Abstract
Osteoclast activation is a critical cellular process for pathological bone resorption, such as erosions in rheumatoid arthritis (RA) or generalized bone loss. Among many factors triggering excessive osteoclast activity, cytokines such as IL-1 or tumour necrosis factor (TNF)-α play a central role. New members of the TNF receptor ligand family (namely receptor activator of nuclear factor-κB [RANK] and RANK ligand [RANKL]) have been discovered whose cross-interaction is mandatory for the differentiation of osteoclasts from hemopoietic precursors, in both physiological and pathological situations. Osteoprotegerin, a decoy receptor which blocks this interaction, decreases osteoclast activity and could have a fascinating therapeutic potential in conditions associated with upregulated bone resorption.
Keywords: bone cytokines, differentiation, osteoclast, osteoprotegerin, RANK, RANKL
Introduction
Bone remodelling is a continuous physiological process that occurs in adult skeleton in which bone resorption is followed by new bone formation, maintaining mechanical strength and structure. Bone cells that are responsible for this coupled process include bone-resorbing cells (osteoclasts, which are derived from haematopoietic cells of the monocyte/macrophage lineage) and bone-forming cells (osteoblasts, which are of mesenchymal origin). The bone resorption process is involved in many clinical situations that are relevant to the work of rheumatologists, such as focal bone destruction or erosion in RA and other inflammatory arthritides, and the diffuse bone loss that is encountered in osteoporosis.
Osteoclast differentiation: basic mechanisms and new insights
Osteoclast progenitor cells are recruited from haematopoietic compartments, and then proliferate and differentiate toward mature osteoclasts. During this multistep differentiation process postmitotic osteoclast precursors progressively express osteoclast-associated markers, such as calcitonin receptor and tartrate-resistant acid phosphatase, as they lose some of their macrophage characteristics. Then, mononuclear preosteoclasts fuse together to form multinucleated giant cells. Terminal osteoclast differentiation eventually leads to active bone-resorbing cells [1].
Role of osteoblast/stromal cells in osteoclast differentiation
Biological models of in vitro osteoclast differentiation have been developed that have facilitated detailed study of many of the factors involved in the regulation of this process. The most commonly studied models are cultures of mouse bone marrow or cocultures of haematopoietic cells with bone-derived stromal cells, which give rise to large numbers of bone-resorbing osteoclasts [2]. Studies based on these models have found that mesenchymally derived stromal cells play a critical role in supporting and stimulating osteoclast differentiation, a process that probably necessitates cell–cell contact between osteoclast precursors and stromal cells [3,4]. In some human models, however, a cellular interaction between osteoclast precursors and stromal cells is not always required [5,6,7].
Local and hormonal factors that are involved in osteoclast differentiation
Bone resorption is closely controlled in vivo by cellular and hormonal factors, which affect not only osteoclast activity, but also osteoclast formation. Parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] increase bone resorption, primarily via an indirect mechanism that is mediated by osteoblasts [2].Oestrogens have a negative impact on osteoclast differentiation, and oestrogen deficiency leads to increased osteoclast differentiation and activation [8]. The cytokines IL-1, IL-6 and TNF-α are known to increase bone resorption by stimulating both osteoclast activity and differentiation. This effect involves, at least in part, prostaglandin production [9,10]. The major role of macrophage colony-stimulating factor (M-CSF) has been pointed out in M-CSF-deficient (op/op) mice, which develop an osteopetrosis that is characterized by the absence of osteoclasts [11]. Studies using murine cocultures [12] have shown that M-CSF acts both on proliferation and on differentiation of precursor cells. Local injections of M-CSF in rat metaphyseal bone also increase in situ osteoclast differentiation and bone resorption [13]. Other cytokines stimulate bone resorption at least partly by increasing osteoclast differentiation, such as leukaemia inhibiting factor and IL-11 [14,15]. Conversely, some cytokines such as IL-4 or IFN-γ have been shown to inhibit osteoclast differentiation in vitro [16]. The role of transforming growth factor-β is more complex; it decreases osteoclast precursor proliferation and bone resorption activity [17,18], but it also increases the expression of two osteoclastic markers — vitronectin receptor and calcitonin receptor [19,20]. Most of the cytokines that regulate osteoclast differentiation are produced in the bone microenvironment, mainly by osteoblast/stromal cells, further emphasizing the key role of these cells in osteoclast differentiation.
