Abstract
We describe a patient with 46,XY partial gonadal dysgenesis (PGD) who presented with polyneuropathy. Sural nerve pathology revealed peculiar findings characterized by extensive minifascicular formation within the endoneurium and with a decreased density of myelinated fibers. We found, in the patient, a homozygous missense mutation (ATG→ACG) at the initiating codon in exon 1 of the desert hedgehog (DHH) gene, which predicts a failure of translation of the gene. The same heterozygous mutation was found in the patient's father. This is the first report of a human DHH gene mutation, and the findings demonstrate that mutation of the DHH gene may cause 46,XY PGD associated with minifascicular neuropathy.
The development of the mature male is a consequence of testis formation in the embryo. Sex reversal in XY females results from the failure of testis determination or differentiation pathways. Gonadal dysgenesis, XY female type (GDXY [MIM 306100]) and XY type [MIM 233420], encompasses a heterogeneous group of different chromosomal, gonadal, and phenotypic abnormalities (Me’ndez et al. 1993). Partial gonadal dysgenesis (PGD) is characterized by the presence of a testis on one side and a streak or an absent gonad at the other, persistence of Müllerian duct structures, and a variable degree of genital ambiguity. Mutations in the sex-determining region Y gene (SRY) have been reported in <20% of these individuals (Jager et al. 1990), and the molecular basis of the disease remains unknown in other patients. Therefore, PGD could be caused by mutation(s) of other genes that are involved in the sex-determination pathways.
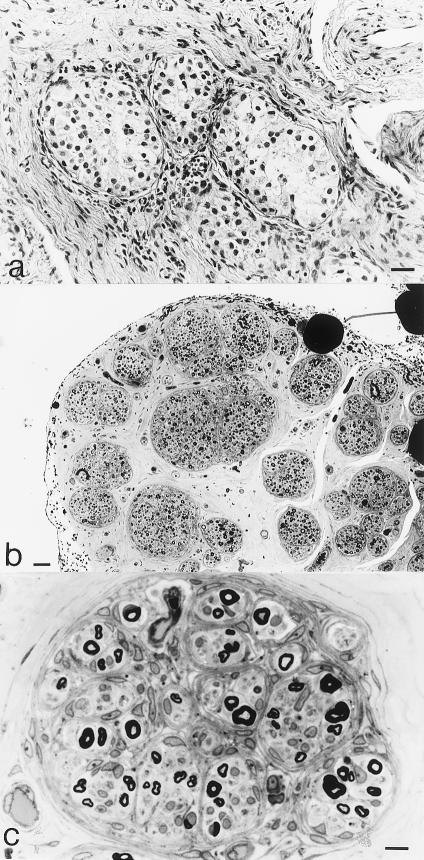
We have reported a patient with 46,XY PGD associated with polyneuropathy (Umehara et al. 1999). The patient was clinically diagnosed as having 46,XY PGD because of the following features: (1) premature female genitalia, with a blinded vagina and an immature uterus; (2) the presence of a testis on one side (fig. 1a) and a streak gonad on the other side; and (3) a 46,XY karyotype, on chromosomal analysis. The details of the clinical and pathological features have been described elsewhere (Umehara et al. 1999). The most peculiar finding in the case was extensive formation of minifascicles within the endoneurium of the sural nerve (fig. 1b). Each minifascicle contained several axon-Schwann cell units that were separated by one-to-several layers of flattened cell processes with the morphology of perineurial cells, which are normally found surrounding large nerve fascicles. We counted ∼100 fascicles, with fascicle diameters in the range of 20–80 μm. Many fascicles contained several smaller fascicles within them (fig. 1c). In addition, the densities of large and small myelinated fibers were moderately decreased in each fascicle. This combination of symptoms suggested that a mutation in a gene that is involved in both male gonadal differentiation and perineurial development might be responsible for this syndrome.
Figure 1.
