Abstract
The contribution of osteoclasts to the process of bone loss in inflammatory arthritis has recently been demonstrated. Studies in osteoclast biology have led to the identification of factors responsible for the differentiation and activation of osteoclasts, the most important of which is the receptor activator of NF-κB ligand/osteoclast differentiation factor (RANKL/ODF), a tumor necrosis factor (TNF)-like protein. The RANKL/ODF receptor, receptor activator of NF-κB (RANK), is a TNF-receptor family member present on both osteoclast precursors and mature osteoclasts. Like other TNF-family receptors and the IL-1 receptor, RANK mediates its signal transduction via TNF receptor-associated factor (TRAF) proteins, suggesting that the signaling pathways activated by RANK and other inflammatory cytokines involved in osteoclast differentiation and activation are interconnected.
Keywords: osteoclasts, RANK, RANKL, TNF-α, TRAF
Introduction
The pathogenesis of focal bone loss in inflammatory processes such as rheumatoid arthritis (RA) is a subject of recent interest. Osteoclasts are known to contribute to focal bone erosion in RA [1,2,3] and in animal models of arthritis [4,5,6]. The role of osteoclasts in normal physiologic bone remodeling is well established. During this process, focal areas of bone are resorbed by osteoclasts and repopulated by osteoblasts, which synthesize new bone matrix [7]. The focal net loss of bone at sites of inflammation in conditions such as RA suggests that there is an imbalance in favor of bone resorption. Considerable effort has been made in elucidating the factors responsible for this increased bone resorption and in defining the mechanisms involved in the differentiation and activation of osteoclasts at sites of inflammation. This review focuses on a newly described and essential factor for osteoclast differentiation-ODF, a member of the TNF ligand family of cytokines [8]. ODF was independently identified as RANKL [9]. We will refer to this factor as RANKL/ODF. Its cognate receptor is RANK. The role of this receptor-ligand pair in bone resorption is reviewed, and the signal transduction pathways involved in signaling through the RANK receptor are discussed in relation to common and interconnected pathways activated by other receptors.
The role of RANKL/ODF in osteoclast differentiation and activation
RANKL/ODF was originally cloned as an essential factor for osteoclastogenesis by two independent research groups [8,10], who named it, respectively, 'osteoclast differentiation factor' (ODF) and 'osteoprotegerin ligand' (OPGL). Using models of osteoclast differentiation in vitro, it has been shown that many of the factors that enhance osteoclast formation or activity, including 1,25(OH)2D3, parathyroid hormone (PTH), interleukin (IL)-11, and prostaglandin E2 (PGE2), mediate these effects at least in part by inducing the expression of RANKL/ODF by osteoblasts and/or bone lining cells [11,12,13,14]. Interestingly, RANKL/ODF had also previously been independently identified as TNF-related activation-induced cytokine (TRANCE) [15], a T cell product upregulated after T cell-receptor stimulation. TRANCE enhances the proliferation of naïve T cells through interactions of T cells with dendritic cells [16].
Activated Tlymphocytes also express membrane-bound RANKL/ODF and can secrete a soluble form of RANKL/ODF [17]. RANKL/ODF messenger RNA is expressed at high levels in cells in trabecular bone and bone marrow, including bone lining cells and osteoblasts, as well as in lymph node, thymus, and Peyer's patches [8,9,10]. RANKL/ODF-/- mice exhibit a dramatic phenotype supporting the essential role of this factor in osteoclast differentiation. These mice have defective tooth eruption and severe osteopetrosis associated with the absence of osteoclasts [18].They also have no peripheral lymph nodes, have defects in B cell and T cell maturation, and have thymic hypoplasia, supporting the argument that this factor plays a role in immune-cell differentiation.
The signaling receptor for RANKL/ODF, a member of the TNF receptor (TNFR) family, was originally described as a receptor on T cells and dendritic cells, and was named RANK [also known as osteoclast differentiation and activation receptor (ODAR) and TRANCE receptor], because binding of RANKL/ODF to this receptor leads to activation of the transcription factor NF-κB [9,14,19]. In addition to being expressed on T cells and dendritic cells, RANK is also expressed on osteoclasts and osteoclast precursor cells, and on certain B cells [9,19,20]. RANK-/- mice have a phenotype similar to that of RANKL/ODF-/- mice, including the presence of osteopetrosis, absence of peripheral lymph nodes, and a deficiency of B cells [21,22].