A new interactive system in osteoclast bone resorption
The recent discovery of new members of the TNF receptor ligand family has pointed out the crucial role of RANK and RANKL in osteoclast differentiation and activation [21*,22*] (Fig. 1).
Figure 1.
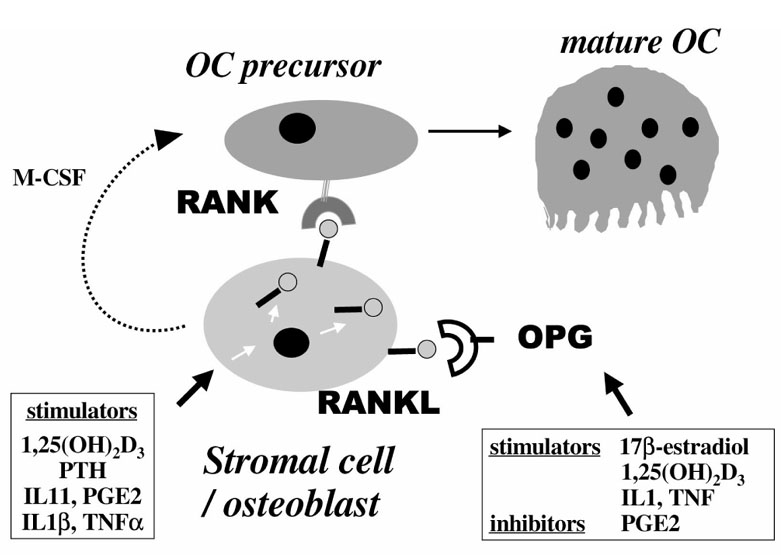
New members of the TNF receptor ligand family: role of RANKL (ODF, TRANCE, OPGL) and its receptor RANK in osteoclast differentiation. RANKL, a membrane-bound TNF-related factor, is expressed by osteoblast/stromal cells and is upregulated by osteotropic factors such as 1,25(OH)2D3, PTH, IL-6 or IL-11. Osteoclast (OC) precursors express RANK, a membrane-bound TNF receptor, that recognizes RANKL through cell-cell interaction with osteoblast/stromal cells. This interaction enables osteoclast precursors to differentiate in the presence of M-CSF. Osteoprotegerin (OPG) is a member of the TNF receptor family that lacks a transmembrane domain and represents a secreted TNF receptor. OPG recognizes RANKL, and this decoy receptor blocks the interaction between RANK and RANKL, leading to inhibition of osteoclast differentiation and activation.
RANKL, also called osteoclast differentiation factor (ODF), TNF-related induced cytokine (TRANCE), or osteoprotegerin ligand (OPGL)
Because osteoblast–stromal cell interactions with osteoclast precursors are required for subsequent osteoclast differentiation, an ODF expressed by these cells and recognized by osteoclast precursors was suspected. Such a factor was identified as RANKL [23**,24**]. RANKL is a membrane-bound TNF-related factor that is expressed by osteoblast/stromal cells. That the presence of RANKL is vital in osteoclast differentiation is now well established, and its soluble recombinant form has been tested in a number of in vitro and in vivo studies. In in vitro murine or human osteoclast differentiation models, soluble RANKL enables osteoclast precursors to differentiate in the presence of M-CSF, even in the absence of osteoblast/stromal cells [25**,26]. Bone resorption activity is increased, as well as the osteoclast survival [23**,26]. Mice that are defective for RANKL develop a form of osteopetrosis. They are characterized by the absence of osteoclasts, although osteoclast progenitors are present and are able to differentiate into bone-resorbing osteoclasts in the presence of normal osteoblast/stromal cells [27**].
In addition, the soluble form of RANKL has been shown to be produced by human fibroblasts transfected with an expression vector for RANKL and by in vitro activated murine T cells [23**,28]. However, it is not clear whether this soluble form plays a role in vivo in normal bone homeostasis or in pathological processes that are characterized by increased bone resorption.
