Introduction
The synovium lines the noncartilaginous surfaces of the diarthrodial joints, and synovial tissue is also found in tendon sheaths and bursae [1]. Several rheumatic diseases are characterized by synovial inflammation. In these conditions, descriptive studies of synovial biopsy specimens may contribute to an understanding of the events that take place in vivo, and they complement experimental animal studies as well as in-vitro studies. Examination of synovial tissue is generally more relevant than synovial fluid analysis, except, for example, the analysis of neutrophils and platelets, and studies of soluble mediators. Recently, there has been an enormous upsurge in investigations of the pathological changes in the synovium [2] because of the availability of new methods to obtain synovial biopsy samples [3,4] and because of the development of immunohistological methods, in-situ hybridization, and the polymerase chain reaction. Moreover, the complementary DNA microarray technology may hold great promise for synovial tissue analysis in the future [5].
Methods to obtain and evaluate synovial biopsy samples
Synovial tissue may be obtained either at surgery, by blind needle biopsy, or at arthroscopy. It is likely that tissue obtained at joint replacement differs from that obtained by blind-needle biopsy or arthroscopy because of clear differences in patient selection. Obviously, surgery is inappropriate for studies on early rheumatoid arthritis (RA) or for serial investigations. The blind-needle biopsy technique is safe, well tolerated and is technically easy to perform. A limitation of this method is that its use in clinical practice is often restricted to the suprapatellar pouch of the knee joint. In addition, it is more difficult to obtain sufficient tissue from clinically uninvolved joints, for example after successful treatment. Arthroscopic sampling of synovial tissue under direct vision is a similarly safe and well tolerated procedure, but is more complicated and expensive [3,6,7]. Most measures of inflammation in needle biopsies are similar to those selected at arthroscopy [4]. An advantage of arthroscopy is that it is always possible to obtain tissue in adequate amounts, even in clinically quiescent joints. Moreover, arthroscopy allows access to most joints and to most regions within the joint, including the pannus-cartilage junction.
There is large variability of synovial inflammation between individuals, different joints, and even within joints [2]. The degree of morphologic heterogeneity in synovial tissue samples obtained from a single joint could suggest that evaluation of synovial tissue is unreliable because of unavoidable sampling error. Several studies [8,9,10], however, have shown that, despite the degree of histologic variation, representative measures of several parameters of synovial inflammation may be obtained by examining a limited number of samples. For example, quantification of T-cell infiltration and activation in sections derived from at least six different biopsy specimens results in variance of less than 10% [10]. It is generally not necessary to know the macroscopic appearance of the rheumatoid synovium in order to obtain representative samples [4,8].
There are essentially three methods to quantify the features of synovial inflammation in biopsy samples: semi-quantitative analysis, quantitative analysis and computer-assisted analysis [11,12,13]. All three methods are reliable in experienced hands. It can be anticipated that digital image analysis will be increasingly important with the use of more advanced computer systems.
Clinical studies
Histological features of the synovium have been documented in various clinical studies, describing associations with disease activity [14,15] and prognosis [16,17]. These studies underscore the important role of macrophages and macrophage-derived mediators of inflammation and destruction in RA. In addition, systematic comparison of synovial tissue from RA patients in different phases of the disease made it possible to define the cell infiltrate [14,15,18,19,20], as well as the expression of adhesion molecules [21], cytokines [14,22,23] and degrading enzymes [17,20,24] in early disease. A major conclusion from this work is that so-called early RA is already a chronic disease. This may explain the observation that a notable percentage of RA patients have signs of joint destruction at the time of initial diagnosis [25]. Preliminary work [26] has identified some immunohistological features that are characteristic for rheumatoid synovial tissue. More extensive future studies may provide helpful markers, which could be used for routine clinical practice.
Studies of synovial tissue may also play an important role in the development of rational therapies in which biotechnology products are used to influence defined pathogenetic mechanisms [27]. The design of optimal treatment regimens for interventions with agents such as monoclonal antibodies, soluble receptors, cytokines and peptides can be facilitated by information regarding the actual achievement of the biological effect at the site of inflammation. Such studies will also provide insight into the mode of action of such agents. Additionally, analysis of serial biopsy samples during treatment may provide useful alternative end points for both joint inflammation and joint destruction. This approach could lead to a rapid screening method that would require relatively low numbers of patients to predict the effects of novel antirheumatic strategies. Studies of the relation between a defined modification of inflammation and the clinical course could also produce information about the pathogenesis of rheumatic diseases.