Pathological features. a, Pathology of resected immature testis. Hematoxylin and eosin staining. Bar = 20 μm. b, c, Light microscopic findings of sural nerve, with toluidine blue staining. b, Many small fascicles. Bar = 50 μm. c, Several prominent minifascicles within a larger fascicle. Bar = 10 μm.
Recent reports suggested to us that the gene involved might be desert hedgehog (DHH). Dhh (the mouse homologue of human DHH) is a member of the hedgehog family of signaling proteins, which also includes sonic hedgehog and indian hedgehog (Ingham 1997). Mammalian hedgehog family members share a striking homology with the Drosophilia segment polarity gene hedgehog, a key regulator of pattern formation in the embryonic and adult fly (Hammerschmidt et al. 1997), and Dhh and its relatives have important signaling functions during early development. Bitgood and McMahon (1995) and Parmantier et al. (1999) reported that during development, Dhh mRNA showed a very restricted distribution, being expressed primarily in the Sertoli cells of developing testes and in Schwann cells in peripheral nerves. Male Dhh-null mutant mice were sterile and failed to produce mature spermatozoa (Bitgood et al. 1996). Furthermore, the peripheral nerves of Dhh-null mutant mice were highly abnormal. The perineurial sheaths surrounding the nerve fascicles were abnormally thin, and extensive minifascicles consisting of perineurial-like cells were formed within the endoneurium. The nerve-tissue barrier was permeable, and the tight-junctional arrays between adjacent perineurial cells were abnormal and incomplete (Parmantier et al. 1999). These findings suggest that Dhh plays crucial roles both in peripheral nerve sheath development and in the regulation of spermatogenesis.
We therefore investigated the structure of the DHH gene (exon 1, AB010581; exon 2, AB010993; and exon 3, AB010994 [DNA Data Bank of Japan]) in the patient and in her family. The human DHH gene contains three exons that encode a polypeptide of 397 amino acids and has been mapped to chromosome 12q12-q13.1 (Tate et al. 2000b). We screened exons 1–3 for DNA sequence differences by direct sequencing of genomic DNA samples from the patient, her father, and her younger sister. The parents of the patient were first cousins. The father and younger sister showed normal sexual development and had no clinical signs of neuropathy. Informed consent was obtained from all study participants. Blood samples were obtained from the patient, her father, and her younger sister but were not available from her mother. Genomic DNA was extracted from whole blood by standard methods. DHH genomic structures and sequence were determined by restriction mapping and by sequencing the genomic DHH clone (Tate et al. 2000a). PCR was performed as follows: genomic DNA was precipitated with ethanol and dissolved in TE buffer; 2 μl was used in the primary PCR in a 50-μl reaction volume containing dNTPs (20 nmol each), forward and reverse primers (10 pmol each), 1×buffer and enzyme mix (2.5 U). We used a thermocycler that was programmed as follows: for exons 1 and 3, 30 cycles each at 98°C for 1 min, 65°C for 1 min, and 72°C for 1 min; and, for exon 2, 30 cycles each at 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min. For the primary PCR reactions, for exon 1, we used primer pairs 46 (forward, 5′-AGA GTT AGT GAG GAC AAG AAC GC-3′) and 47 (reverse, 5′-TAG GAA AAC GGA GGA GCT GGC AGT G-3′); for exon 2, we used primer pairs 48 (forward, 5′-GGG TGG TGG TGG GGA GAC TGA CTC-3′) and 49 (reverse, 5′-CCC TTT GGG CGG ATT TTA CCA C-3′); and, for exon 3, we used primer pairs 55 (forward, 5′-CTT GAT TCA ATC CTC CCT GTG GTT G-3′) and 54 (reverse, 5′-CCC GCC GGG CGC CAG CAC CGA GTC-3′) and 52 (forward, 5′-TGG CAC CTG GTG TTT GCC GCT CG-3′) and 51 (reverse, 5′-CTC GGG CGC TTC GAG GTT TCT ATG-3′). DNA sequencing was performed with an ABI PRISMTM 377 DNA sequencer, and direct sequencing by PCR was done.