RANK
Osteoclast precursors express RANK, a membrane-bound TNF receptor that recognizes RANKL through a direct cell-cell interaction with osteoblast/stromal cells [29**]. Recent studies [29**,30] demonstrated that this receptor is essential for the transduction of signals that lead to osteoclast differentiation. An overexpression of soluble RANK results in osteopetrosis, with a decreased number of osteoclasts [30]. Conversely, mice that are deficient for RANK develop a severe osteopetrosis that is characterized by the absence of osteoclasts. In addition, osteoclast precursors in these mice are unable to differentiate to osteoclasts in vitro, in the presence of RANKL and M-CSF [31].
Osteoprotegerin
Osteoprotegerin is a member of the TNF receptor family that lacks a transmembrane domain and represents a secreted receptor. Osteoprotegerin recognizes RANKL, and this decoy receptor blocks the interaction between RANK and RANKL, leading to an inhibition of osteoclast differentiation and activation [32**,33**]. Overexpression of osteoprotegerin in transgenic mice results in a form of osteopetrosis that is characterized by a defect in osteoclast differentiation [32**]. By contrast, osteoprotegerin-deficient mice develop severe osteoporosis because of increased osteoclast differentiation and function [34**]. In vitro studies have demonstrated the strong inhibitory action of osteoprotegerin on osteoclast differentiation, as well as on the bone-resorbing activity of osteoclasts [32**,33**].
Role of RANK/RANKL and osteoprotegerin in osteoclast differentiation
RANKL/osteoprotegerin balance, signal transduction and osteoclast differentiation
Recent data suggest that M-CSF and RANKL are two major factors involved in osteoclast differentiation. M-CSF is required for both proliferation and differentiation, and RANKL (which is not a growth factor) is required for differentiation into mature osteoclasts and for osteoclast activity [26]. In bone tissue, osteoprotegerin and RANKL are expressed by osteoblast/stromal cells, and the ratio of these products may modulate the ability of these cells to stimulate osteoclast differentiation/activity, as well as the rate of bone resorption [21*].
In addition, it has been shown [26] that the interaction between RANKL and RANK results in a transduction signal in preosteoclasts and in mature osteoclasts that may activate nuclear factor-κ (NF-κB). The role of NF-κB in the osteoclast differentiation has been previously demonstrated in mice with a double knockout for the p50 and p52 NF-κB subunits, in which a defect of osteoclast differentiation leads to an osteopetrosis [35,36]. Other intracellular events are activated by transduction signals, such as c-jun terminal kinase, and TNF receptor-associated factors, which regulate activation of NF-κB and/or c-jun terminal kinase [21*].
RANK/RANKL, immune cells and osteoclast differentiation
RANK and RANKL have been shown to be expressed in dendritic cells and T lymphocytes, respectively, in which they appear to be important regulators of the interactions between these cells [37,38]. These data suggest that, apart from osteoclast differentiation and activation, RANK and RANKL are involved in the immune system as suggested by the mice knockout models. RANKL-deficient mice lack lymph nodes and exhibit defects in differentiation of T and B lymphocytes [27**], and RANK-deficient mice exhibit a marked deficiency in B cells in the spleen and lack lymph nodes [31].
Regulation of RANKL and osteoprotegerin expression
Recent studies have demonstrated that osteotropic factors and hormones such as PTH, 1,25(OH)2D3, IL-11, IL-1β, TNF-α or prostaglandin E2 upregulate RANKL expression in osteoblast/stromal cells (Fig. 1). In addition, osteoprotegerin expression is downregulated by prostaglandin E2, and is upregulated by oestrogens [21*]. RANK expression has not yet been extensively studied.
An integrated view and clinical implications
An emerging concept is that cytokines and hormonal factors that are involved in bone resorption may act by a common final pathway involving RANKL and RANK [21*]. In accordance with this concept, a recent in vivo study [39**] has shown that a recombinant chimaeric Fc fusion form of osteoprotegerin inhibited hypercalcaemia and bone resorption induced by IL-1β, TNF-α, PTH and 1,25(OH)2D3 in mice. This convergence theory is probably not exclusive because recent studies [40,41] have suggested that the effects of TNF-α or IL-6 may involve different effectors.
Therapeutic perspectives
The concept presented above will probably lead to new therapeutic approaches in several diseases that are characterized by excessive bone resorption. Thus, osteoprotegerin (a specific inhibitor of RANKL) or an analogue may be used to block the excess of bone resorption in pathological conditions such as hyper-resorption of malignancy, in which this pathway seems to be primarily involved [42**], or in osteoporosis, in which oestrogen deficiency could lead to decreased production of osteoprotegerin and subsequent increased bone resorption.
