Abstract
This commentary is a concise discussion of the interactions between bone morphogenetic proteins (BMPs) and their binding proteins in bone and cartilage morphogenesis. BMPs are a family of growth and differentiation factors, and they act on mesenchymal cells to induce cartilage and bone differentiation in concentration-dependent thresholds. The BMP–BMP receptor binding leads to a cascade of signaling and transcription of BMP response genes. BMP binding proteins, noggin, chordin and DAN, act as antagonists and determine the bioavailability of BMPs for binding to cognate receptors to elicit the biological response. Noggin null mice with unrestricted action of BMPs exhibit defects in joint morphogenesis. BMPs and their binding proteins may reciprocally regulate the dynamic topography of joints, muscle, tendons and ligaments during morphogenesis of the skeleton. In addition, BMP actions may be potentiated by twisted gastrulation. BMPs and their binding proteins may play a critical role in regeneration of cartilage in osteoarthritis.
Keywords: BMP, BMP receptors, extracellular matrix, Smads
Introduction
The sequelae of damage and degradation of articular cartilage is well known in osteoarthritis. Repair and regeneration of articular cartilage in experimental animals is initiated when the subchondral bone is penetrated, as in full thickness defects. On the other hand, there is no repair in partial thickness defects with the defect confined to cartilage only. Subchondral bone with associated matrix and hematopoietic stromal cells may thus play a role in cartilage repair. The difference in regenerative potential between bone and cartilage is immense. The fundamental differences between cartilage and bone in inherent repair potential may be due to differences in concentration of endogeneous growth and morphogenetic factors and their antagonists. The demineralized bone matrix is a repository of BMPs and this might explain, in part, the repair of articular cartilage in full thickness defects. The aim of this commentary is to discuss the interplay between the evolving family of BMPs and cognate BMP binding proteins in bone and cartilage development. In arthritis, with attendant cartilage damage, there is a derangement of the morphogenetic signals. It is thus possible to harness the recent progress in developmental biology of morphogens to design rational therapeutic approaches to cartilage regeneration. Regeneration is, after all, a recapitulation of embryonic development and morphogenesis, and includes redeployment of morphogens in regeneration.
Bone morphogenetic proteins
BMPs are a family of growth and differentiation factors [1,2]. BMPs are pleiotropic morphogens, and they induce new cartilage and bone formation in ectopic sites by a developmental sequence that mimics limb development and morphogenesis. BMPs have chemotactic, mitogenic and differentiation-inducing properties. The actions of BMPs are concentration dependent, and are based on thresholds. BMPs are dimeric proteins with a single interchain disulfide bond. The dimeric conformation is an absolute requirement for the biological action and interaction with receptors.
BMPs were initially identified as bioactive molecules in the demineralized bone matrix responsible for cartilage and bone differentiation [1,2]. BMPs regulate the lineage, pattern and differentiation of bone, and over 15 have been cloned and expressed in humans and mice. Genetic evidence points to actions of BMPs in tissues beyond bone. Knockout of BMP 2 and BMP 4 results in defects in mesoderm formation. Mutations in BMP 5 in mice are responsible for the short ear phenotype. BMP 7 null mice exhibit defects in eye, kidney and skeleton [2]. BMP 4 stimulates chondrogenesis in limb bud mesenchymal cells [3] and maintains articular cartilage phenotype [4].
Cartilage-derived morphogenetic proteins
Luyten and coworkers identified cartilage-derived morphogenetic protein-1 (CDMP-1) in articular cartilage [5] and found it identical to growth/differentiation factor 5. The incisive work of Lee and coworkers cloned growth/differentiation factors 5, 6, and 7, with mutations in growth/differentiation factor 5 identified in mouse brachypodism [6]. It is noteworthy that mutations in human CDMP-1 were identified in patients with Hunter-Thompson type chondrodysplasia, type C Brachydactyly and Grebe type chondrodysplasia with severe limb shortening and impaired morphogenesis [7,8]. BMPs and CDMPs are thus critical for bone and cartilage morphogenesis and beyond. BMPs should perhaps be called body morphogenetic proteins, which would take into account the wide-ranging actions of BMPs and obviate the need for tinkering with terminology.
