To the Editor:
A complete set of telomere region–specific FISH probes designed to hybridize to the unique subtelomeric regions of every human chromosome was initially described in 1996 (National Institutes of Health et al. 1996), and an update was recently reported in the Journal (Knight et al. 2000). It was anticipated that these probes would be extremely valuable in the identification of submicroscopic telomeric aberrations that were thought to account for a substantial yet previously underrecognized proportion of cases of mental retardation in the population. Recently, a version of these probes was made commercially available as part of a diagnostic device that allows for simultaneous analysis of the telomeric regions of every human chromosome, except the p arms of the acrocentric chromosomes, on a single microscope slide (Cytocell) (Knight et al. 1997). The utility of these probes is evident in that numerous reports now exist describing cryptic telomere rearrangements or submicroscopic telomeric deletions that were undetectable by standard cytogenetic banding techniques but that were revealed by these FISH probes (Horsley et al. 1998; Ballif et al. 2000; reviewed in Knight and Flint 2000). Furthermore, several recent studies that have used these probes to investigate the telomeric regions of patients who have idiopathic mental retardation with apparently normal karyotypes indicate that ⩽23% of such cases have cryptic telomeric aberrations (Knight et al. 1999; reviewed in Knight and Flint 2000). This suggests that telomeric anomalies may be second only to Down syndrome as the most common cause of mental retardation (Knight and Flint 2000).
In our clinical cytogenetics laboratory, we have used telomere region–specific probes to examine the telomeric regions of 154 unrelated patients with apparently normal karyotypes, submitted for a variety of clinical indications. The recent report, in the Journal, by Knight et al. (2000) prompted us to examine the results, to date, of our telomeric FISH assay. This is not a controlled study of a selected population but, rather, a glance at the telomeric anomalies identified since the inception of the telomeric assay in the laboratory. Metaphase chromosomes obtained from peripheral blood specimens sent by the referring physician were analyzed in all cases. Of these patients, 15/154 (9.7%) had either submicroscopic telomeric deletions or cryptic telomeric rearrangements identified. However, only 4/15 (27%) telomeric abnormalities were shown to potentially contribute to the phenotype, since 11/15 (73%) patients inherited apparently benign telomeric variants from a phenotypically normal parent who carried the same cytogenetic “anomaly” (fig. 1). This reduces the percentage of clinically significant subtelomeric aberrations to 4/154 (2.6%) in our study population.
Figure 1.
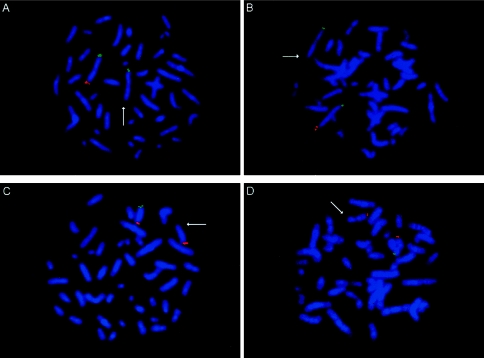
Subtelomeric polymorphisms detected by telomere region–specific FISH probes. Probes specific for p arms fluoresce green, and probes specific for q arms fluoresce red. A, Metaphase from a phenotypically normal parent, showing a deletion of the 2q telomere region–specific FISH probe (arrow). Note a normal hybridization pattern for the 2p telomere region–specific FISH probe. B, Metaphase from the child of the parent shown in A, indicating an inherited deletion of the 2q FISH probe (arrow). C, Metaphase from a patient with a polymorphic deletion of the Xp subtelomeric probe (arrow) that was paternally inherited (parental data not shown). D, Metaphase from a patient showing a polymorphic deletion, of the 9p FISH probe (arrow), that was paternally inherited (parental data not shown).
The four clinically significant telomeric abnormalities are listed in table 1. Patients included in this study underwent diagnostic study because of a variety of clinical indications, including developmental delay, mental retardation, dysmorphic features, and/or multiple congenital anomalies. However, the precise details of the clinical diagnoses were not available to the diagnostic laboratory, which limited further extrapolation of these telomeric abnormalities being associated with a particular phenotype or subset of patients. All 11 observed telomeric polymorphisms are listed in table 2. Our data indicate that telomeric polymorphisms may be quite common (occurring in ∼7% of patients studied), with a deletion in the 2q subtelomeric region occurring in 8/154 patients (∼5% of the population). By means of a cosmid (2112b2), this 2q polymorphism has been detected elsewhere (Knight and Flint 2000; Knight et al. 2000). However, it was noted that the 2q probe present on the commercial telomere device had been recently updated, by the manufacturer, to a PAC probe (Genome Systems PAC 1011O17). Although the updated probe is larger than the first-generation cosmid used and is located <240 kb from the true telomere, it still detects the polymorphism (fig. 1A and B) (Knight and Flint 2000; Knight et al. 2000). For those patients who show a 2q deletion with PAC 1011O17, FISH using another version of the 2q probe (P1 210E14) (National Institutes of Health et al. 1996; Knight et al. 1997) demonstrated signals on both chromosomes, indicating nondeletion of this locus (data not shown). Although the parents of three patients with 2q deletions were unavailable for study, these patients showed the presence of the previously reported 2q subtelomeric probe (P1 210E14) on both homologues, making it highly likely that the anomalies seen in these patients also represent 2q polymorphisms. In addition, the XpYp subtelomeric cosmid probe (CY29), designed to hybridize to the pseudoautosomal regions of both sex chromosomes, has been shown to detect polymorphic sequences (Knight and Flint 2000; Knight et al. 2000), as found in one of our cases (fig. 1C). Detection of a 9pter polymorphism by telomere region–specific probes has not been previously reported (fig. 1D). It is expected that, as the limits of the technology are pushed farther toward the ends of the chromosome, more polymorphisms are likely to be identified.