Inflammatory cells in the synovium
The synovium comprises the intimal lining layer and the synovial sublining [1]. The intimal lining layer consists mainly of intimal macrophages and fibroblast-like synoviocytes. The synovium becomes hypertrophic and edematous in various arthritides. Angioneogenesis, recruitment of inflammatory cells under influence of chemokines, local retention and cell proliferation all contribute to the accumulation of cells in the inflamed synovium. The following discussion focuses on the major infiltrating cell populations.
Rheumatoid synovial tissue is characterized by marked intimal lining hyperplasia and by accumulation of T cells, plasma cells, macrophages, B cells, mast cells, natural killer cells and dendritic cells in the synovial sublining (Table 1, Fig. 1) [14,23,28,29,30]. Distinct patterns of lymphoid organization can occur in the synovium; diffuse, follicular and granulomatous variants have been distinguished [31]. In contrast to general belief, proliferation of synovial tissue is mainly due to changes in the synovial sublining (Fig. 1). Important contributors are angioneogenesis, oedema, massive cell infiltration and fibrosis. The differences with other forms of arthritis are only gradual. There is, for example, on average stronger infiltration by macrophages, plasma cells and granzyme-positive cytotoxic cells in RA [23,26,30]. So-called pannocytes have been observed at the pannus-cartilage junction [32,33]. These cells exhibit phenotypic and functional features of both fibroblast-like synoviocytes and chondrocytes. Furthermore, cells with features of osteoclasts have been identified at the junction [34]; they are probably derived from the monocyte/macrophage lineage.
Table 1.
Inflammatory cells in synovial tissue from patients with rheumatoid arthritis
| Macrophages |
| Fibroblast-like synoviocytes |
| T cells |
| Plasma cells |
| B cells |
| Interdigitating dendritic cells |
| Follicular dendritic cells |
| Natural killer cells |
| Mast cells |
| Neutrophils |
Figure 1.
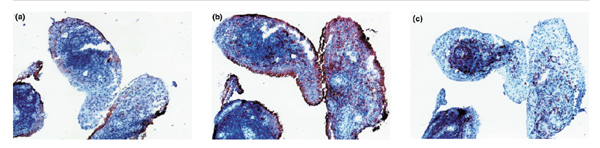
Rheumatoid synovial tissue showing (a) CD55+ fibroblast-like synoviocytes in the intimal lining layer; (b) CD68+ macrophages in the intimal lining layer and in the synovial sublining; and (c) CD3+ T cells in lymphocyte aggregates and in the diffuse leucocyte infiltrate in the synovial sublining. Original magnification ×100.
Pathogenetic mechanisms in rheumatoid arthritis
Although the aetiology of RA remains elusive, immune-mediated mechanisms are probably of crucial importance. The evidence to support a role of CD4+ T cells in the immune response in RA patients [35,36] is substantial, but circumstantial. A subset of the CD4+ cells in the synovium shows phenotypic evidence of prior activation, but many of the T cells are small and few of them express activation molecules such as transferrin and the interleukin-2 receptor [22,37,38]. Of interest is that the percentage of interferon-γ producing T cells [22,39] and the detectable levels of T cell receptor-ζ protein [40,41] are significantly lower in RA synovium than in a chronic T-cell-mediated immunological reaction, such as tonsillitis or tuberculous pleuritis. These data indicate that T cells in RA synovium are in a peculiar activation state.
Lymphocyte aggregates (Fig. 1c) are observed in 50-60% of RA patients, and can be surrounded by coronas of plasma cells [14]. In these areas interdigitating dendritic cells are observed in proximity to CD4+ T-cells [42,43,44,45]. Human leucocyte antigen class II molecules and the costimulatory molecule CD86 (B7-2), which has an important role in antigen presentation, are expressed on these cells [46,47,48], suggesting that interdigitating dendritic cells could present antigen to CD4+ T cells. Whether this involves mainly endogenous autoantigens [49,50] or exogenous agents, such as bacteria [51] and viruses [52], remains to be elucidated. Recent studies [51,53,54] have shown that there is a much higher load of bacterial DNA and peptidoglycans in the synovium than previously expected. Conceivably, the T-cell response is directed at an array of different antigens, which might well be a secondary phenomenon.
When the perivascular lymphocyte aggregates are large, substantial numbers of B cells can be found in close association with CD4+ cells and follicular dendritic cells [14,28]. Of importance is that fibroblast-like synoviocytes also have intrinsic properties of follicular dendritic cells [55]. The aggregates that consist mainly of CD4+ T cells and B cells resemble germinal centers, although they are morphologically not identical to the germinal centers in lymphatic organs [45,56]. The microenvironment suggests a close functional relationship between follicular dendritic cells and B cells in RA synovium, allowing activation and maturation of the humoral immune response.