Osteoprotegerin inhibits the actions of RANKL/ODF
A decoy receptor for RANKL/ODF has been identified and named osteoprotegerin (OPG) and osteoclastogenesis inhibitory factor (OCIF) [23,24]. OPG is a secreted member of the TNFR family that lacks a transmembrane domain and is structurally distinct from RANK. It is active as either a soluble monomer or a disulfide-linked homodimer [25]. OPG binds RANKL/ODF with high affinity, thereby preventing RANKL/ODF from interacting with its cognate receptor (RANK) (Fig. 1). Overexpression of OPG in transgenic mice blocks the activity of endogenous RANKL/ODF, resulting in the development of osteopetrosis, although there does not appear to be a defect in lymphoid tissue development [23]. OPG-/- mice demonstrate severe osteoporosis, which is a result of the unopposed activity of endogenous RANKL/ODF, leading to excessive osteoclast differentiation and activity [26,27]. Under physiologic conditions and in disorders associated with disturbed bone remodeling, osteoclast-mediated bone resorption can be modulated by altering the balance between OPG and RANKL/ODF. This has obvious implications for the development of therapeutic strategies for controlling physiologic and pathologic bone loss [11].
Figure 1.
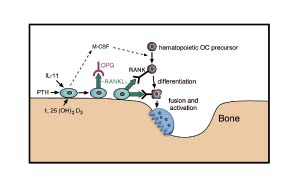
Simplified schematic view of the interactions between membrane-bound RANKL on osteoblast/bone stromal cells and its receptor RANK on osteoclast precursor cells. This interaction leads to the differentiation of osteoclast precursor cells and the activation of osteoclasts to resorb bone. OPG can inhibit this interaction by binding to membrane-bound RANKL and blocking the RANKL-RANK interaction. D3, vitamin D3; M-CSF, macrophage-colony stimulating factor; OC, osteoclast.
Two classes of tumor necrosis factor receptors regulate life and death
Binding of RANKL/ODF to RANK activates signal transduction pathways that ultimately lead to osteoclast differentiation and increased osteoclastic activity. It is useful to review other members of the TNFR family and their associated signal transduction pathways to examine the common and interconnected pathways by which these receptors regulate diverse cellular activities. Members of the TNFR family all share an extracellular ligand-binding domain containing at least two repeats of a signature module consisting of a disulfide-rich anti-parallel beta-strand structure of approximately 40 amino acids. The structural analysis of the ligand interaction reveals that a trimeric ligand interacts intimately with two of these modules from each of three receptors, resulting in a 3:3 complex [28] that leads to intracellular signal transduction.
With regard to intracellular signaling, the TNFR family can be subdivided into two groups, those that directly induce apoptosis (eg TNFRI/p55 TNF-α receptor and Fas), and those that typically do not (eg RANK, TNFRII/p75 TNF-α receptor, and CD40) (Fig. 2). The apoptotic response is dependent upon the presence of death domains (DDs) within the cytoplasmic region of the receptor. These domains mediate protein-protein interactions resulting in dimeric and tetrameric complexes among proteins containing nonidentical DDs [29]. The nonapoptotic TNFRs do not contain DDs but, in contrast to the apoptotic forms, do contain a short-sequence motif [30] necessary for the recruitment of a family of related TRAFs essential for many downstream intracellular signaling events [31,32]. These sequence motifs are referred to here as TRAF interaction motifs (TIMs). Activation of TRAFs appears to be dependent upon membrane localization [33] and trimerization of amino-terminal RING-zinc finger (RZF) domains mediated by the carboxyl-terminal ligand-interaction region [34,35]. Although apoptotic TNFRs do not contain any TIMs, they can recruit TRAFs indirectly via DD-containing adapter molecules.
Figure 2.
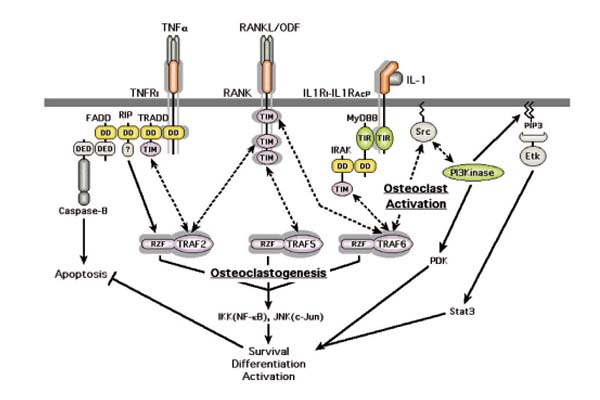
Interrelationships among receptors that signal via TRAFs. Double-headed, dashed arrows indicate known direct protein-protein interactions; solid arrows represent enzymatic and functional pathways. Emphasis is placed on distinctions between osteoclastogenesis and osteoclast activation. Abbreviations and terms are defined in the text.
