PTCH and SMOH (Sasaki et al. 1999). In the homologous Drosophila system, this process involves the serine-threonine kinase Fused (Fu), the kinesin-related protein Costal-2 (Cos-2), and a physical interaction with microtubules (Robbins et al. 1997; Sisson et al. 1997). However, Cos-2 and Fu have not yet been demonstrated in vertebrates. The only cytoplasmic element that has been shown to function upstream of the GLI genes in human cells is Suppressor of Fused (Su[fu]), which cooperates with the F-box–containing protein Slimb to inhibit transactivation by GLI1 (Stone et al. 1999). Su(fu) can inhibit SHH-induced osteogenic differentiation and can sequester GLI1 in the cytoplasm (Kogerman et al. 1999), but it is currently unclear whether Su(fu) acts immediately downstream of PATCHED within the SHH pathway or, rather, as an independent regulator of GLI-mediated signaling. No human diseases have been associated, to date, with defects in Su(fu).
CREB-Binding Protein (CBP)
Studies of human GLI3 protein have shown that it is bound and coactivated by mouse CBP (MIM 600140), which is a common transcriptional coactivator (Akimaru et al. 1997; Dai et al. 1999). Several studies have shown that human mutations leading to haploinsufficiency of CBP cause Rubinstein-Taybi syndrome (RTS [MIM 180849]) (Petrij et al. 1995; Blough et al. 2000). Rubinstein-Taybi syndrome is a developmental disorder with symptoms that include broad thumbs and first toes, syndactyly, short stature, delayed osseous maturation, vertebral anomalies, mental retardation, dysmorphic facies, cryptorchidism, and an increased risk of neural and developmental tumors (Rubinstein 1990; Miller and Rubinstein 1995; Jones 1997). The similarity between the Rubinstein-Taybi phenotype and diseases caused by GLI3 mutations suggests that the biochemical interaction between CBP and GLI3 may significantly influence SHH signaling.
TWIST
A novel interaction between the SHH-PTCH-GLI pathway and TWIST (MIM 601622), the developmental regulatory gene and potential oncogene (Gitelman 1997; Bourgeois et al. 1998; Maestro et al. 1999), has been demonstrated (authors' unpublished observation). Mouse Twist protein can activate human GLI1 at the transcriptional level by interacting with E-boxes in GLI1’s first intron. Nonsense, missense, deletion, and insertion mutations in several regions of the human TWIST gene have been shown to cause Saethre-Chotzen syndrome (SCS [MIM 101400]), an autosomal dominant disease characterized by craniosynostosis, asymmetrical facies, syndactyly, broad thumbs and first toes with or without partial reduplication, ossification defects, and prominent ear crura (El Ghouzzi et al. 1997; Howard et al. 1997). Alternatively, some patients with Saethre-Chotzen have TWIST mutations that appear to disrupt either the stability of TWIST protein or its ability to correctly localize to the nucleus (El Ghouzzi et al. 2000). The clinical phenotype of Saethre-Chotzen syndrome partially overlaps with those of other Shh-pathway–related human diseases discussed above, indicating that dysregulation of Gli-family transcription factors by either Shh-signaling defects or TWIST mutations causes similar downstream effects. It has not yet been determined whether TWIST is regulated by SHH signaling, but TWIST does appear to be linked to SHH signal transduction.
GLI Research: Progress and Controversies
As the most downstream elements within the SHH-PTCH-GLI signaling cascade, GLI proteins are the key regulators of target-gene expression in response to SHH signaling. Although it is known that the three GLI genes encode zinc-finger transcription factors in the SHH-PTCH-GLI pathway, there are many controversies and unanswered questions.
There has been a great deal of discussion in recent years as to whether the Gli proteins function as transcriptional activators, repressors, or both. In several experimental systems, human GLI1 has been shown to contain a VP16-like, C-terminal activation domain and to be a transcriptional activator of both endogenous genes such as HNF-3β (MIM 600288) and reporter constructs containing the GLI1 binding site. GLI1 repressor function has never been observed (Lee et al. 1997; Yoon et al. 1998; Dai et al. 1999).