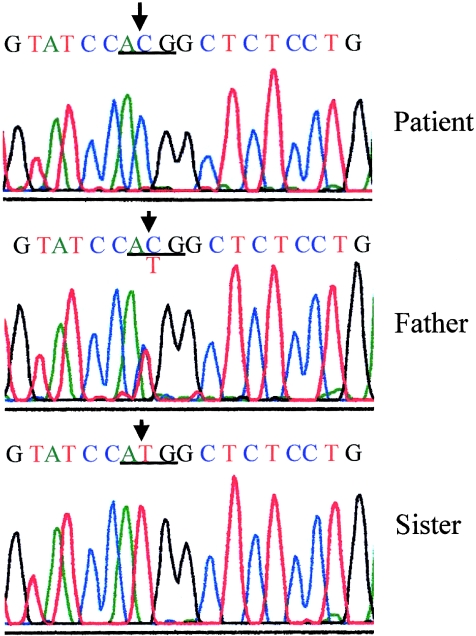
A single base-pair substitution (ATG→ACG) in the initiation codon, which will abolish initiation of translation at the normal start site, was found in the patient (fig. 2). The sequences of exons 2 and 3 were normal (data not shown). The same heterozygous mutation was found in her father but not in her sister. To exclude an additional abnormality in the SRY gene, we analyzed the sequence of the open reading frame of the SRY gene, and no mutations were found.
Figure 2.
DNA sequence of the mutant DHH gene. Upper panel, The homozygous T→C substitution at the initiation codon (underlined) of exon 1 is indicated by an arrow. Middle panel, The father of the patient showed T→T/C heterozygous mutation. Lower panel, The younger sister of the patient showed no mutated sequence.
The striking concordance between the dual pathological features of the patient and Dhh-null mutant mice suggests that a homozygous missense mutation at the initiation codon of the DHH gene may be causative in the patient. As mentioned above, Dhh, in mice, encodes a signaling molecule in the testis but not in the ovary, and it plays a role in the regulation of spermatogenesis (Bitgood et al. 1996). In mammals, testis development is initiated in the embryo in response to the expression of the Sry (the mouse homologue of the human SRY) in Sertoli cell precursors. Subsequently, Sertoli cells are thought to play a central role in the proper development of the male gonads. In Sertoli cells, the activation of Dhh transcription occurs shortly after the initiation of Sry expression, and lack of Dhh results in the absence of mature sperm and abnormally small testes in mature Dhh-null male mice. These results demonstrate that Dhh signaling plays an essential role in the regulation of mammalian spermatogenesis and may be directly regulated by the sex-determining pathway genes. Therefore, in humans, failure of translation of the DHH gene may disturb the differentiation of male gonads and may result in 46,XY PGD in the case reported in this study.
As in the patient, Dhh-null mutant mice have peripheral nerve abnormalities. These include perineurial cells, ectopically located within the endoneurial space, which formed minifascicles around small groups of nerve fibers. In electrophysiological studies, the nerve fibers in Dhh-null mutant mice showed, on average, a lowered conduction velocity (Parmantier et al. 1999). These features resemble those found in the present case in which, as in the Dhh-null mouse, absence of Dhh signals may disturb the perineurial sheaths at a relatively early stage, which results in minifascicles formation. In the mouse, the minifascicles formation is probably caused by the absence of a perineurial tight-junctional barrier, and the DHH mutation may lead to a similar abnormality in humans. Disturbance in the internal milieu of the nerve may then eventually lead to nerve-fiber dysfunction and neuropathy.
Among the human hedgehog family members, mutations in the sonic hedgehog (SHH) gene, which maps to chromosome 7q36, cause holoprosencephaly (HPE) (Belloni et al. 1996, Roessler et al. 1996). Haploinsufficiency of the SHH gene is the genetic correlate of HPE with autosomal dominant inheritance. In contrast, heterozygous mutations of DHH were insufficient to cause PGD in the present family, again consistent with the findings from Dhh-null mutant mice, in which loss of Dhh activity resulted in a recessive, sex-specific phenotype (Bitgood et al. 1996).