Bone erosions in rheumatoid arthritis
Rheumatoid arthritis is another interesting clinical model for the study of the role of RANK/RANKL in bone erosions, and as a therapeutic target for osteoprotegerin. Rheumatoid arthritis is characterized by progressive bone and cartilage destruction as a result of chronic synovitis. Numerous studies have pointed out the role of cytokines such as TNF-α or IL-1 in the joint destruction [43]. Recent studies suggest that RANKL mRNA is highly expressed in synovial tissues from patients with RA, but not in normal synovial tissues. This expression is detected in synovial fibroblasts, as well as in activated T cells derived from RA synovial tissues, suggesting that these cells may contribute to osteoclast formation at the specific sites of bone destruction in RA [44*,45*]. In addition, in rat adjuvant-induced arthritis, RANKL is expressed on the surface of activated T cells isolated from affected rats, and may be secreted in T cell cultures. Activated T cells could therefore directly induce osteoclastogenesis through membrane-bound and soluble RANKL [28]. These data suggest that RANKL may have a major pathophysiological importance in the bone and joint destruction observed in inflammatory arthritides such as RA. Activated T cells, which play a central role in the pathogenesis of RA, may (in addition to stromal cells) contribute to the osteoclast-mediated bone resorption via RANKL expression [46**].
Conclusion
Osteoclasts are multinucleated cells that are formed by fusion of osteoclast precursors from haematopoietic origin. These cells are responsible for bone resorption and osteoclast differentiation and represent an evident point of control of bone resorption. Bone resorption is closely regulated in vivo by many cellular and hormonal factors, which affect not only osteoclast activity, but also osteoclast formation. The recent discovery of new members of the TNF receptor ligand family (ODF, TNF-related induced cytokine, osteoprotegerin ligand) have emphasized the crucial role of RANKL, which is expressed by osteoblast/stromal cells, and its receptor RANK, which is expressed by osteoclast cells, in osteoclast differentiation and activation. This system is completed by osteoprotegerin, which is a secreted TNF receptor. Osteoprotegerin recognizes RANKL, and this decoy receptor blocks the interaction between RANK and RANKL. A number of osteotropic factors and hormones may modulate bone resorption via this common final pathway, which may represent a potential therapeutic target in pathologic processes that are characterized by excessive bone resorption.
References
- Takahashi N, Udagawa N, Tanaka S, Murakami H, Owan I, Tamura T, Suda T. Postmitotic osteoclast precursors are mononuclear cells which express macrophage-associated phenotypes. Dev Biol. 1994;163:212–221. doi: 10.1006/dbio.1994.1137. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Quinn JM, McGee JO, Athanasou NA. Cellular and hormonal factors influencing monocyte differentiation to osteoclastic bone-resorbing cells. . Endocrinology. 1994;134:2416–2423. doi: 10.1210/endo.134.6.8194468. [DOI] [PubMed] [Google Scholar]
- Kurihara N, Civin C, Roodman GD. Osteotropic factor responsiveness of highly purified populations of early and late precursors for human multinucleated cells expressing the osteoclast phenotype. . J Bone Miner Res. 1991;6:257–261. doi: 10.1002/jbmr.5650060307. [DOI] [PubMed] [Google Scholar]
- Matayoshi A, Brown C, DiPersio JF, Haug J, Abu-Amer Y, Liapis H, Kuestner R, Pacifici R. Human blood-mobilized hematopoietic precursors differentiate into osteoclasts in the absence of stromal cells. . Proc Natl Acad Sci USA. 1996;93:10785–10790. doi: 10.1073/pnas.93.20.10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S, Quinn J, Pichaud F, Orcel P, Chastre E, Jullienne A, De Vernejoul MC. Human cord blood monocytes undergo terminal osteoclast differentiation in vitro in the presence of culture medium conditioned by giant cell tumor of bone. J Cell Physiol. 1996;168:489–498. doi: 10.1002/(SICI)1097-4652(199609)168:3<489::AID-JCP1>3.3.CO;2-R. [DOI] [PubMed] [Google Scholar]
