Abstract
In rheumatoid arthritis, T cells and B cells participate in the immune responses evolving in the synovial lesions. Interaction between T cells and B cells is probably antigen specific because complex microstructures typical of secondary lymphoid organs are generated. Differences between patients in forming follicles with germinal centers, T-cell–B-cell aggregates without germinal center reactions, or loosely organized T-cell–B-cell infiltrates might reflect the presence of different antigens or a heterogeneity in host response patterns to immune injury. Tertiary lymphoid microstructures in the rheumatoid lesions can enhance the sensitivity of antigen recognition, optimize the collaboration of immunoregulatory and effector cells, and support the interaction between the tissue site and the aberrant immune response. The molecular basis of lymphoid organogenesis studied in gene-targeted mice will provide clues to why the synovium is a preferred site for tertiary lymphoid tissue. B cells have a critical role in lymphoid organogenesis. Their contribution to synovial inflammation extends beyond antibody secretion and includes the activation and regulation of effector T cells.
Keywords: antigen-presenting cells, autoimmunity, germinal center, lymphoid organogenesis, pathogenesis, tertiary lymphoid tissue
Introduction
Protective immune responses directed against infections and malignancies are generated in specialized lymphoid organs localized at strategic sites [1,2]. Their main purpose is to optimize immune reactions by bringing together rare antigen-specific T cells and B cells and by providing the infrastructure for the optimal pairing of lymphocytes and accessory cells [3]. The degeneration of such secondary lymphoid organs causes severe immunodeficiency, fortifying the concept that spatial arrangements, in addition to frequencies of immune cells, are critical for immunoresponsiveness. Although lymph nodes, spleen, tonsils, and Peyer's patches are the main sites of host immune reactions, lymphoid microstructures can also emerge, aberrantly creating novel sites for the activation and maturation of T cells and B cells. Aberrant lymphoid organogenesis is generally associated with pathologic immune responses [4]. A prime example is rheumatoid arthritis (RA), in which complex lymphoid microstructures are created in the inflamed synovial membrane [5,6]. Specifically, synovial lesions form highly sophisticated spatial arrangements of T cells and B cells, at times proceeding to the generation of germinal centers (GCs). Extranodal GCs can occur in other autoimmune diseases, but they are encountered infrequently. Tertiary lymphoid organizations have been described in some patients with Sjögren's syndrome and occasionally in the pancreas of mice with type I diabetes mellitus [7,8].
The presence of GCs in the rheumatoid joint emphasizes that these inflammatory lesions are involved in generating strong immune responses [1,9], almost certainly to specific antigens. In addition, it lends support to the critical role of both T cells and B cells in rheumatoid pathogenesis. The impetus for understanding how these extranodal lymphoid organs arise is obvious: they might provide clues to the antigens driving the immune process. New therapeutic targets might be identified when the cellular and non-cellular components of synovial GC reactions have been identified, and fundamental insights into the process of lymphoid neogenesis might be gained. In addition, because only a subset of RA patients is able to generate GCs, a molecular understanding of this pathway might lead to the identification of markers useful in dissecting the heterogeneity of the RA syndromes.
B-cell follicles in rheumatoid synovitis
Synovial infiltrates in rheumatoid synovitis produce an array of lesions [10]. Regular participants in the tissue infiltrates are T cells, macrophages, and hyperplastic synoviocytes [5,11]. In many, but not all, patients, B cells also accumulate in the synovial membrane. In a series of 173 RA patients, 60% of synovial biopsies were classified as having diffuse infiltrates with T cells and B cells and no distinct topography. In 34% of patients, tissue-invading T cells and B cells were compartmentalized. As in secondary lymphoid organs, B cells cluster in follicle-like structures in a close spatial relationship to CD4+ T cells and CD8+ T cells. The ratio of T cells to B cells accumulating in the follicles is strictly regulated, indicating a well-choreographed chain of events in the formation of this anatomy [6].