It has become clear that cells other than lymphocytes, in particular activated macrophages (Fig. 1b) and fibroblast-like synoviocytes (Fig. 1a), play a critical role as effector cells in chronic disease [14,37,57,58]. Both cell types are highly activated and secrete a variety of cytokines [59,60], as well as matrix metalloproteinases [61,62]. Fibroblast-like synoviocytes can also produce other factors, such as proteoglycans and arachidonic acid metabolites [58,63]. The increase in the numbers of fibroblast-like synoviocytes can be explained in part by proliferation and by impaired apoptosis. Although proliferation probably contributes to some extent [64,65], inhibition of apoptosis in particular provides an important explanation for the increased cellularity [66]. Very few apoptotic cells are found in the synovium of RA patients [67,68], despite the presence of fragmented DNA in the intimal lining layer [67,69]. Various mechanisms may be involved in causing inadequate apoptosis: the development of mutations of the p53 suppressor gene [70,71,72]; deficient functional Fas ligand expression [73], overexpression of antiapoptotic molecules, such as sentrin [74]; and activation of nuclear factor-κB [75,76,77]. The marked increase in the expression of granzymes A and B in RA patients [30,78,79] could be a reactive attempt to induce apoptosis in synovial cells. Interestingly, fibroblast-like synoviocytes from RA patients exhibit many features of transformed cells [58,72]. The presence of these 'transformed' cells in the synovium may contribute to the autonomous progression of pannus and joint destruction in a subset of RA patients.
Two-thirds or more of the cells in the hyperplastic intimal lining layer in RA are macrophages, where they are particularly observed in the more superficial parts [80]. It is generally believed that they originate from bone marrow-derived monocytes that have migrated in response to chemotactic factors [81]. Relatively little is known about the factors that influence the specific retention of macrophages in the intimal lining layer. It has recently been suggested that the ligand pair CD55-CD97 could be involved [82]. Fibroblast-like synoviocytes can be distinguished from other fibroblasts by the marked expression of CD55 or complement decay accelerating factor. CD55 can act as a cellular ligand for the sevenspan-transmembrane molecule CD97, which is expressed by nearly all intimal macrophages [82]. The microarchitecture of the intimal lining layer strongly suggests that intimal macrophages and fibroblast-like synoviocytes may specifically interact via this ligand pair. Of note is that intimal macrophages exhibit stronger expression of CD97 than macrophages in the synovial sublining, illustrating the highly activated phenotype of the intimal macrophages in rheumatoid synovial tissue. The exact role of the CD55-CD97 interaction in the pathogenesis of RA remains to be elucidated.
The macrophages often also constitute the majority of the inflammatory cells in the synovial sublining [14]. Macrophage infiltration occurs preferentially in areas adjacent to the articular cartilage [4]. Of interest is that most cells in areas where synovial cells display tumour-like morphology are macrophages [83]. The preferential accumulation of macrophages at the pannus-cartilage junction is probably related to the expression of a range of adhesion molecules by macrophages [84,85,86] and to the effects of selective chemotactic factors [87,88,89].
The importance of these cells and their soluble mediators is supported by clinical observations. Local disease activity is particularly associated with the number of macrophages and the expression of cytokines, such as tumour necrosis factor (TNF)-α and interleukin-6, in synovial tissue [14,15]. There is also a significant positive correlation between intimal lining layer depth and cell counts for macrophages in the synovial sublining on the one hand, and radiographic signs of joint destruction after follow up on the other [16]. The pivotal role of TNF-α, at least in the majority of RA patients, has been confirmed by the impressive effects of specific therapeutic strategies targeting the TNF-α molecule [90,91,92]. The importance of cytokines, which are mainly derived from macrophages, is also illustrated by the effects of treatment aimed at blocking the effects of interleukin-1 [93,94] and interleukin-6 [95].
These observations have stimulated studies of the factors that drive the production of proinflammatory cytokines, such as TNF-α. It has been suggested [96] that cytokinestimulated T cells may contribute to the excessive production of TNF-α in synovial tissue. Among the cytokines that can promote a Th1-like proinflammatory response in the synovium are interleukin-12 [97,98] and probably also IL-18 [99]. Interleukin-15 is another cytokine that has drawn a lot of attention as a potential factor implicated in the interaction between T cells and TNF-α-producing macrophages [100,101,102]. In addition to cytokines, cell-surface molecules may also play a role in driving the production of proinflammatory cytokines and matrix metalloproteinases by macrophages and fibroblast-like synoviocytes [103,104].