In conclusion, this is the first report, to our knowledge, of mutation in a human DHH gene. The patient studied showed that the peculiar syndrome consisted of 46,XY PGD, accompanied by minifascicular neuropathy. These data demonstrate that DHH is a key molecule in both male gonadal differentiation and perineurial formation in peripheral nerves.
Acknowledgments
We thank Professor Rhona Mirsky (Department of Anatomy and Developmental Biology, University College London) and Dr. Raymond L. Rosales, for critical reading of the manuscript. We also thank Ms. Shoko Taniguchi, Mihioko Teshima, and Noriko Hirata, for their technical assistance. This work was supported, in part, by the High-Technology Research Center project from the Ministry of Education, Science, Sports and Culture of Japan (support to G.T.).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for gonadal dysgenesis, XY female type [MIM 306100], and gonadal dysgenesis, XY type [MIM 233420])
- DNA Data Bank of Japan, http://www.ddbj.nig.ac.jp/ (for genomic DNA sequencing of human DHH)
References
- Belloni E, Muenke M, Roessler E, Traverso G, Siegel-Bartelt J, Frumkin A, Mitchell HF, Donis-Keller H, Helms C, Hing AV, Heng HHQ, Koop B, Martindale D, Rommens JM, Tsui LC, Scherer SW (1996) Identification of sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet 14:353–356 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP (1995) Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol 172:126–138 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP (1996) Sertoli cell signaling by desert hedgehog regulates the male germline. Curr Biol 6:298–304 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Brook A, McMahon AP (1997) The world according to hedgehog. Trends Genet 13:14–21 [DOI] [PubMed] [Google Scholar]
- Ingham PW (1998) Transducing hedgehog: the story so far. EMBO J 17:3505–3511 [DOI] [PMC free article] [PubMed]
- Jager RJ, Anvret M, Hall K, Scherer G (1990) A human XY female with a frameshift mutation in the candidate testis determining gene SRY. Nature 348:452–454 [DOI] [PubMed] [Google Scholar]
- Me’ndez, JP, Ulloa-Aguirre A, Kofman-Alfaro S, Mutchinick O, Fernandez-del-Castillo C, Reyes E, Perez-Palacios G (1993) Mixed gonadal dysgenesis: clinical, cytogenetic, endocrinological, and histological findings in 16 patients. Am J Med Genet 46:263–267 [DOI] [PubMed] [Google Scholar]
- Parmantier E, Lynn B, Lawson D, Turmaine M, Namini SS, Chakrabarti L, McMahon AP, Jessen KR, Mirsky R (1999) Schwann cell-derived desert hedgehog controls the development of peripheral nerve sheath. Neuron 23:713–724 [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M (1996) Mutations in the human sonic hedgehog gene cause holoprosencephaly. Nat Genet 14:357–360 [DOI] [PubMed] [Google Scholar]
- Tate G, Kishimoto K, Mitsuya T (2000a) Expression of sonic hedgehog and its receptor patched/smoothened in human cancer cell lines and embryonic organs. J Biochem Mol Biol Biophys 4:27–34 [Google Scholar]
- Tate G, Satoh H, Endo Y, Mitsuya T (2000b) Assignment of desert hedgehog to human chromosome bands 12q12→q13.1 by in situ hybridization. Cytogenet Cell Genet 88:93–94 [DOI] [PubMed] [Google Scholar]
- Umehara F, Yamaguchi N, Kodama D, Takenaga S, Kiwaki T, Sonoda Y, Arimura Y, Yamada H, Arimura K, Osame M (1999) Polyneuropathy with minifascicle formation in a patient with 46XY mixed gonadal dysgenesis. Acta Neuropathol (Berl) 98:309–312 [DOI] [PubMed]