BMP receptors
There are seven canonical conserved cysteines in the mature monomer, six of which are involved in intrachain disulfide bonds. The seventh is key for the interchain disulfide bond. The BMP monomer forms two finger-like projections from the palm of the hand. BMPs interact with specific receptors. Recombinant human BMP 4 and BMP 7 bind to two distinct type I BMP receptors, BMPR-IA and BMPR-IB, and BMP 7 also appears to bind to activintype I receptor. BMPs also bind to type II BMP receptors, with both BMP receptors I and II being serine/threonine kinases. A gene from Caenorhabditis elegans, daf 4, binds to recombinant human BMP 4. Expression of daf 4 inhibits dauer larva formation. Three genes, Sma 2, Sma 3 and Sma 4, were identified in daf 4 signaling in C. elegans. Systematic genetic screens in Drosophila melanogaster identified a gene mother against decapentaplegic (Mad). There was sequence homology between Sma 2, Sma 3, and Sma 4 in C. elegans and Mad in Drosophila, and the scientists have fused the terms Sma and Mad to Smad in mammals. There are three classes of Smads. The receptor-regulated R-Smad 1, R-Smad 5 and R-Smad 8 are phosphorylated by BMP receptor kinases [9]. X-Ray crystallography has revealed the trimeric nature of Smads. The R-Smads interact with a common signaling partner Co-Smad, Smad 4. The multimeric protein complex of Smad 1/5/8 and Smad 4 is translocated into the nucleus and initiates transcription of BMP response genes. The type I receptor kinase catalyzed phosphorylation of Smad 1 and Smad 5 is inhibited by inhibitory Smads, Smad 6 and Smad 7. These inhibitory Smads are normally resident in the nucleus, act as a homeostatic relay upon BMP stimulation of cells and are translocated into the cytosol to inhibit type I BMP receptor kinase catalyzed phosphorylation of Smad 1/5. This intricate signaling is dependent on the bioavailability of BMPs at steady state to the cognate receptors. BMP levels and interaction with receptors is dependent on binding to the extracellular matrix and BMP binding proteins (Fig. 1). BMPs bind to extracellular matrix components and thus the availability of BMP for receptor binding is restricted [10]. The extracellular matrix may potentiate the biological actions of BMPs [10].
Figure 1.
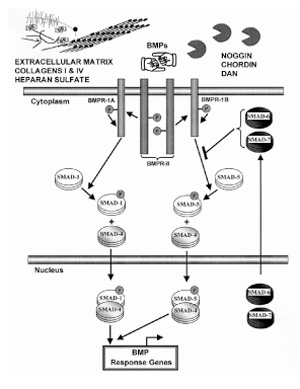
BMP receptors and signaling cascades. BMPs are dimeric ligands with a cysteine knot in each monomer fold. Each monomer has two β sheets (represented as two pointing fingers). These fingers in the functional dimer are oriented in opposite directions. BMPs interact with both type I and type II BMP receptors (BMPR-I and BMPR-II). The exact stoichiometry of the receptor complex is currently being elucidated. BMPR-II phosphorylates the glycine-serine (GS) domain of BMPR-I. The collaboration between type I and type II receptors forms the signal transducing complex. The BMP type I receptor kinase complex phosphorylates the trimeric signaling substrate Smad 1 or Smad 5. This phosphorylation is inhibited and modulated by inhibitory Smad 6 and Smad 7. Phosphorylated Smad 1 or Smad 5 interacts with Smad 4 (functional partner) and enters the nucleus to activate the transcriptional machinery for early BMP response genes. A novel Smad interacting protein may interact and modulate the binding of heteromeric Smad 1/Smad 4 complexes to the DNA. The bioavailability of BMP for interaction with BMP receptors is determined by binding to extracellular matrix components such as heparan sulfate and collagen IV. The BMP antagonists noggin, chordin and DAN can also bind with high affinity to BMP and prevent interaction with receptors. There is thus a very intricate regulation of BMP biological actions.
BMP binding proteins as antagonists
Noggin
During the course of a search for the elusive neural inducer, noggin was isolated from Xenopus based on rescue of dorsal development in ultraviolet-induced ventralized embryos [11]. Injection of the putative cloned RNA into embryos resulted in large heads, hence the name noggin. Noggin is produced by the Spemann organizer and antagonizes the action of BMPs, induces neural tissue and dorsalizes ventral mesoderm. Noggin binds to BMP 2 and BMP 4 with high affinity and blocks interaction with BMP receptor [12]. The bioavailability receptor is thus modulated by BMP antagonist noggin.