In the case of the apoptotic receptors, TRAFs appear to be important for cell death, probably by supporting the action of Fas-associated death domain protein (FADD), which is critical for caspase-8 activation. As shown in Fig. 2, the apoptotic TNFRs (eg TNFRI) have the potential to induce both death and survival signals. The specific outcome (ie survival versus death) appears to depend upon mechanisms that shift the balance of cytoplasmic signaling components. For example, the activation of caspase-8 not only activates apoptosis, but also inhibits the NF-κB survival pathway by degrading receptor-interacting protein (RIP). RIP is a DD kinase essential for TNF-α-dependent activation of NF-κB [36], possibly through the TRAF amino-terminal RZF domain, in a kinase-independent manner [37].
Lipopolysaccharides and IL-1 receptors also signal via TRAF
TRAF recruitment and activation are also mediated by the Toll/IL-1/IL-18 receptor family (TIR), which is distinct from the TNFR family and includes the homodimeric Toll-like receptors (TLRs) that bind microbiological products (eg the lipopolysaccharide receptor TLR4); the heterodimeric IL-1 receptor (consisting of two related molecules, IL-1RI and IL-1RAcP); and receptors for IL-18 (Fig. 2). The TIRs all contain a specific domain structure (TIR domain) that is critical for the recruitment of MyD88, an adapter molecule that possesses both a TIR and a DD [38,39]. The MyD88 DD can recruit one or more IL-1R-associated kinases (IRAKs) that bind TRAF6. The kinase function of IRAK appears not to be important for TRAF recruitment but may serve to facilitate receptor recycling [40]. Therefore, IL-1, LPS, and TNF-α, which have long been known to have overlapping functions, may mediate these shared activities via the recruitment of TRAFs.
There are at least six distinct TRAF forms, not all of which induce intracellular signaling. TRAF2, 5, and 6 have been shown to be involved in TNFR and TIR downstream activation of NF-κB and the c-jun amino-terminal kinase (JNK), whereas TRAF1, 3, and 4 have not. TRAF4 is restricted to the nucleus, and TRAF1, which can heterotrimerize with TRAF2, is deficient in an amino-terminal region necessary for NF-κB activation [31]. TRAF3, however, which can be activated by engineered membrane localization [33], binds to the CD40 TNF receptor [34], and is required for T cell-dependent immune responses [41]. Although the receptor-binding specificities for TRAF2, 3, and 5 appear to be similar, TRAF6 possesses a distinct binding specificity. Therefore, although TRAF2, 3, and 5 bind either directly or indirectly to most TNFRs, TRAF6 is unique in its ability to bind directly only to IRAK, RANK, and CD40 [30].
RANK binds and activates a full complement of TRAFs and activates Src
The cytoplasmic domain of RANK is unique in that it has three independent TIMs, for binding TRAF2, 5, and 6 [42]. The membrane-proximal TRAF6 binding site appears to be highly specific. The other two sites each bind TRAF2 and 5. However, the carboxyl-terminal site is most specific for TRAF5 and the more amino-proximal site is most specific for TRAF2 [43]. The amino-terminal RZF domain of TRAF2 and 6 has been shown to be capable of activating downstream signals to I-κB kinase (IKK), JNK, and p38 kinase [35]. Osteoclast differentiation is blocked in mice deficient in the p50 and p52 forms of NF-κB, demonstrating the critical role of this factor [44,45]. However, RANK activation of both the JNK and p38 kinase pathways has also been demonstrated to be important for osteoclast differentiation and function [46,47]. TRAF2 and 5 appear to have similar activities. Interestingly, TRAF2-/- mice do not exhibit osteopetrosis [48], an observation indicating that this TRAF is either not important for osteoclastogenesis or, more likely, is complemented by other TRAFs.