Synovial T-cell–B-cell follicles occur in two mutually exclusive and equally frequent forms (Figure 1). The first are T-cell–B-cell aggregates that lack proliferating B cells in the center, are composed mostly of IgD+ B cells, do not have follicular dendritic cell (FDC) networks, and therefore have not generated GC reactions; the second are T-cell–B-cell aggregates that have all the morphologic characteristics of classical GCs, including well-developed FDC networks and Ki-67+ proliferating B-cell blasts that have downregulated IgD. These FDC+ T-cell–B-cell aggregates have all the components for GC reactions, and molecular studies have documented that immunoglobulin gene hypermutations are generated [12,13,14]. In essence, the rheumatoid synovium can serve as a site for aberrant lymphoid organogenesis. However, some observations have identified special features of synovial GCs in comparison with their classical counterparts in secondary lymphoid organs (Table 1). A recent study has used micro-manipulation to isolate CD20+ B cells and tissue-residing plasma cells [14]. B cells were activated, underwent clonal expansion, and shared identical rearrangements. Surprisingly, these rearrangements, supposedly directed at generating high-affinity antibodies against the initiating antigen, were not detected in the plasma cells. It is possible that events in synovial GCs do not totally mimic similar pathways in lymph nodes.
Figure 1.
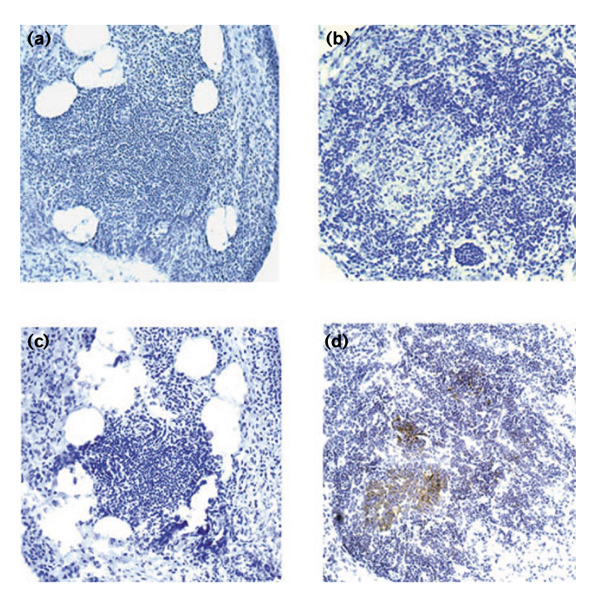
The formation of lymphoid microstructures by T cells and B cells in rheumatoid synovitis. T cells and B cells infiltrating the synovial membrane are arranged into highly organized structures. Synovial lymphoid microstructures include two mutually exclusive types of T-cell–B-cell clusters, those lacking GCs (a, c) and follicles with central GCs (b, d). In both microstructures, T cells and B cells are mixed in a well-maintained ratio. Centroblasts and centrocytes in the follicular sample indicate a GC reaction that is missing from the aggregate sample. The stain in (a) and (b) was hematoxylin. CD23-expressing FDC networks were detected by immunoperoxidase technique with 3',3'-diaminobenzidine tetrahydrochloride substrate (c, d). Centers of follicles with GCs were occupied by FDC networks, reminiscent of typical secondary follicles in lymph nodes. No FDCs were identified in T-cell–B-cell aggregates that lacked GCs. It is not known whether synovial T-cell–B-cell aggregates resemble structures represented in secondary lymphoid tissues.
Table 1.