Different groups have reported strong data suggesting that GLI3 either activates (Dai et al. 1999) or represses (Wang et al. 2000) GLI1 transcription or protein activity. Human GLI3 appears to contain the C-terminal activation domain, in addition to an N-terminal repression domain, and can, in model systems, apparently function as either an activator or repressor of downstream genes such as HNF-3β (Sasaki et al. 1999), Gli1, and Ptch-1. The mechanism of switching between activation and repression has not been unequivocally determined, although cleavage of the GLI3 protein to release activating or repressing fragments has been suggested as one possibility. Current data suggest that human GLI2 works only as an activator, whereas mouse gli2 can function as either an activator or a repressor (Lee et al. 1997; Sasaki et al. 1997, 1999; Tanimura et al. 1998; Dai et al. 1999; Ruiz i Altaba 1999; Shin et al. 1999; von Mering and Basler 1999).
The homologue of GLI in Drosophila, cubitus interruptus (ci), is localized in the cytoplasm, and its translocation to the nucleus, where it functions as a transcription factor, is part of the Hedgehog-signaling response. The subcellular localization of Gli proteins within the cell is a matter of debate. Immunohistochemistry of several human tumor cell lines has demonstrated GLI1 protein in both the nucleus and the cytoplasm, in varying proportions in different cell lines (Stein et al. 1999). Other reports have noted GLI1 only in the nucleus of Tera-1 and D259MG cells (Kinzler and Vogelstein 1990) or mainly in the cytoplasm in basal cell carcinomas (Ghali et al. 1999).
Nonhuman experimental systems have also been used to study the localization of human GLI proteins, with varying results. In transfection studies of monkey COS cells, GLI1 accumulated primarily in the nucleus and, to a limited extent, in the cytoplasm (Dahmane et al. 1997; Lee et al. 1997). However, in transfected 10T1/2 mouse cells, human GLI1 localized primarily to the cytoplasm, particularly in the presence of transfected Su(fu) (Stone et al. 1999). GLI3 localization also differed between cell lines, with mainly cytoplasmic labeling in COS cells (Lee et al. 1997) and nuclear staining in 10T1/2 cells (Ruiz i Altaba 1999). In one study, transfected Hela cells showed full-length GLI3 in the cytoplasm, GLI3 with a PHS-type frameshift in the nucleus, and GLI3 with a Greig syndrome–type frameshift in both the cytoplasm and nucleus (Shin et al. 1999). It has been suggested, on the basis of experiments with mouse fibroblasts and frog tissues, that N-terminal GLI-protein motifs target proteins to the nucleus, whereas the C-terminal regions of GLI proteins contain a cytoplasmic tethering domain (Ruiz i Altaba 1999). The discrepancies between these experiments may reflect differences in localization of GLI that are based on different tissues or different cell-cycle states. Furthermore, it is unclear whether the subcellular localization of Gli proteins observed in nonhuman transfected cells accurately reflects the subcellular localization of human GLI in wild-type human cells and whether human tumor cells localize GLI in the same manner as do wild-type cells. Another unresolved issue in human GLI studies is whether any of the GLI proteins are cleaved, since GLI’s homologue in Drosophila, ci, requires cleavage into separate activator and repressor forms (Methot and Basler 1999).
There has been little convincing evidence to suggest that human GLI1 is cleaved in vivo. GLI1 antibody has demonstrated a major 150-kD band in human Tera-1 (teratocarcinoma) and human RMS (rhabdomyosarcoma) cells and, independently, a single 150-kD band in human D259 (glioblastoma) cells (Kinzler and Vogelstein 1990; Ruppert et al. 1991). Mouse-embryo extracts have demonstrated a single gli1 protein band (Dai et al. 1999), but frog gli1 has yielded multiple protein bands when transfected into human cells (Ruiz i Altaba 1999).
Reports from different groups studying different cell types have yielded conflicting data regarding GLI3. Human GLI3 transfected into 10T1/2 cells has produced a single full-length band, whereas the same construct transfected into COS cells has shown a full-length band, as well as an ∼100-kD band (Ruiz i Altaba 1999). In mouse cells, human GLI3 also produced a full-length band at all times, but additional processed forms were observed in the absence of Shh (Dai et al. 1999). Recently, PKA-dependent cleavage of human GLI3 to an 83-kD form was reported in transfected COS, mouse limb, and chick limb cells, as well as in endogenous mouse and chick limb buds. Phosphorylation of full-length GLI3 was apparently necessary for cleavage. No cleavage or phosphorylation of GLI2 was observed (Wang et al. 2000).