Table 1.
Clinically Significant Telomeric Aberrations Detected Using Telomere Region–Specific FISH Probes
| Telomeric Aberration | No. Observed | Probe(s) Used |
| ish del(1)(qter) | 1 | PAC 160H23 |
| ish der(2)t(2q;17q)pat | 1a | P1 210E14, cosmid B37c1 |
| ish der(18)t(7p;18q)mat | 1 | PAC 164D18, PAC 964M9 |
| ish der(22)t(14q;22p)mat | 1 | PAC 820M16, D14Z1/D22Z1b |
Source: Bacino et al. (2000).
Probe hybridizes to the centromeres of chromosomes 14 and 22.
Table 2.
Telomeric Polymorphisms Detected Using Telomere Region–Specific FISH Probes
| Telomeric Polymorphism | No. Observed | Probe(s) Used |
| ish add(1)(qter)(13qtel+)pat | 1a | PAC 163C9 |
| ish del(2)(qter)mat or pat | 8 | PAC 1011O17, P1 210E14b |
| ish del(9)(pter)pat | 1 | PAC 43N6 |
| ish del(X)(pter)pat | 1 | Cosmid CY29 |
Source: Shaffer et al. (1999).
PAC 1011O17 was deleted in all patients, and P1 210E14 was not deleted in all patients.
The American College of Medical Genetics, in conjunction with the College of American Pathologists, has set forth guidelines for validation of FISH probes (Watson 1999). These guidelines suggest hybridizing five normal specimens with each new FISH probe. This approach will not uncover the frequency of these subtelomeric polymorphisms, and large numbers of normal individuals need to be tested to gather the frequencies of these polymorphic variants in the population. Although identifying these polymorphisms and the frequency with which they occur may help in the understanding of telomere structure and function, as well as in the understanding of the mechanisms that underlie the formation of terminal deletions and subtelomeric rearrangements, polymorphic subtelomeric probes are tenuous for diagnostic purposes. Whenever possible, when abnormalities are observed, parental samples should be tested with the same telomere region–specific probes, prior to the interpretation of the results from the child, to exclude the possibility of a benign familial polymorphism segregating in the family (Shaffer et al. 1999). This approach will improve the usefulness of these probes in the identification of telomeric alterations with true clinical significance.
Acknowledgments
We thank the following clinicians and counselors for submission of cases for clinical study: P. Benke (University of Miami School of Medicine, Miami); L. Celle, K. Russell, and E. Zackai (Children’s Hospital of Philadelphia, Philadelphia); J. Graham (Cedar-Sinai Medical Center, Los Angeles); L. Sadler (Children’s Hospital of Buffalo, Buffalo); and C. Bacino, A. Beaudet, B. Bejjani, W. Craigen, S. Fernbach, F. Scaglia, R. Sutton, and J. Towbin (Baylor College of Medicine, Houston).
References
- Bacino CA, Kashork CD, Davino NA, Shaffer LG (2000) Detection of a cryptic translocation in a family with mental retardation using FISH and telomere region-specific probes. Am J Med Genet 92:250–255 [PubMed] [Google Scholar]
- Ballif BC, Kashork CD, Shaffer LG (2000) FISHing for mechanisms of cytogenetically defined terminal deletions using chromosome-specific subtelomeric probes. Eur J Hum Genet 8:764–770 [DOI] [PubMed] [Google Scholar]
- Horsley SW, Knight SJL, Nixon J, Huson S, Fitchett M, Boone RA, Hilton-Jones D, Flint J, Kearney L (1998) Del(18p) shown to be a cryptic translocation using a multiprobe FISH assay for subtelomeric chromosome rearrangements. J Med Genet 35:722–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SJL, Flint J (2000) Perfect endings: a review of subtelomeric probes and their use in clinical diagnosis. J Med Genet 37:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SJL, Horsley SW, Regan R, Lawrie NM, Maher EJ, Cardy DLN, Flint J, Kearney L (1997) Development and clinical application of an innovative fluorescence in situ hybridization technique which detects submicroscopic rearrangements involving telomeres. Eur J Hum Genet 5:1–8 [PubMed] [Google Scholar]
- Knight SJL, Lese CM, Precht KS, Kuc J, Ning Y, Lucas S, Regan R, Brenan M, Nicod A, Lawrie NM, Cardy DLN, Nguyen H, Hudson TJ, Riethman HC, Ledbetter DH, Flint J (2000) An optimized set of human telomere clones for studying telomere integrity and architecture. Am J Hum Genet 67:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SJL, Regan R, Nicod A, Horsley SW, Kearney L, Homfray T, Winter RM, Bolton P, Flint J (1999) Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 354:1676–1681 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health, Institute of Molecular Medicine Collaboration, Ning Y, Roschke A, Smith AC, Macha M, Precht K, Riethman H, Ledbetter DH, Flint J, Horsley S, Regan R, Kearney L, Knight S, Kvaloy K, Brown WRA (1996) A complete set of human telomeric probes and their clinical application. Nat Genet 14:86–89 [DOI] [PubMed] [Google Scholar]
- Shaffer LG, Kashork CD, Bacino CA, Benke PJ (1999) Caution: telomere crossing. Am J Med Genet 87:278–280 [DOI] [PubMed] [Google Scholar]
- Watson MS (ed) (1999) Standards and guidelines for clinical genetics laboratories, 2d ed. American College of Medical Genetics, Bethesda, MD [Google Scholar]