Conclusion
There has been increased interest in the pathological changes at the site of inflammation in patients with various forms of arthritis. Several studies have focused on methodological matters concerning synovial biopsy procedures, sampling error and the methods used to quantify synovial inflammation. This has led to the first steps in the development of quality control systems and the standardization of methodology.
Preliminary studies have identified features of synovial tissue that are associated with specific arthritides. More extensive studies could yield important information for differential diagnosis and estimating prognosis. Moreover, studies on serial biopsy samples after experimental therapy may help to understand the mechanism of action of specific interventions. Such studies may also provide insight into the role of specific cells and molecules in the pathogenesis. Based on these and other investigations, macrophages and fibroblast-like synoviocytes have been recognized as key players in the effector phase of rheumatoid arthritis.
References
- Tak PP. Examination of the synovium and synovial fluid. Rheumatoid Arthritis: The New Frontiers in Pathogenesis and Treatment. Edited by Wollheim FA, Firestein GS, Panayi GS. Oxford: Oxford University Press.
- Bresnihan B, Tak PP. The role of synovial biopsy. . Baillieres Clin Rheumatol.
- Reece R, Emery P. Needle arthroscopy. Br J Rheumatol . 1995;34:1102–1104. doi: 10.1093/rheumatology/34.12.1102. [DOI] [PubMed] [Google Scholar]
- Youssef PP, Kraan M, Breedveld F, et al. Quantitative microscopic analysis of inflammation in rheumatoid arthritis synovial membrane samples selected at arthroscopy compared with samples obtained blindly by needle biopsy. Arthritis Rheum. 1998;41:663–669. doi: 10.1002/1529-0131(199804)41:4<663::AID-ART13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Heller RA, Schena M, Chai A, et al. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc Natl Acad Sci USA. 1997;94:2150–2155. doi: 10.1073/pnas.94.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad S, Hedfors E. Intraarticular variation in synovitis. Local macroscopic and microscopic signs of inflammatory activity are significantly correlated. Arthritis Rheum. 1985;28:977–986. doi: 10.1002/art.1780280904. [DOI] [PubMed] [Google Scholar]
- Ike RW. Diagnostic arthroscopy. Baillieres Clin Rheumatol. 1996;10:495–517. doi: 10.1016/s0950-3579(96)80046-x. [DOI] [PubMed] [Google Scholar]
- Rooney M, Condell D, Daly L, et al. Analysis of the histologic variation of synovitis in rheumatoid arthritis. . Arthritis Rheum. 1988;31:956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Bresnihan B, Cunnane G, Youssef PP, et al. Microscopic measurement of synovial membrane inflammation in rheumatoid arthritis: proposals for the evaluation of tissue samples by quantitative analysis. . Br J Rheumatol. 1998;37:636–642. doi: 10.1093/rheumatology/37.6.636. [DOI] [PubMed] [Google Scholar]
- Dolhain RJEM, Ter Haar NT, De Kuiper R, et al. Distribution of T cells and signs of T-cell activation in the rheumatoid joint: implications for semiquantitative comparative histology. . Br J Rheumatol. 1998;37:324–330. doi: 10.1093/rheumatology/37.3.324. [DOI] [PubMed] [Google Scholar]
- Youssef PP, Smeets TJM, Bresnihan B, et al. Microscopic measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: a comparison of semiquantitative and quantitative analysis. . Br J Rheumatol. 1998;37:1003–1007. doi: 10.1093/rheumatology/37.9.1003. [DOI] [PubMed] [Google Scholar]
- Youssef PP, Triantafillou S, Parker A, et al. Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis. J Rheumatol. 1997;24:2291–2298. [PubMed] [Google Scholar]
- Kraan MC, Haringman JJ, Ahern MJ, et al. Digital image analysis for the quantification of the cell infiltrate in synovial tissue. . Rheumatology. [DOI] [PubMed]
- Tak PP, Smeets TJM, Daha MR, et al. Analysis of the synovial cellular infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- Kraan MC, Versendaal H, Jonker M, et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum . 1998;41:1481–1488. doi: 10.1002/1529-0131(199808)41:8<1481::AID-ART19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mulherin D, FitzGerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. . Arthritis Rheum. 1996;39:115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- Cunnane G, FitzGerald O, Hummel KM, et al. Collagenase, cathepsin B and cathepsin L gene expression in the synovial membrane of patients with early inflammatory arthritis. Rheumatology. 1999;38:34–42. doi: 10.1093/rheumatology/38.1.34. [DOI] [PubMed] [Google Scholar]