Conclusions
Normal development is regulated by the complex interaction of gene hierarchies, and the central role of signal-transduction pathways in these gene hierarchies is well established. The Shh-Ptch-Gli pathway is one of the most critical of these during early development. Although some components and functions of the Shh-Ptch-Gli pathway are highly conserved, the implications of species-specific differences should not be overlooked. In humans, genetic disruption of SHH-PTCH-GLI–pathway components leads to a range of developmental defects, and aberrant SHH signaling in postnatal life leads to dysregulated cell growth, as in basal cell carcinomas. As such, study of the human SHH-PTCH-GLI pathway is critical for the understanding of the pathogenesis of SHH-related human diseases, as well as in the determination of potential therapeutic targets.
Electronic-Database Information
The URL and accession numbers for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for Shh [MIM 600725], GLI1 [MIM 165220], GLI2 [MIM 165230], GLI3 [MIM 165240], HPE [MIM 236100], SLOS [MIM 270400], PTCH-1 [MIM 601309], PTCH-2 [MIM 603673], SMOH [MIM 601500], medulloblastomas [MIM 155255], esophageal squamous cell carcinomas [MIM 133239], transitional cell carcinomas of the bladder [MIM 109800], BCNS [MIM 109400], GLI4 [MIM 165280], MDM2 [MIM 164785], CDK4 [MIM 123829], GCPS [MIM 175700], PHS [MIM 146510], PAPA [MIM 174200], preaxial polydactyly type IV [MIM 174700], CBP [MIM 600140], RTS [MIM 180849], Twist [MIM 601622], and SCS [MIM 101400])
References
- Akimaru H, Chen Y, Dai P, Hou DX, Nonaka M, Smolik SM, Armstrong S, Goodman RH, Ishii S (1997) Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature 386:735–738 [DOI] [PubMed] [Google Scholar]
- Arheden K, Ronne M, Mandahl N, Heim S, Kinzler KW, Vogelstein B, Mitelman F (1989) In situ hybridization localizes the human putative oncogene GLI to chromosome subbands 12q13.3-14.1. Hum Genet 82:1–2 [DOI] [PubMed] [Google Scholar]
- Bale SJ, Amos CI, Parry DM, Bale AE (1991) Relationship between head circumference and height in normal adults and in the nevoid basal cell carcinoma syndrome and neurofibromatosis type I. Am J Med Genet 40:206–210 [DOI] [PubMed] [Google Scholar]
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HH, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW (1996) Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet 14:353–356 [DOI] [PubMed] [Google Scholar]
- Blough RI, Petrij F, Dauwerse JG, Milatovich-Cherry A, Weiss L, Saal HM, Rubinstein JH (2000) Variation in microdeletions of the cyclic AMP-responsive element-binding protein gene at chromosome band 16p13.3 in the Rubinstein-Taybi syndrome. Am J Med Genet 90:29–34 [DOI] [PubMed] [Google Scholar]
- Bourgeois P, Bolcato-Bellemin AL, Danse JM, Bloch-Zupan A, Yoshiba K, Stoetzel C, Perrin-Schmitt F (1998) The variable expressivity and incomplete penetrance of the twist-null heterozygous mouse phenotype resemble those of human Saethre-Chotzen syndrome. Hum Mol Genet 7:945–957 [DOI] [PubMed] [Google Scholar]
- Brewster R, Lee J, Ruiz i Altaba A (1998) Gli/Zic factors pattern the neural plate by defining domains of cell differentiation. Nature 393:579–583 [DOI] [PubMed] [Google Scholar]
- Cohen MM Jr (1989) Perspectives on holoprosencephaly. I. Epidemiology, genetics, and syndromology. Teratology 40:211–235 [DOI] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A (1997) Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389:876–881 (erratum: Nature 390:536 [1997]) [DOI] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S (1999) Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 274:8143–8152 [DOI] [PubMed] [Google Scholar]
- Donnai D, Burn J, Hughes H (1987) Smith-Lemli-Opitz syndromes: do they include the Pallister-Hall syndrome? Am J Med Genet 28:741–743 [DOI] [PubMed] [Google Scholar]
- El Ghouzzi V, Legeai-Mallet L, Aresta S, Benoist C, Munnich A, Gunzburg J, Bonaventure J (2000) Saethre-Chotzen mutations cause TWIST protein degradation or impaired nuclear location. Hum Mol Genet 9:813–819 [DOI] [PubMed] [Google Scholar]