What has noggin to do with arthritis and articular cartilage? The precise boundary between muscle and cartilage with the interspersed perichondrium, the tendon/ligament interface, requires precisely regulated boundary conditions during joint morphogenesis. One can envisage, given the role of dominant morphogens such as BMPs and CDMPs, BMP/CDMP binding proteins (antagonists) to play a role in defining boundaries. Experimental evidence has in fact been adduced to precisely demonstrate this, using noggin null mice generated by homologous recombination [13]. Regulated cell death and joint cavitation follow mesenchymal cell condensation in regions of presumptive joint morphogenesis, and CDMP-1 has been implicated in this process [13]. It is possible in mice lacking noggin that the unfettered actions of BMPs/CDMPs may lead to impaired joint formation. Defects in vertebrae, ribs and limbs were observed in homozygous mutants; heterozygous mice appeared normal. The limbs are shorter in the mutant, the joints were not demarcated and fusion of the joints was commonplace. Optimal expression of BMPs/CDMP is thus needed for the normal joint morphogenesis. Future investigations with conditional knockouts of noggin in mice will shed more light on the role of noggin in joint morphogenesis. BMP 2 induces the expression of noggin in osteoblasts [14] and bone marrow cells [15], implying regulated expression of the BMP antagonist, perhaps to downregulate the agonist response to BMP 2. This is akin to a molecular thermostat to maintain skeletal homeostasis.
Chordin
During the course of investigation on Xenopus pattern formation, chordin was identified as BMP 4 binding protein [16]. Chordin has a homolog in Drosophila, short gastrulation (sog), known to bind to decapentaplegic, a BMP 2/4 homolog. These BMP antagonists have thus been conserved for several million years. Chordin binds to BMP 2 and BMP 4, and is further regulated by a metalloprotease BMP 1 and tolloid and Xenopus xolloid [17,18,19]. Antagonists such as chordin thus govern the intricate pattern formation by morphogens such as BMP, and in turn are proteolytically inactivated by a metalloprotease BMP 1, related to the astacin family. BMP 1 was originally identified in osteogenic extracts derived from bovine bone as it copurified with bonafide bone morphogenetic proteins 2–7 [1]. BMP 1 also functions as a procollagen c-protease. In retrospect, the term BMP-1 is a misnomer, as this BMP does not induce bone morphogenesis.
DAN
The DAN gene was isolated as a candidate tumor suppressor gene in a differential hybridization screen [20]. Secreted DAN suppressed DNA synthesis in transformed cells. The head inducer gene Cereberus codes for a secreted protein and can induce heads in Xenopus embryos, and it is related to DAN in the cysteine rich domain [21]. Gremlin is a Xenopus homolog related to DAN that inhibits BMP 2 action and was identified by screening an ovarian cDNA library for activities inducing the secondary axis [22]. DAN family members are thus newly identified BMP antagonists [23,24,25]. It is not yet clear if they play a role in articular cartilage and in arthritis.
BMP potentiating agonist: twisted gastrulation
One of the emerging themes in developmental biology is the regulation of pattern formation and morphogenesis by sets of genes. The anterior–posterior axis, for example, is governed by hox genes. The dorsal–ventral axis is the result of the activity and expression pattern of bone morphogenetic proteins, BMP antagonist chordin and metalloprotease xolloid. Recent work in Xenopus has identified a gene 'twisted gastrulation' (xTsg), as an agonist of BMP actions [26]. In vertebrates, the dorsal–ventral pattern is regulated by a gradient of BMP activity. Although BMPs are expressed uniformly, the expression of BMP antagonists such as chordin generates the dorsal–ventral gradients. Further control is exerted by xolloid, a zinc-dependent metalloprotease. The xTsg binds to BMPs with a dissociation constant in the low nanomolar range. It is noteworthy that microinjection of xTsg results in potentiation of BMP signaling, leading to ventralization of Xenopus embryo. Twisted gastrulation is thus an agonist of BMP signaling. xTsg may thus activate the inactive BMP–chordin cysteine rich domain complex by releasing the bioactive BMPs. It is important to bear in mind, given BMPs bind to extracellular matrix [10,27] components such as collagens, heparan sulfate and heparin, additional regulation is inevitable, and is a question of time and space!
Conclusion
The recent advances in the developmental biology of BMP binding proteins will allow a rational approach to the challenges of articular cartilage repair by morphogens. It is remarkable how Nature zealously regulates the intricate interplay between BMPs, BMP receptors, extracellular matrix, BMP antagonists such as noggin, chordin and the DAN family, and BMP potentiating twisted gastrulation.