Features of secondary lymphoid tissues in lymph nodes and tertiary lymphoid tissues in rheumatoid synovium
| Tissue | Lymph node primary follicles | Lymph node secondary follicles | Synovial CD23-null T-cell–B-cell aggregates | Synovial CD23+ follicles |
| Organized T-cell zones | + | + | + | + |
| Follicular clustering of B cells | + | + | + | + |
| Proliferating B cells | - | + | - | + |
| Hypermutated immunoglobulin genes | - | + | ? | + |
| Plasma cells with identical rearrangements | - | + | ? | - |
| CD23+ FDC networks | + | - | - | + |
| CD8+CD40L+ cells | - | - | - | + |
The question is also raised as to the origin of synovial plasma cells, which have a critical role in secreting disease-relevant antibodies. Are they simply outliving rapidly cycling CD20+ B cells? Are they recruited to the lesions instead of being generated in situ? What are the mechanisms attracting, retaining, and guiding B cells and plasma cells in the synovial tissue? Another typical feature of synovial GCs is the presence of CD8+ T cells expressing CD40 ligand (CD40L) [6]. CD8 T cells have not yet been implicated in the series of events leading to GC reactions [15]. When synovial tissues with lymphoid follicles were analyzed, frequencies of tissue-residing CD8 T cells were identified as a distinguishing factor. CD8+CD40L+ T cells were absent from tissues with T-cell–B-cell aggregates and no GC formation, but they were enriched in synovial samples with typical GCs. With the use of microscopic imaging, these cells were localized at the outskirts of the T-cell–B-cell clusters. Preliminary functional data from these CD8 T cells are available. They were described as lacking the pore-forming enzyme perforin, a molecular marker associated with cytolytic capability, and instead transcribed high concentrations of interferon-γ (IFN-γ) [6]. Why would CD8 T cells, instead of CD4 T cells, have a role in regulating synovial GCs? One possible interpretation could be that the antigens recognized in these lymphoid microstructures preferentially target CD8 T cells responsive to endogenously processed antigens.
The choreography of lymphoid organogenesis
The complexity of the topographic arrangement and function of GCs is the result of an orchestrated effort by lymphocytes, non-lymphoid stromal cells, cytokines, chemokines, and growth factors controlling the traffic of leukocytes into compartments of lymphoid organs [16,17], optimizing contact to antigens and providing the infrastructure for optimal cooperation to yield effective immune responses (Figure 2). GCs are critical for the affinity maturation of antibodies and the generation of B-cell memory. As they progress through the GC, B cells selectively mutate their immunoglobulin genes and are selected on antigen presented on FDCs, ultimately resulting in the production of high-affinity neutralizing antibodies specific for viral antigens and bacterial exotoxins. By receiving appropriate signals from T cells participating in the GC reaction, mutated B cells expressing high-affinity antibodies differentiate to become antibody-forming plasma cells or memory B cells; B cells with low affinity are eliminated. GCs also have a role in removing B cells that form self-reactive antibody by forcing them to undergo apoptosis. In general, GCs cannot be formed unless supported by antigen-specific CD4 T cells. Although a recent report indicates that under unusual circumstances, high-affinity B cells can be induced to generate GCs in response to (4-hydroxy-3-nitrophenyl)acetyl Ficoll in the absence of T cells. GCs formed without T-cell help were possible only when B-cell receptors were extensively crosslinked and when high frequencies of antigen-specific B cells were available [18]. These GCs also had a short lifespan and aborted conspicuously at the time when B cells are normally selected by antigen-reactive T cells. Two receptor-ligand pairs are crucial in mediating the T-cell–B-cell contact that is essential for successful GC reactions. GC T cells and B cells communicate through the interaction of CD28 and CD80/CD86 and through the crosslinking of CD40 on the surface of B cells by CD40L expressed on activated T cells [19,20,21,22].
Figure 2.
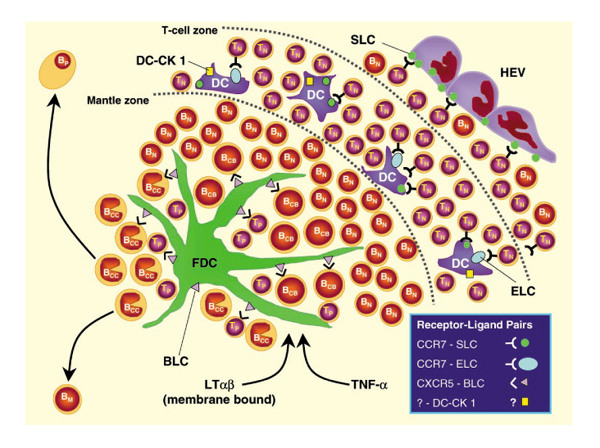
Schematic model of specialized cell populations and chemokines instrumental in the formation of GCs in secondary and tertiary lymphoid tissues. BCB, centroblastic B cell; BCC, centroclastic B cell; BM, memory B cell; BP, plasma B cell; DC, dendritic cell; HEV, high endothelial venule; TN, naïve T cell; TP, primed T cell.