- de Vernejoul MC, Cohen-Solal M, Orcel P. Bone cytokines. . Curr Opin Rheumatol. 1993;5:332–338. doi: 10.1097/00002281-199305030-00012. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Interleukin-6: an osteotropic factor? J Bone Miner Res. 1992;7:475–478. doi: 10.1002/jbmr.5650070502. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Chenu C, Bird A, Mundy GR, Roodman GD. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989;4:113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S. The murine mutation osteopetrosis is in the coding region of macrophage colony stimulating factor gene. . Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, Kurokawa T, Suda T. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993;91:257–263. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcel P, Feuga M, Bielakoff J, de Vernejoul MC. Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol. 1993;264:E391–E397. doi: 10.1152/ajpendo.1993.264.3.E391. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Abe E, Jin CH, Miyaura C, Hong MH, Oshida M, Kurosawa H, Yamaguchi Y, Tomida M, Hozumi M. Leukemia inhibitory factor/ differentiation-stimulating factor (LIF/D-Factor): regulation of its production and possible roles in bone metabolism. J Cell Physiol . 1992;152:71–78. doi: 10.1002/jcp.1041520110. [DOI] [PubMed] [Google Scholar]
- Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: a new cytokine critical for osteoclast development. J Clin Invest . 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey DL, Erdmann JM, Teitelbaum SL, Tan H, Ohara J, Shioi A. Interleukin 4, Interferon-γ and prostaglandin E impact the osteoclastic cell-forming potential of murine bone marrow macrophages. Endocrinology. 1995;136:2367–2376. doi: 10.1210/endo.136.6.7750457. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Bonewald L, Kukita A, Garrett IR, Seyedin SM, Rosen D, Mundy GR. Inhibitory effects of bone-derived growth factors, osteoinductive factor and transforming growth factor-β on isolated osteoclasts. Endocrinology. 1990;126:3069–3075. doi: 10.1210/endo-126-6-3069. [DOI] [PubMed] [Google Scholar]
- Chenu C, Pfeilschifter J, Mundy GR, Roodman GD. Tansforming growth factor β inhibits formation of osteoclast-like cells in long-term human marrow cultures. Proc Natl Acad Sci USA . 1988;85:5683–5687. doi: 10.1073/pnas.85.15.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbalaviele G, Orcel P, Bouizar Z, Julienne A, de Vernejoul MC. Transforming growth factor β enhances calcitonin-induced cyclic AMP production and the number of calcitonin receptors in long term cultures of human umbilical cord blood monocytes in the presence of 1,25-dihydroxycholecalciferol. J Cell Physiol . 1992;152:486–494. doi: 10.1002/jcp.1041520307. [DOI] [PubMed] [Google Scholar]
- Orcel P, Bielakoff J, de Vernejoul MC. Effect of transforming growth factor β on long-term human cord blood monocytes cultures. J Cell Physiol. 1990;142:293–298. doi: 10.1002/jcp.1041420211. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. . Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclasto-genesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JM, Elliott J, Gillespie MT, Martin TJ. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. . Endocrinology. 1998;139:4424–4427. doi: 10.1210/endo.139.10.6331. [DOI] [PubMed] [Google Scholar]
- Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999;163:434–442. [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. . Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. . Proc Natl Acad Sci USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev . 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell . 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997;234:137–142. doi: 10.1006/bbrc.1997.6603. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. . Nature Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, 3rd, Frankel WN, Lee SY, Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey DL, Dunstan CR. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha, PTH, PTHrP, and 1, 25(OH)2D3. J Bone Miner Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin- 6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–259. doi: 10.1016/S8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikatsu N, Takeuchi Y, Tamura Y, Fukumoto S, Yano K, Tsuda E, Ogata E, Fujita T. Interactions between cancer and bone marrow cells induce osteoclast differentiation factor expression and osteoclast-like cell formation in vitro. Biochem Biophys Res Commun. 2000;267:632–637. doi: 10.1006/bbrc.1999.2008. [DOI] [PubMed] [Google Scholar]
- Duff GW. Cytokines and anti-cytokines. Br J Rheumatol . 1993;32(Suppl 1):15–20. [PubMed] [Google Scholar]
- Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. . Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. . Biochem Biophys Res Commun . 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]