- El Ghouzzi V, Le Merrer M, Perrin-Schmitt F, Lajeunie E, Benit P, Renier D, Bourgeois P, Bolcato-Bellemin AL, Munnich A, Bonaventure J (1997) Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat Genet 15:42–46 [DOI] [PubMed] [Google Scholar]
- Ghali L, Wong ST, Green J, Tidman N, Quinn AG (1999) Gli1 protein is expressed in basal cell carcinomas, outer root sheath keratinocytes and a subpopulation of mesenchymal cells in normal human skin. J Invest Dermatol 113:595–599 [DOI] [PubMed] [Google Scholar]
- Gitelman I (1997) Twist protein in mouse embryogenesis. Dev Biol 189:205–214 [DOI] [PubMed] [Google Scholar]
- Gorlin RJ (1995) Nevoid basal cell carcinoma syndrome. Dermatol Clin 13:113–125 [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz A (2000) Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 24:216–217 [DOI] [PubMed] [Google Scholar]
- Howard TD, Paznekas WA, Green ED, Chiang LC, Ma N, Ortiz de Luna RI, Garcia Delgado C, Gonzalez-Ramos M, Kline AD, Jabs EW (1997) Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet 15:36–41 [DOI] [PubMed] [Google Scholar]
- Jones KL (1997) Smith’s recognizable patterns of human malformation, 5th ed. WB Saunders, Philadelphia [Google Scholar]
- Kalff-Suske M, Wild A, Topp J, Wessling M, Jacobsen EM, Bornholdt D, Engel H, Heuer H, Aalfs CM, Ausems MG, Barone R, Herzog A, Heutink P, Homfray T, Gillessen-Kaesbach G, Konig R, Kunze J, Meinecke P, Muller D, Rizzo R, Strenge S, Superti-Furga A, Grzeschik KH (1999) Point mutations throughout the GLI3 gene cause Greig cephalopolysyndactyly syndrome. Hum Mol Genet 8:1769–1777 [DOI] [PubMed] [Google Scholar]
- Kang S, Graham JM Jr, Olney AH, Biesecker LG (1997) GLI3 frameshift mutations cause autosomal dominant Pallister-Hall syndrome. Nat Genet 15:266–268 [DOI] [PubMed] [Google Scholar]
- Kas K, Wlodarska I, Meyen E, Van den Berghe H, Van de Ven WJ (1996) Assignment of the gene encoding human Kruppel-related zinc finger protein 4 (GLI4) to 8q24.3 by fluorescent in situ hybridization. Cytogenet Cell Genet 72:297–298 [DOI] [PubMed] [Google Scholar]
- Kelley RL, Roessler E, Hennekam RC, Feldman GL, Kosaki K, Jones MC, Palumbos JC, Muenke M (1996) Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am J Med Genet 66:478–484 [DOI] [PubMed] [Google Scholar]
- Keys DN, Lewis DL, Selegue JE, Pearson BJ, Goodrich LV, Johnson RL, Gates J, Scott MP, Carroll SB (1999) Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science 283:532–534 [DOI] [PubMed] [Google Scholar]
- King T, Brown NA (1999) Developmental biology: antagonists on the left flank. Nature 401:222–223 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, Wong AJ, Vogelstein B (1987) Identification of an amplified, highly expressed gene in a human glioma. Science 236:70–73 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Ruppert JM, Bigner SH, Vogelstein B (1988) The GLI gene is a member of the Kruppel family of zinc finger proteins. Nature 332:371–374 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B (1990) The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol 10:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG (1999) Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol 1:312–319 [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A (1997) Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 124:2537–2552 [DOI] [PubMed] [Google Scholar]
- Maesawa C, Tamura G, Iwaya T, Ogasawara S, Ishida K, Sato N, Nishizuka S, Suzuki Y, Ikeda K, Aoki K, Saito K, Satodate R (1998) Mutations in the human homologue of the Drosophila patched gene in esophageal squamous cell carcinoma. Genes Chromosomes Cancer 21:276–279 [PubMed] [Google Scholar]
- Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH, Hannon GJ (1999) Twist is a potential oncogene that inhibits apoptosis. Genes Dev 13:2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Fujimoto M, Kato R, Niikawa N (1996) Assignment of the human GLI2 gene to 2q14 by fluorescence in situ hybridization. Genomics 36:220–221 [DOI] [PubMed] [Google Scholar]
- McGarvey TW, Maruta Y, Tomaszewski JE, Linnenbach AJ, Malkowicz SB (1998) PTCH gene mutations in invasive transitional cell carcinoma of the bladder. Oncogene 17:1167–1172 [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K (1999) Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96:819–831 [DOI] [PubMed] [Google Scholar]