Significant progress in understanding the events leading to the recruitment, retention, compartmentalization, activation, and recirculation of lymphocytes in and out of secondary lymphoid tissues has been made in recent years. Mice with spontaneous abnormalities in lymphoid organogenesis or with targeted homologous deletions of specific mediators have been instrumental in developing a model of how immune cells are brought together in defined spaces to generate immune responses. After being released from primary lymphoid organs, such as bone marrow or the thymus, lymphocytes migrate into lymph nodes by crossing the endothelium in a multistep process. Supported by selectins, lymphocytes start to roll on the endothelial layer and then adhere firmly via integrins [23]. It is currently believed that the mass transit of resting lymphocytes across specialized high endothelial venules is facilitated by the expression of secondary lymphoid tissue chemokine (SLC) on the endothelial cells [24,25,26]. Mice homozygous for a spontaneous mutation plt (paucity of lymph node T cells) lack SLC expression and have defective trafficking of T cells into lymph nodes [27,28,29]. In addition, T cells lacking the SLC receptor, C-C chemokine receptor-7 (CCR7), are markedly impaired in their ability to enter lymph nodes and Peyer's patches [30]. B-cell trafficking in plt mice and in CCR7-deficient mice is affected much less, which is compatible with a dichotomy in regulating T-cell and B-cell migration. Once lymphocytes have accessed lymphoid tissues, they effectively search out certain subcompartments; B cells accumulate in B-cell follicles and T cells home to areas rich in T cells. This process is at least partly under the control of chemokines.
The current model is that the movement of T cells is guided by two ligands that both bind to CCR7: SLC and Epstein-Barr virus-induced molecule 1 ligand chemokine (ELC) [29,31,32,33,34]. These ligands can probably be produced by dendritic cells, macrophages, and other non-hematopoietic cells residing in the T-cell zones [35,36,37]. Mice with the plt mutation, which have a defect in expressing SLC and ELC, have impairment not only in T-cell entry into lymph nodes but also in organizing T cells in the T-cell areas. In addition, trafficking of T cells through secondary lymphoid tissues is severely disturbed in CCR7-deficient mice [27]. Two molecular pathways have been implicated in regulating SLC and ELC production: signaling by lymphotoxin-αβ (LTαβ) seems to be necessary for the induction of SLC and ELC [1,38,39], and mice deficient in Re1 B, a member of the nuclear transcription factor NF-κB family, have an impairment in secreting SLC [40].
T-cell–B-cell follicles would not be preferred sites of immune recognition unless chances for antigen encounter were markedly enhanced [41]. This is achieved by transporting antigen by specialized cells to the T-cell and B-cell zones of secondary lymphoid organs. Dendritic cells (DCs) are positioned in peripheral tissue; there they capture antigen, transform into activated DCs, become migratory, access lymphatic vessels, and travel to the draining lymph nodes, where they migrate deep into the T-cell areas [42,43]. Recruitment of DCs to the lymph nodes, and the concomitant ferrying of antigens to sites of critical mass, involves the upregulation of CCR7, rendering these specialized cells responsive to SLC and ELC [44,45,46]. It is now believed that SLC and ELC facilitate the entry of DCs into lymphatics and their trafficking to the T-cell zones [26]. DCs in T-cell zones are highly effective antigen-presenting cells (APCs), permitting the priming of naïve T cells. Interestingly, such DCs have been identified as the cellular source of ELC and also of the chemoattractant dendritic cell-derived C-C chemokine-1 (DC-CK 1), raising the possibility that they attract naïve T cells to enhance the opportunity for APC-T cell interaction [47,48]. Once T cells have been primed, they become responsive to other chemokines, guiding their movements in the search for their B-cell partners [49]. B cells that have bound sufficient antigen are redirected from the migration route of naïve B cells and move to the boundary of the B-cell and T-cell zones [50].