- Schumacher HR, Jr, Kitridou RC. Synovitis of recent onset. A clinicopathologic study during the first month of disease. . Arthritis Rheum. 1972;15:465–485. doi: 10.1002/art.1780150502. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Bergroth V, Nordstrom D, et al. Cellular immunohistopathology of acute, subacute, and chronic synovitis in rheumatoid arthritis. Ann Rheum Dis. 1985;44:549–555. doi: 10.1136/ard.44.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler NJ, Boyle D, Firestein GS. Early synovitis-synoviocytes and mononuclear cells. Semin Arthritis Rheum. 1994:11–16. doi: 10.1016/0049-0172(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Tak PP, Thurkow EW, Daha MR, et al. Expression of adhesion molecules in early rheumatoid synovial tissue. Clin Immunol Immunopathol. 1995;77:236–242. doi: 10.1006/clin.1995.1149. [DOI] [PubMed] [Google Scholar]
- Smeets TJM, Dolhain RJEM, Miltenburg AMM, et al. Poor expression of T cell-derived cytokines and activation and proliferation markers in early rheumatoid synovial tissue. Clin Immunol Immunopathol. 1998;88:84–90. doi: 10.1006/clin.1998.4525. [DOI] [PubMed] [Google Scholar]
- Smeets TJM, Dolhain RJEM, Breedveld FC, Tak PP. Analysis of the cellular infiltrates and expression of cytokines in synovial tissue from patients with rheumatoid arthritis and reactive arthritis. 1998. pp. 75–81. [DOI] [PubMed]
- Fearon U, Reece R, Smith J, Emery P, Veale DJ. Synovial cytokine and growth factor regulation of MMPs/TIMPs: implications for erosions and angiogenesis in early rheumatoid and psoriatic arthritis patients. . Ann N Y Acad Sci. 1999;878:619–621. doi: 10.1111/j.1749-6632.1999.tb07743.x. [DOI] [PubMed] [Google Scholar]
- van der Heijde DM. Joint erosions and patients with early rheumatoid arthritis. Br J Rheumatol. 1995;34 (suppl 2):74–78. [PubMed] [Google Scholar]
- Kraan MC, Haringman JJ, Post W, et al. Immunohistologic analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology. [DOI] [PubMed]
- Tak PP, Breedveld FC. Analysis of serial synovial biopsies as a screening method for predicting the effects of therapeutic interventions. . J Clin Rheumatol. 1997;3:186–187. doi: 10.1097/00124743-199708000-00002. [DOI] [PubMed] [Google Scholar]
- Yanni G, Whelan A, Feighery C, Bresnihan B. Analysis of cell populations in rheumatoid arthritis synovial tissues. Semin Arthritis Rheum. 1992;21:393–399. doi: 10.1016/0049-0172(92)90040-k. [DOI] [PubMed] [Google Scholar]
- De Paulis A, Marino I, Ciccarelli A, et al. Human synovial mast cells.1. Ultrastructural in situ and in vitro immunologic characterization. Arthritis Rheum. 1996;39:1222–1233. doi: 10.1002/art.1780390723. [DOI] [PubMed] [Google Scholar]
- Tak PP, Kummer JA, Hack CE, et al. Granzyme positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum. 1994;37:1735–1743. doi: 10.1002/art.1780371205. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Klimiuk PA, Goronzy JJ. Heterogeneity of rheumatoid arthritis: from phenotypes to genotypes. Springer Semin Immunopathol. 1998;20:5–22. doi: 10.1007/BF00831996. [DOI] [PubMed] [Google Scholar]
- Zvaifler NJ, Tsai V, Alsalameh S, et al. Pannocytes: distinctive cells found in rheumatoid arthritis articular cartilage erosions. Am J Pathol. 1997;150:1125–1138. [PMC free article] [PubMed] [Google Scholar]
- Xue C, Takahashi M, Hasunuma T, et al. Characterisation of fibroblast-like cells in pannus lesions of patients with rheumatoid arthritis sharing properties of fibroblasts and chondrocytes. Ann Rheum Dis. 1997;56:262–267. doi: 10.1136/ard.56.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese EM, Harada Y, Wang JT, et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- Panayi GS, Lanchbury JS, Kingsley GH. The importance of the T cell in initiating and mainaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992;35:729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Fox DA. The role of T cells in the immunopathogenesis of rheumatoid arthritis. New perspectives. Arthritis Rheum. 1997;40:598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Zvaifler NJ. How important are T cells in chronic rheumatoid synovitis. Arthritis Rheum. 1990;33:768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Smith MD, O'Donnell J, Highton J, et al. Immunohistochemical analysis of synovial membranes from inflammatory and non-inflammatory arthritides: scarcity of CD5 positive B cells and IL2 receptor bearing T cells. Pathology. 1992;24:19–26. doi: 10.3109/00313029209063615. [DOI] [PubMed] [Google Scholar]