- Miller RW, Rubinstein JH (1995) Tumors in Rubinstein-Taybi syndrome. Am J Med Genet 56:112–115 [DOI] [PubMed] [Google Scholar]
- Murone M, Rosenthal A, de Sauvage FJ (1999) Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol 9:76–84 [DOI] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, Giannotti A, Imaizumi K, Jones KL, Campo MD, Martin RA, Meinecke P, Pierpont ME, Robin NH, Young ID, Roessler E, Muenke M (1999) The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet 8:2479–2488 [DOI] [PubMed] [Google Scholar]
- Odent S, Atti-Bitach T, Blayau M, Mathieu M, Aug J, Delezo de AL, Gall JY, Le Marec B, Munnich A, David V, Vekemans M (1999) Expression of the Sonic hedgehog (SHH) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum Mol Genet 8:1683–1689 [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui C, Nakashima M, Joyner AL (2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127:1593–1605 [DOI] [PubMed] [Google Scholar]
- Pavletich NP, Pabo CO (1993) Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science 261:1701–1707 [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, Taylor FR, Wang EA, Galdes A (1998) Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem 273:14037–14045 [DOI] [PubMed] [Google Scholar]
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ, Breuning MH (1995) Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376:348–351 [DOI] [PubMed] [Google Scholar]
- Radhakrishna U, Bornholdt D, Scott HS, Patel UC, Rossier C, Engel H, Bottani A, Chandal D, Blouin JL, Solanki JV, Grzeschik KH, Antonarakis SE (1999) The phenotypic spectrum of GLI3 morphopathies includes autosomal dominant preaxial polydactyly type-IV and postaxial polydactyly type-A/B: no phenotype prediction from the position of GLI3 mutations. Am J Hum Genet 65:645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna U, Wild A, Grzeschik KH, Antonarakis SE (1997) Mutation in GLI3 in postaxial polydactyly type A. Nat Genet 17:269–271 [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP (1997) Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90:225–234 [DOI] [PubMed] [Google Scholar]
- Roberts WM, Douglass EC, Peiper SC, Houghton PJ, Look AT (1989) Amplification of the gli gene in childhood sarcomas. Cancer Res 49:5407–5413 [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M (1996) Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet 14:357–360 [DOI] [PubMed] [Google Scholar]
- Roessler E, Ward DE, Gaudenz K, Belloni E, Scherer SW, Donnai D, Siegel-Bartelt J, Tsui LC, Muenke M (1997) Cytogenetic rearrangements involving the loss of the Sonic Hedgehog gene at 7q36 cause holoprosencephaly. Hum Genet 100:172–181 [DOI] [PubMed] [Google Scholar]
- Rubinstein JH (1990) Broad thumb-hallux (Rubinstein-Taybi) syndrome 1957-1988. Am J Med Genet Suppl 6:3–16 [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A (1998) Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development 125:2203–2212 [DOI] [PubMed] [Google Scholar]
- ——— (1999) Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development 126:3205–3216 [DOI] [PubMed] [Google Scholar]
- Ruppert JM, Kinzler KW, Wong AJ, Bigner SH, Kao FT, Law ML, Seuanez HN, O’Brien SJ, Vogelstein B (1988) The GLI-Kruppel family of human genes. Mol Cell Biol 8:3104–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert JM, Vogelstein B, Arheden K, Kinzler KW (1990) GLI3 encodes a 190-kilodalton protein with multiple regions of GLI similarity. Mol Cell Biol 10:5408–5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert JM, Vogelstein B, Kinzler KW (1991) The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol Cell Biol 11:1724–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Hui C, Nakafuku M, Kondoh H (1997) A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 124:1313–1322 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H (1999) Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126:3915–3924 [DOI] [PubMed] [Google Scholar]