A critical role in the compartmental homing of B cells has been assigned to the ligand binding C-X-C chemokine receptor-5 (CXCR5). CXCR5-deficient B cells lack the ability to migrate to splenic follicles, and targeted disruption of CXCR5 prevents the development of B-cell follicles in the spleen [51,52]. The ligand for this receptor has been named B-lymphocyte chemoattractant (BLC). This CXC chemokine is probably constitutively expressed by stromal cells in the center of follicles, presumably by FDCs [53]. Thus, B-cell recruitment seems to be controlled by follicular structures. This model does not provide an explanation for the initial establishment of follicles unless the assumption is made that FDCs are present before B cells are recruited. If that were so, follicle formation would ultimately depend on whether FDCs can be developed or selectively attracted to tissue sites.
Some experimental evidence suggests that antigen-specific T cells, when activated by DCs, upregulate the expression of CXCR5, rendering them responsive to BLC [46]. T cells can then selectively migrate towards the BLC-producing FDCs, meet B cells specific for the same antigen, and support the generation of a GC. This model would re-emphasize the critical position of FDCs in the choreography of lymphoid organogenesis [54]. The development and the function of FDCs has been linked with the presence of the cytokines LTαβ and tumour necrosis factor-α (TNF-α) [55,56,57]. One of the activities mediated by these cytokines seems to be the induction of BLC. Given the critical participation of BLC-producing FDCs, LTαβ and TNF-α could also be instrumental in regulating aberrant lymphoid neogenesis.
Aberrant lymphoid organogenesis in rheumatoid synovitis
Synovial membrane is ordinarily not a site for lymphoid structures to emerge. In many RA patients, inflammation can persist for years and infiltrating lymphocytes do not acquire defined topographical arrangements. The subset of RA patients with classical GC reactions usually generate numerous follicles, possess this pattern of inflammation in essentially all affected joints, and maintain it over time without switching to other microstructures. Most patients with follicular synovitis form rheumatoid nodules outside the joint. These structures closely resemble granulomatous reactions but, remarkably, in the synovial membrane, follicular arrangements are not mixed with granulomas [10].
It has been proposed that priming of the tissue site has to be achieved during a window of embryonic development to permit the subsequent appearance of primary follicles [1]. The mechanisms of priming are not understood, but it is difficult to envisage that such a priming event would condition the synovium in some patients while sparing others. Nurse-like cells that are able to activate B cells and provide survival signals have been described in rheumatoid synovitis, but it is currently unclear whether their presence correlates with extranodal GCs [58]. Patterns of cytokine production in the synovial microenvironment are clearly distinct in patients with diffuse, follicular, or granulomatous synovitis [10]. Tissue levels of TNF-α, interleukin (IL)-1β, and IFN-γ are low in samples with diffuse synovitis. Follicular synovitis is characterized by an increased transcription of IFN-γ and is typically associated with high concentrations of IL-10. Formation of granulomas is correlated with the production of the highest tissue concentrations of IFN-γ and IFN-γ transcripts and, interestingly, lacks the production of IL-10 in situ. Synovial IL-4 mRNA was found in some patients, indicating that patterns similar to those for both T helper 1 (TH1) and T helper 2 (TH2) cells might be present in rheumatoid synovitis [59]. T cells with the TH1 and TH2 phenotypes have been described to express distinct profiles of chemokine receptors [60,61,62].
Given the central role of chemokines in the establishment of lymphoid microstructures, it needs to be examined whether the differences in the inflammatory cytokines are a reflection of the selective recruitment of distinct populations of T cells into the synovial membrane. Another possibility is that chemokine receptor expression on activated T cells is influenced by the site of initial priming. Support for this model has come from the observation that CCR4+ T cells accumulate preferentially at sites of cutaneous inflammation but avoid the intestinal tract [63]. Finally, it could be proposed that the site of priming and also the nature of the antigen have a direct impact on modulating the chemokine receptor patterning of T cells, thus dictating their trafficking to and retention in inflammatory lesions.