- Firestein GS, Xu WD, Townsend K, et al. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988;168:1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice MM, Lankester AC, Bezemer AC, et al. Defective TCR-mediated signaling in synovial T cells in rheumatoid arthritis. . J Immunol. 1997;159:2973–2978. [PubMed] [Google Scholar]
- Matsuda M, Ulfgren AK, Lenkei R, et al. Decreased expression of signal-transducing CD3 zeta chains in T cells from the joints and peripheral blood of rheumatoid arthritis patients. Scand J Immunol. 1998;47:254–262. doi: 10.1046/j.1365-3083.1998.00296.x. [DOI] [PubMed] [Google Scholar]
- Duke O, Panayi GS, Janossy G, Poulter LW. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982;49:22–30. [PMC free article] [PubMed] [Google Scholar]
- Janossy G, Panayi GS, Duke O, et al. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. . Lancet. 1981;2:839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Klareskog L, Forsum U, Scheynius A, Kabelitz D, Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci USA. 1982;79:3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenn V, Schalhorn N, Greiner A, et al. Immunohistochemical analysis of proliferating and antigen-presenting cells in rheumatoid synovial tissue. Rheumatol Int. 1996;15:239–247. doi: 10.1007/BF00290377. [DOI] [PubMed] [Google Scholar]
- Balsa A, Dixey J, Sansom DM, Maddison PJ, Hall ND. Differential expression of the costimulatory molecules B7.1 (CD80) and B7.2 (CD86) in rheumatoid synovial tissue. Br J Rheumatol. 1996;35:33–37. doi: 10.1093/rheumatology/35.1.33. [DOI] [PubMed] [Google Scholar]
- Thomas R, Quinn C. Functional differentiation of dendritic cells in rheumatoid arthritis: role of CD86 in the synovium. J Immunol. 1996;156:3074–3086. [PubMed] [Google Scholar]
- Thomas R, Lipsky PE. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol Today. 1996;17:559–564. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- Thomas R, Lipsky PE. Presentation of self peptides by dendritic cells: possible implications for the pathogenesis of rheumatoid arthritis. . Arthritis Rheum. 1996;39:183–190. doi: 10.1002/art.1780390202. [DOI] [PubMed] [Google Scholar]
- Verheijden GFM, Rijnders AWM, Bos E, et al. Human cartilage glycoprotein-39 as a candidate autoantigen in rheumatoid arthritis. Arthritis Rheum. 1997;40:1115–1125. doi: 10.1002/art.1780400616. [DOI] [PubMed] [Google Scholar]
- Van der Heijden IM, Wilbrink B, Tchetverikov I, et al. The presence of bacterial DNA and bacterial peptidoglycans in the joints of patients with rheumatoid arthritis and other arthritides [abstract]. Arthritis Rheum. 1998;41 (suppl 9):S162. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Brusic V, McColl G, Harrison LC. Direct evidence for the expression of multiple endogenous retroviruses in the synovial compartment in rheumatoid arthritis. Arthritis Rheum. 1997;40:627–638. doi: 10.1002/art.1780400407. [DOI] [PubMed] [Google Scholar]
- Wilbrink B, Van der Heijden IM, Schouls LM, et al. Detection of bacterial DNA in joint samples from patients with undifferentiated arthritis and reactive arthritis, using polymerase chain reaction with universal 16S ribosomal RNA primers. Arthritis Rheum . 1998;41:535–543. doi: 10.1002/1529-0131(199803)41:3<535::AID-ART20>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson NZ, Kingsley GH, Jones HW, et al. The detection of DNA from a range of bacterial species in the joints of patients with a variety of arthritides using a nested, broad-range polymerase chain reaction. Rheumatology. 1999;38:260–266. doi: 10.1093/rheumatology/38.3.260. [DOI] [PubMed] [Google Scholar]
- Lindhout E, Van Eijk M, Van Pel M, et al. Fibroblast-like synoviocytes from rheumatoid arthritis patients have intrinsic properties of follicular dendritic cells. J Immunol . 1999;162:5949–5956. [PubMed] [Google Scholar]
- Randen I, Mellbye OJ, Forre O, Natvig JB. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand J Immunol. 1995;41:481–486. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- Burmester GR, Stuhlmuller B, Keyszer G, Kinne RW. Mononuclear phagocytes and rheumatoid synovitis. Mastermind or workhorse in arthritis? . Arthritis Rheum. 1997;40:5–18. doi: 10.1002/art.1780400104. [DOI] [PubMed] [Google Scholar]