The carboxy-terminal receptor-binding/trimerization domain of TRAF6 is distinct from that of other TRAFs in that it contains a short, proline-rich loop capable of binding to the SH3 domain of the Src tyrosine kinase [42]. This loop provides a means for the activation of Src, which, though constitutively membrane-associated via amino-terminal myristylation, is inhibited by intramolecular SH2 and SH3 interactions [49]. The activation of Src by TRAF6-mediated SH3 competition provides a mechanism for the reported activation of phosphatidylinositol 3-kinase (PI3 kinase) by both RANK [42] and IL-1R [39]. This activation may not require involvement of the Src kinase function and may depend only upon interaction between the SH3 domain of Src and the proline-rich sequence of the PI3 kinase p85 regulatory subunit [50]. This could explain how src-/- mice, which exhibit a severe osteopetrotic phenotype [51], can be rescued by a kinase-defective Src [52]. The functional association between TRAF6 and Src is also supported by the observation that both src-/- and TRAF6-/- mice exhibit a similar phenotype of osteopetrosis in which there are abundant osteoclasts, but a defect in osteoclastic bone resorption [52,53]. This is in contrast to both the RANK-/- and RANKL/ODF-/- mice, which lack osteoclasts [18,21].
Src is also capable of direct activation of the Stat3 transcription factor via tyrosine phosphorylation as well as indirect activation via Tec tyrosine kinases like Etk [54]. The tyrosine kinase Etk is activated by binding to phosphatidylinositol-(3,4,5)-triphosphate (PIP3), a product of activated PI3 kinase, through an amino-terminal plextrin homology domain [49]. The Src-Etk pathway could provide a mechanism for the reported tyrosine phosphorylation-dependent activation of the Stat3 transcription factor by IL-1 via the TRAF6-dependent IL-1R [39]. Also, PI3 kinase phospholipid products can activate many other kinases via PI3 kinase-dependent kinase (PDK) and protein kinases A, B, and C. PI3 kinase may therefore activate many pathways that are probably essential to osteoclast development and activation, including apoptosis inhibition, cell proliferation, endocytosis, and vesicular trafficking [55].
Cross-talk between TNF and Toll/IL-1 receptors probably modulates osteoclast action
The distinction between the activation of osteoclasts to resorb bone and osteoclast differentiation (osteoclasto-genesis) is underscored by the observed differences between, on the one hand, src-/- and TRAF6-/- mice (which exhibit abundant but poorly functioning osteoclasts) and, on the other hand, RANK-/- and RANKL/ODF-/- mice (which lack osteoclasts). RANKL/ODF can support both osteoclast differentiation and activation. In contrast, TNF-α, primarily through TNFRI, supports osteoclast differentiation, while both IL-1 and LPS can activate preformed osteoclasts to resorb bone [56,57,58,59,60]. Interestingly, TNFRI directly recruits only TRAF2 via the TNFR-associated death domain protein (TRADD) adapter [61], whereas both the LPS TLR4 [38] and IL-1R recruit only TRAF6 via IRAK [31] (Fig. 2). Therefore, because only TRAF6 activates Src, TRAF6 (and receptor ligands that can effect its signaling) may be a key component for the activation of osteoclasts to resorb bone. In this model, RANK induction of osteoclast differentiation does not require TRAF6 when other TRAFs are present. It is not yet clear whether TNF-α/TNFRI can replace all of the osteoclastogenic activities of RANK. Similarly, it is not yet known whether TRAF5 plays a distinct role in osteoclast differentiation.
A recent publication supports the involvement of TNFRI but not TNFRII in enhancing both basal and soluble TNF-α-induced osteoclastogenesis in marrow cultures [56], suggesting that TNF-α can synergize with RANKL. Consequently, if differences exist between RANK- and TNFRI-induced osteoclastogenesis, they may be due either to another element in the pathway (such as RIP) or to a dosage effect resulting from the activation of more than one TRAF. Regardless, the ability to fine-tune osteoclast differentiation and activation by TNFRI and the TRAF6-specific receptors for either IL-1 or LPS, which can each act to deliver a portion of the complete signal that is provided via activation of the RANK signal transduction pathway, may be an important mechanism for regulating osteoclast-mediated bone resorption.