Little is known about the molecular components of the synovial microenvironment that determine lymphoid neo-organogenesis. Cell migration in tissue sites is ultimately regulated by chemokine gradients that are established by local deposits but also by the binding of these chemokines, highly basic proteins, to sulfated matrix components and proteoglycans. The final decision about the specific arrangement of lymphocytes in the inflamed synovial membrane might also depend on the composition of extracellular matrix proteins and their distribution in the soft tissue layer.
An important aspect of T-cell–B-cell physiology in rheumatoid synovitis is related to the precise function that these immune cells have in the disease process. The focus of GCs in generating high-affinity antibodies suggests that the major function of synovial B cells is the secretion of immunoglobulins, probably including rheumatoid factor [64,65]. Recent data suggest that B cells might contribute to disease mechanisms through pathways not directly dependent on the release of disease-relevant antibodies. In murine models of autoimmune disease, including MRL/lpr mice and non-obese diabetic mice, B cells have been found to be instrumental in the disease process [66,67]. The accumulation of activated T cells, of both the CD4+and the CD8+ subsets, in MRL/lpr mice is highly B-cell dependent [68,69]. The underlying mechanism does not involve antibody-mediated activation because mIgM.MRL/lpr mice, which have B cells but are deficient in serum Ig, continue to generate manifestations of lupus [67]. B cells in the rheumatoid synovium seem to have similar functions. Depletion of B cells from a rheumatoid synovium–severe combined immunodeficiency (SCID) mouse chimera not only destroyed the lymphoid follicles but also abrogated IFN-γ production by T cells and IL-1/TNF-α production. In these experiments, rheumatoid synovium was grafted into SCID mice, the animals were treated with anti-CD20 monoclonal antibody (Rituxan), and transcription of cytokines in situ was quantified from the retrieved grafts [70].
It is possible that B cells could facilitate T-cell activation and inflammatory tissue damage by secreting cytokines and chemokines. A more likely model is that B cells function as critical APCs, taking up antigens through their immunoglobulin receptor and presenting it to CD4 T cells [71,72]. Antigens driving rheumatoid synovitis might be particularly susceptible to capture by B cells in the synovial membrane, favoring B cells as APCs for recruited T cells. The implications of such a mechanism are obvious because the immune response could be disrupted by eliminating B cells from the inflammatory lesions.
Acknowledgments
Acknowledgements
We thank James W Fulbright for contributions to the preparation of the graphics and manuscript. This work was supported by grants from the NIH (AR41974, AR42527, and AI44142) and the Mayo Foundation.
References
- Fu YX, Chaplin DD. Development and maturation of secondary lymphoid tissues. Annu Rev Immunol. 1999;17:399–433. doi: 10.1146/annurev.immunol.17.1.399. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Kaldjian EP, Anderson AO, Shaw S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. . J Immunol. 1996;157:495–499. [PubMed] [Google Scholar]
- Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol. 2000;156:1133–1138. doi: 10.1016/S0002-9440(10)64981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CL, Adamson TC3, Vaughan JH, Fox RI. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. . Arthritis Rheum. 1984;27:32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- Wagner UG, Kurtin PJ, Wahner A, Brackertz M, Berry DJ, Goronzy JJ, Weyand CM. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. 1998;161:6390–6397. [PubMed] [Google Scholar]
- Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjögren's syndrome. J Clin Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Wong FS, Burkly L, Altieri M, Mamalaki C, Kioussis D, Flavell RA, Sherwin RS. Induction of insulitis by glutamic acid decarboxylase peptide-specific and HLA-DQ8-restricted CD4(+) T cells from human DQ transgenic mice. J Clin Invest. 1998;102:947–957. doi: 10.1172/JCI2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. . Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- Klimiuk PA, Goronzy JJ, Bjornsson J, Beckenbaugh RD, Weyand CM. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am J Pathol. 1997;151:1311–1319. [PMC free article] [PubMed] [Google Scholar]