- Shin SH, Kogerman P, Lindstrom E, Toftgard R, Biesecker LG (1999) GLI3 mutations in human disorders mimic Drosophila cubitus interruptus protein functions and localization. Proc Natl Acad Sci USA 96:2880–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JC, Ho KS, Suyama K, Scott MP (1997) Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90:235–245 [DOI] [PubMed] [Google Scholar]
- Smyth I, Narang MA, Evans T, Heimann C, Nakamura Y, Chenevix-Trench G, Pietsch T, Wicking C, Wainwright BJ (1999) Isolation and characterization of human patched 2 (PTCH2), a putative tumour suppressor gene inbasal cell carcinoma and medulloblastoma on chromosome 1p32. Hum Mol Genet 8:291–297 [DOI] [PubMed] [Google Scholar]
- Stein U, Eder C, Karsten U, Haensch W, Walther W, Schlag PM (1999) GLI gene expression in bone and soft tissue sarcomas of adult patients correlates with tumor grade. Cancer Res 59:1890–1895 [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, de Sauvage F, Rosenthal A (1996) The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384:129–134 [DOI] [PubMed] [Google Scholar]
- Stone DM, Murone M, Luoh S, Ye W, Armanini MP, Gurney A, Phillips H, Brush J, Goddard A, de Sauvage FJ, Rosenthal A (1999) Characterization of the human suppressor of fused, a negative regulator of the zinc-finger transcription factor Gli. J Cell Sci 112:4437–4448 [DOI] [PubMed] [Google Scholar]
- Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K (2000) The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development 127:1971–1980 [DOI] [PubMed] [Google Scholar]
- Tanimura A, Dan S, Yoshida M (1998) Cloning of novel isoforms of the human Gli2 oncogene and their activities to enhance tax-dependent transcription of the human T-cell leukemia virus type 1 genome. J Virol 72:3958–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G (1994) Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N Engl J Med 330:107–113 [DOI] [PubMed] [Google Scholar]
- von Mering C, Basler K (1999) Distinct and regulated activities of human Gli proteins in Drosophila. Curr Biol 9:1319–1322 [DOI] [PubMed] [Google Scholar]
- Vorechovsky I, Unden AB, Sandstedt B, Toftgard R, Stahle-Backdahl M (1997) Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res 57:4677–4681 [PubMed] [Google Scholar]
- Vortkamp A, Gessler M, Grzeschik KH (1991) GLI3 zinc-finger gene interrupted by translocations in Greig syndrome families. Nature 352:539–540 [DOI] [PubMed] [Google Scholar]
- ——— (1995) Identification of optimized target sequences for the GLI3 zinc finger protein. DNA Cell Biol 14:629–634 [DOI] [PubMed] [Google Scholar]
- Walterhouse D, Ahmed M, Slusarski D, Kalamaras J, Boucher D, Holmgren R, Iannaccone P (1993) gli, a zinc finger transcription factor and oncogene, is expressed during normal mouse development. Dev Dyn 196:91–102 [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA (2000) Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100:423–434 [DOI] [PubMed] [Google Scholar]
- Werner CA, Dohner H, Joos S, Trumper LH, Baudis M, Barth TF, Ott G, Moller P, Lichter P, Bentz M (1997) High-level DNA amplifications are common genetic aberrations in B-cell neoplasms. Am J Pathol 151:335–342 [PMC free article] [PubMed] [Google Scholar]
- Wild A, Kalff-Suske M, Vortkamp A, Bornholdt D, Konig R, Grzeschik KH (1997) Point mutations in human GLI3 cause Greig syndrome. Hum Mol Genet 6:1979–1984 [DOI] [PubMed] [Google Scholar]
- Williams KP, Rayhorn P, Chi-Rosso G, Garber EA, Strauch KL, Horan GS, Reilly JO, Baker DP, Taylor FR, Koteliansky V, Pepinsky RB (1999) Functional antagonists of sonic hedgehog reveal the importance of the N terminus for activity. J Cell Sci 112:4405–4414 [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, Jr, de Sauvage FJ (1998) Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391:90–92 [DOI] [PubMed] [Google Scholar]
- Yang JT, Liu CZ, Villavicencio EH, Yoon JW, Walterhouse, D, Iannaccone PM (1997) Expression of human GLI in mice results in failure to thrive, early death, and patchy Hirschsprung-like gastrointestinal dilatation. Mol Med 3:826–835 [PMC free article] [PubMed] [Google Scholar]
- Yoon JW, Liu CZ, Yang JT, Swart R, Iannaccone P, Walterhouse D (1998) GLI activates transcription through a herpes simplex viral protein 16-like activation domain. J Biol Chem 273:3496–3501 [DOI] [PubMed] [Google Scholar]