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed agressors? . Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Ziff M. Role of endothelium in chronic inflammation. . Springer Semin Immunopathol. 1989;11:199–214. doi: 10.1007/BF00197189. [DOI] [PubMed] [Google Scholar]
- Zvaifler NJ. Rheumatoid arthritis: the multiple pathways to chronic synovitis. Lab Invest. 1995;73:307–310. [PubMed] [Google Scholar]
- Murphy G, Hembry RM. Proteinases in rheumatoid arthritis. . J Rheumatol. 1992;19:61–64. [PubMed] [Google Scholar]
- Hembry RM, Bagga MR, Reynolds JJ, Hamblen DL. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis. 1995;54:25–32. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Paine MM, Littman BH. Gene expression (collagenase, tissue inhibitor of metalloproteinases, complement, and HLA-DR) in rheumatoid arthritis and osteoarthritis synovium. Quantitative analysis and effect of intraarticular corticosteroids. Arthritis Rheum. 1991;34:1094–1105. doi: 10.1002/art.1780340905. [DOI] [PubMed] [Google Scholar]
- Lalor PA, Mapp PI, Hall PA, Revell PA. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7:183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- Qu ZH, Garcia CH, O'Rourke LM, et al. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia: results of proliferation cell nuclear antigen/cyclin, C-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994;37:212–220. doi: 10.1002/art.1780370210. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. Apoptosis in rheumatoid arthritis. Apoptosis and Inflammation Edited by Winkler JD Basel: Birkhauser Publishing Ltd. 1999:149–162. [Google Scholar]
- Nakajima T, Aono H, Hasunuma T, et al. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. . Arthritis Rheum. 1995;38:485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Muller-Ladner U, Gay RE, Nishioka K, Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996;23:1345–1352. [PubMed] [Google Scholar]
- Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest . 1995;96:1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. . Proc Natl Acad Sci USA. 1997;94:10895–10900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reme T, Travaglio A, Gueydon E, et al. Mutations of the p53 tumour suppressor gene in erosive rheumatoid synovial tissue. Clin Exp Immunol. 1998;111:353–358. doi: 10.1046/j.1365-2249.1998.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. [DOI] [PubMed]
- Cantwell MJ, Hua T, Zvaifler NJ, Kipps TJ. Deficient Fas ligand expression by synovial lymphocytes from patients with rheumatoid arthritis. . Arthritis Rheum. 1997;40:1644–1652. doi: 10.1002/art.1780400914. [DOI] [PubMed] [Google Scholar]
- Franz JK, Humeel KM, Aicher WK, et al. Invasive synovial fibroblasts express the novel anti-apoptotic molecule sentrin in the SCID mouse model of rheumatoid arthritis [abstract]. Arthritis Rheum. 1998;41 (suppl 9):S238. [Google Scholar]
- Miagkov AV, Kovalenko DV, Brown CE, et al. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel ML, Mcmorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium: localization of p50 and p65. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- Marok R, Winyard PG, Coumbe A, et al. Activation of the transcription factor nuclear factor-kappa B in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- Muller-Ladner U, Kriegsmann J, Tschopp J, Gay RE, Gay S. Demonstration of granzyme A and perforin messenger RNA in the synovium of patients with rheumatoid arthritis. Arthritis Rheum . 1995;38:477–484. doi: 10.1002/art.1780380404. [DOI] [PubMed] [Google Scholar]
- Tak PP, Spaeny-Dekking L, Kraan MC, et al. The levels of soluble granzyme A and B are elevated in plasma and synovial fluid of patients with rheumatoid arthritis (RA). Clin Exp Immunol. 1999;116:366–370. doi: 10.1046/j.1365-2249.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broker BM, Edwards JC, Fanger MW, Lydyard PM. The prevalence and distribution of macrophages bearing Fc gamma R I, Fc gamma R II, and Fc gamma R III in synovium. Scand J Rheumatol. 1990;19:123–135. doi: 10.3109/03009749009102116. [DOI] [PubMed] [Google Scholar]
- Henderson B, Revell PA, Edwards JC. Synovial lining cell hyperplasia in rheumatoid arthritis: dogma and fact. Ann Rheum Dis . 1988;47:348–349. doi: 10.1136/ard.47.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J, Wishaupt JO, Van Lier RAW, et al. Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum. 1999;42:650–658. doi: 10.1002/1529-0131(199904)42:4<650::AID-ANR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Sack U, Stiehl P, Geiler G. Distribution of macrophages in rheumatoid synovial membrane and its association with basic activity. Rheumatol Int. 1994;13:181–186. doi: 10.1007/BF00390265. [DOI] [PubMed] [Google Scholar]