- Krenn V, Schalhorn N, Greiner A, Molitoris R, Konig A, Gohlke F, Muller-Hermelink HK. Immunohistochemical analysis of proliferating and antigen-presenting cells in rheumatoid synovial tissue. . Rheumatol Int. 1996;15:239–247. doi: 10.1007/BF00290377. [DOI] [PubMed] [Google Scholar]
- Schroder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berek C, Kim HJ. B-cell activation and development within chronically inflamed synovium in rheumatoid and reactive arthritis. . Semin Immunol. 1997;9:261–268. doi: 10.1006/smim.1997.0076. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Krenn V, Steinhauser G, Berek C. Plasma cell development in synovial germinal centers in patients with rheumatoid and reactive arthritis. J Immunol. 1999;162:3053–3062. [PubMed] [Google Scholar]
- Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Cyster JG. Lymphocyte homing: the scent of a follicle. Curr Biol. 1997;7:R219–R222. doi: 10.1016/s0960-9822(06)00105-9. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Ngo VN, Ekland EH, Gunn MD, Sedgwick JD, Ansel KM. Chemokines and B-cell homing to follicles. Curr Top Microbiol Immunol. 1999;246:87–92. doi: 10.1007/978-3-642-60162-0_11. [DOI] [PubMed] [Google Scholar]
- de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur J Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.3.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. . Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J Exp Med. 2000;191:61–76. doi: 10.1084/jem.191.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Leukocyte migration: scent of the T zone. . Curr Biol. 2000;10:R30–R33. doi: 10.1016/s0960-9822(99)00253-5. [DOI] [PubMed] [Google Scholar]
- Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Mori S, Yonekawa H, Nariuchi H, Matsuzawa A, Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. . Blood. 1998;91:2886–2895. [PubMed] [Google Scholar]
- Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, Hedrick JA, Lira SA. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. . J Exp Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell . 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Nagira M, Imai T, Yoshida R, Takagi S, Iwasaki M, Baba M, Tabira Y, Akagi J, Nomiyama H, Yoshie O. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur J Immunol . 1998;28:1516–1523. doi: 10.1002/(SICI)1521-4141(199805)28:05<1516::AID-IMMU1516>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Nagira M, Imai T, Baba M, Takagi S, Tabira Y, Akagi J, Nomiyama H, Yoshie O. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. . Int Immunol. 1998;10:901–910. doi: 10.1093/intimm/10.7.901. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. . J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- Kim CH, Pelus LM, White JR, Applebaum E, Johanson K, Broxmeyer HE. CK beta-11/macrophage inflammatory protein-3 beta/EBI1-ligand chemokine is an efficacious chemoattractant for T and B cells. J Immunol. 1998;160:2418–2424. [PubMed] [Google Scholar]
- Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med . 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. . Eur J Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin's role in inflammation and development. Immunol Res. 1999;19:119–125. doi: 10.1007/BF02786481. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Korner H, Gunn MD, Schmidt KN, Riminton DS, Cooper MD, Browning JL, Sedgwick JD, Cyster JG. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Lu Z, Luo Y, Quackenbush EJ, Berman MA, Collins-Racie LA, Mi S, Reilly C, Lo D, Jacobs KA, Dorf ME. Identification of a new mouse beta-chemokine, thymus-derived chemotactic agent 4, with activity on T lymphocytes and mesangial cells. J Immunol. 1997;159:5671–5679. [PubMed] [Google Scholar]
- Allavena P, Luini W, Bonecchi R, D'Amico G, Bianchi G, Longoni D, Vecchi A, Mantovani A, Sozzani S. Chemokines and chemokine receptors in the regulation of dendritic cell trafficking. Chem Immunol. 1999;72:69–85. doi: 10.1159/000058727. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Mantovani A, Allavena P. Control of dendritic cell migration by chemokines. Forum (Genova) 1999;9:325–338. [PubMed] [Google Scholar]