Acknowledgments
Acknowledgements
The author wishes to thank Mrs Rita Rowlands for outstanding assistance in the preparation of this manuscript. This research is supported by grants from Shriners Hospitals and the Lawrence Ellison Chair in Musculoskeletal Molecular Biology. The author also thanks the anonymous reviewers for their invaluable critique that improved our manuscript immensely.
References
- Reddi AH. Bone morphogenetic proteins: an unconventional approach to isolation of first mammalian morphogens. Cytokine Growth Factor Rev. 1997;8:11–20. doi: 10.1016/s1359-6101(96)00049-4. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- Chen P, Carrington JL, Hammonds RG, Reddi AH. Stimulation of chondrogenesis in limb bud mesodermal cells by recombinant human BMP-2B and modulation by TGF-β1 and TGF-β2. Exp Cell Res. 1991;195:509–515. doi: 10.1016/0014-4827(91)90403-h. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant BMP-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267:3685–3691. [PubMed] [Google Scholar]
- Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, Kozak CA, Reddi AH, Moos M., Jr Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley EM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGFβ superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Lin K, Nandedkar M, Camargo M, Cerrenka J, Luyten FP. A human chondrodysplasia due to a mutation in a TGFβ superfamily member. Nat Genet. 1996;12:315–317. doi: 10.1038/ng0396-315. [DOI] [PubMed] [Google Scholar]
- Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP. Disruption of human limb morphogenesis by a dominant negative mutation in CDMP-1. Nat Genet. 1997;17:58–64. doi: 10.1038/ng0997-58. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGFβ signaling from cell membrane to nucleus through Smad proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Paralkar VM, Weeks BS, Yu YM, Kleinman HK, Reddi AH. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. J Cell Biol. 1992;119:1721–1728. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of Noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, Jesus-Escobar JM, Harland RM. The Spemann organizer signal Noggin binds and inactivates bone morphogenetic protein-4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of Noggin, which limits their activity in cultured rat osteoblast. J Clin Invest. 1998;102:2106–2114. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O'Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by Noggin. J Bone Miner Res. 2000;5:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus : inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz IL, Shimmi O, Wunnenberg-Stapleton K, O'Connor MB, Cho KWY. Is Chordin a long-range or short-range-acting factor? Roles for BMP1-related metalloproteases in Chordin and BMP4 autofeedback loop regulation. Dev Biol. 2000;223:120–138. doi: 10.1006/dbio.2000.9740. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KWY, Greenspan DS. Mammalian BMP-1/tolloid-related metalloproteinases, including novel family member mammalian tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- Wardle FC, Welch JV, Dale L. Bone morphogenetic protein 1 regulates dorsal-ventral patterning in early Xenopus embryos by degrading Chordin, a BMP-4 antagonist. Mech Dev. 1999;86:75–85. doi: 10.1016/s0925-4773(99)00114-8. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ozaki T, Nakagawara A, Sakiyama S. A product of DAN, a novel candidate tumour suppressor gene, is secreted into culture medium and suppresses DNA synthesis. Eur J Cancer. 1997;33:1986–1990. doi: 10.1016/s0959-8049(97)00333-x. [DOI] [PubMed] [Google Scholar]
- Picollo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Stanley E, Biben C, Kotecha S, Fabri L, Tajbakhsh S, Wang CC, Hatzistavrou T, Roberts B, Drinkwater C, Lah M, Buckingham M, Hilton D, Nash A, Mohun T, Harvey RP. DAN is a secreted glycoprotein related to Xenopus cerberus. Mech Dev. 1998;77:173–184. doi: 10.1016/s0925-4773(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Pearce JJH, Penny G, Rossant J. A mouse Cerberus/Dan-related gene family. Dev Biol. 1999;209:98–110. doi: 10.1006/dbio.1999.9240. [DOI] [PubMed] [Google Scholar]
- Merino R, Rodriguez-Leon J, Macias D, Gañan Y, Economides AN, Hurle JM. The BMP antagonist Gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development. 1999;126:5515–5522. doi: 10.1242/dev.126.23.5515. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Larrain J, Geissert D, DeRobertis EM. The evolutionarily conserved BMP-binding protein twisted gastrulation promotes BMP signaling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGFβ and BMP 2. J Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]