- Allen CA, Highton J, Palmer DG. Increased expression of p150,95 and CR3 leukocyte adhesion molecules by mononuclear phagocytes in rheumatoid synovial membranes. Comparison with osteoarthritic and normal synovial membranes. Arthritis Rheum. 1989;32:947–954. doi: 10.1002/anr.1780320803. [DOI] [PubMed] [Google Scholar]
- El-Gabalawy HS, Gallatin M, Vazeux R, Peterman G, Wilkins J. Expression of ICAM-R (ICAM-3), a novel counter-receptor for LFA-1, in rheumatoid and nonrheumatoid synovium: comparison with other adhesion molecules. Arthritis Rheum. 1994;37:846–854. doi: 10.1002/art.1780370612. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Hirata S, Andoh Y, et al. An immunohistochemical and immunoelectron microscopic study of adhesion molecules in synovial pannus formation in rheumatoid arthritis. . Rheumatol Int. 1996;16:53–60. doi: 10.1007/BF01816436. [DOI] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Harlow LA, et al. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. . J Clin Invest. 1992;90:772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CQ, Field M, Allard S, et al. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992;31:653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Harlow LA, et al. Macrophage inflammatory protein-1 alpha: a novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–928. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med . 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- Tak PP, Taylor PC, Breedveld FC, et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha treatment in patients with rheumatoid arthritis. . Arthritis Rheum. 1996;39:1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- Gabay C, Arend WP. Treatment of rheumatoid arthritis with IL-1 inhibitors. Springer Semin Immunopathol. 1998;20:229–246. doi: 10.1007/s002810050032. [DOI] [PubMed] [Google Scholar]
- Bresnihan B, Alvaro-Gracia JM, Cobby M, et al. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41:2196–2204. doi: 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Nishimoto N, Mihara M, Kishimoto T. Therapy of rheumatoid arthritis by blocking IL-6 signal transduction with a humanized anti-IL-6 receptor antibody. Springer Semin Immunopathol. 1998;20:247–259. doi: 10.1007/s002810050033. [DOI] [PubMed] [Google Scholar]
- Sebbag M, Parry SL, Brennan FM, Feldmann M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor alpha, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- Morita Y, Yamamura M, Nishida K, et al. Expression of interleukin-12 in synovial tissue from patients with rheumatoid arthritis. . Arthritis Rheum. 1998;41:306–314. doi: 10.1002/1529-0131(199802)41:2<306::AID-ART15>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sakkas LI, Johanson NA, Scanzello CR, Platsoucas CD. Interleukin-12 is expressed by infiltrating macrophages and synovial lining cells in rheumatoid arthritis and osteoarthritis. Cell Immunol. 1998;188:105–110. doi: 10.1006/cimm.1998.1363. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Almughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. . Nature Med. 1996;2:175–182. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- Thurkow EW, Van der Heijden IM, Breedveld FC, et al. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared to patients with Yersinia-induced arthritis and osteoarthritis. J Pathol. 1997;181:444–450. doi: 10.1002/(SICI)1096-9896(199704)181:4<444::AID-PATH778>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor alpha production in rheumatoid arthritis. Nature Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- Vey E, Dayer JM, Burger D. Direct contact with stimulated T cells induces the expression of IL-1 beta and IL-1 receptor antagonist in human monocytes. Involvement of serine/threonine phosphatases in differential regulation. Cytokine . 1997;9:480–487. doi: 10.1006/cyto.1997.0191. [DOI] [PubMed] [Google Scholar]
- Burger D, Rezzonico R, Li JM, et al. Imbalance between interstitial collagenase and tissue inhibitor of metalloproteinases 1 in synoviocytes and fibroblasts upon direct contact with stimulated T lymphocytes: involvement of membrane-associated cytokines. Arthritis Rheum. 1998;41:1748–1759. doi: 10.1002/1529-0131(199810)41:10<1748::AID-ART7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]