- Kimber I, Cumberbatch M, Dearman RJ, Bhushan M, Griffiths CE. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–412. doi: 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- Chan VW, Kothakota S, Rohan MC, Panganiban-Lustan L, Gardner JP, Wachowicz MS, Winter JA, Williams LT. Secondary lymphoid-tissue chemokine (SLC) is chemotactic for mature dendritic cells. Blood . 1999;93:3610–3616. [PubMed] [Google Scholar]
- Kellermann SA, Hudak S, Oldham ER, Liu YJ, McEvoy LM. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J Immunol. 1999;162:3859–3864. [PubMed] [Google Scholar]
- Saeki H, Moore AM, Brown MJ, Hwang ST. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon KB, Figdor CG. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med . 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. . Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. . J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DD, Fu Y. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–297. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- Fu YX, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-alpha (LTalpha) supports development of splenic follicular structure that is required for IgG responses. J Exp Med . 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Fu YX, Molina H, Huang G, Kim J, Thomas DA, Nahm MH, Chaplin DD. Distinct roles of lymphotoxin alpha and the type I tumor necrosis factor (TNF) receptor in the establishment of follicular dendritic cells from non-bone marrow-derived cells. J Exp Med. 1997;186:1997–2004. doi: 10.1084/jem.186.12.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka Y, Attrep JF, Hirano T, Ishihara K, Suzuki R, Toyosaki T, Ochi T, Lipsky PE. Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest. 1998;102:606–618. doi: 10.1172/JCI3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Klimiuk PA, Goronzy JJ. Heterogeneity of rheumatoid arthritis: from phenotypes to genotypes. Springer Semin Immunopathol. 1998;20:5–22. doi: 10.1007/BF00831996. [DOI] [PubMed] [Google Scholar]
- Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol. 1999;21:263–285. doi: 10.1007/s002810050067. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Pan J, Butcher EC. Cutting edge: developmental switches in chemokine responses during T cell maturation. J Immunol . 1999;163:2353–2357. [PubMed] [Google Scholar]
- Williams DG, Moyes SP, Mageed RA. Rheumatoid factor isotype switch and somatic mutation variants within rheumatoid arthritis synovium. . Immunology. 1999;98:123–136. doi: 10.1046/j.1365-2567.1999.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi U, Rzepka R, Batsford S, Kaufmann SH, von der MK, Peter HH, Melchers I. The B cell repertoire of patients with rheumatoid arthritis. II. Increased frequencies of IgG+ and IgA+ B cells specific for mycobacterial heat-shock protein 60 or human type II collagen in synovial fluid and tissue. Arthritis Rheum. 1997;40:1409–1419. doi: 10.1002/art.1780400808. [DOI] [PubMed] [Google Scholar]
- Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–1168. [PubMed] [Google Scholar]
- Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Schultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new 'speed congenic' stock of NOD.Ig mu null mice. J Exp Med. 1996;184:2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TO, Madaio MP, Shlomchik MJ. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. . J Immunol. 1999;163:3592–3596. [PubMed] [Google Scholar]
- Chan TO, Shlomchik MJ. Cutting edge: B cells promote CD8+ T cell activation in MRL-Fas(lpr) mice independently of MHC class I antigen presentation. J Immunol. 2000;164:1658–1662. doi: 10.4049/jimmunol.164.4.1658. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Klimiuk PA, Takemura S, Goronzy JJ. T cell activation in rheumatoid synovitis is B cell dependent. Arthritis Rheum. 1999;42:S389. [Google Scholar]
- Hodgkin PD, Basten A. B cell activation, tolerance and antigen-presenting function. Curr Opin Immunol. 1995;7:121–129. doi: 10.1016/0952-7915(95)80037-9. [DOI] [PubMed] [Google Scholar]
- Nussenzweig MC. Immune responses: tails to teach a B cell. . Curr Biol. 1997;7:R355–R357. doi: 10.1016/s0960-9822(06)00173-4. [DOI] [PubMed] [Google Scholar]