Potential contributions of RANKL/ODF to bone erosions in rheumatoid arthritis
Given the critical role of RANKL/ODF in the regulation of osteoclastogenesis in physiologic bone remodeling, the potential role of interactions between RANKL/ODF and RANK in the generation of bone erosions in RA has been explored. We and others have shown that RANKL/ODF is expressed in cultured synovial fibroblasts from patients with RA [62,63]. This factor is also expressed in CD4+ and CD8+ T lymphocyte subsets in RA synovium [17,64] and in activated CD4+ lymphocytes derived from RA synovium [62]. A potential direct role for RA synovial fibroblasts and/or Tlymphocytes in inducing osteoclast differentiation is suggested by recent studies in which co-culture of either synovial fibroblasts or Tlymphocytes with osteoclast precursors in the presence of cofactors resulted in the generation ofmultinucleated cells with the phenotypic features of osteoclasts [17,63,64].
Important evidence suggesting that RANKL/ODF plays a role in the pathogenesis of osteoclastic resorption in inflammatory arthritis comes from studies in the rat adjuvant-arthritis model [17]. Arthritic rats treated with OPG at disease onset had minimal loss of cortical and trabecular bone, whereas bone loss was severe in untreated control animals. A dramatic decrease in osteoclast numbers was also observed in the OPG-treated animals [17]. OPG treatment did not appear to decrease joint inflammation, suggesting that the prevention of focal bone destruction was related to a specific effect on osteoclast-mediated bone resorption. These findings are important with respect to treatment strategies that target RANKL-RANK signaling pathways to prevent focal bone destruction in RA. The use of OPG, which binds RANKL and prevents activation of the RANK signaling pathway, represents one therapeutic approach. Additional strategies could target signal transduction pathways shared by TNF-α, IL-1, and RANKL, with the goal of inhibiting more broadly both inflammatory cascades and osteoclast-mediated bone resorption.
Acknowledgments
Acknowledgement
The authors thank Jason Boch for his input and fruitful discussions.
References
- Leisen JCC, Duncan H, Riddle JM, Pitchford WC. The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J Rheumatol. 1988;15:17–22. [PubMed] [Google Scholar]
- Bromley M, Woolley DE. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 1984;27:968–975. doi: 10.1002/art.1780270902. [DOI] [PubMed] [Google Scholar]
- Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- Romas E, Bakharevski O, Hards DK, Kartsogiannis V, Quinn JMW, Ryan PFJ, Martin J, Gillespie MT. Expression of osteoclast differentiation factor at sites of bone erosion in collagen-induced arthritis. Arthritis Rheum. 2000;43:821–826. doi: 10.1002/1529-0131(200004)43:4<821::AID-ANR12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nishikaku F, Nakatuka M, Koga Y. Osteoclast-like cells in murine collagen induced arthritis. J Rheumatol. 1998;25:1154–1160. [PubMed] [Google Scholar]
- Kuratani T, Nagata K, Kukita T, Hotokebuchi T, Nakasima A, Iijima T. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol. 1998;13:751–759. doi: 10.14670/HH-13.751. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki SI, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprote-gerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem Biophys Res Commun. 1998;250:776–781. doi: 10.1006/bbrc.1998.9394. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Elliott J, Martin TJ, Gillespie MT. Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology. 1998;139:4743–4746. doi: 10.1210/endo.139.11.6433. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Goto M, Mochizuki SI, Tsuda E, Morinaga T, Udagawa N, Takahashi N, Suda T, Higashio K. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–113. doi: 10.1016/S8756-3282(99)00121-0. [DOI] [PubMed] [Google Scholar]
- Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, 3rd, Frankel WN, Lee SY, Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–25194. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, Morinaga T, Higashio K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Amgen ProgramEST, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Kinosaki M, Goto M, Kobayashi F, Tsuda E, Morinaga T, Higashio K. Characterization of structural domains of human osteoclastogenesis inhibitory factor. J Biol Chem. 1998;273:5117–5123. doi: 10.1074/jbc.273.9.5117. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun. 1998;247:610–615. doi: 10.1006/bbrc.1998.8697. [DOI] [PubMed] [Google Scholar]
- Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W. Crystal structure of the soluble human 55 kd TNF receptor-human TNFβ complex: Implications for TNF receptor activation. Cell. 1993;73:431–445. doi: 10.1016/0092-8674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Xiao T, Towb P, Wasserman SA, Sprang SR. Three-dimensional structure of a complex between the death domains of Pelle and Tube. Cell. 1999;99:545–555. doi: 10.1016/s0092-8674(00)81542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen SS, Dang TTA, Crute JJ, Kehry MR. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). J Biol Chem. 1999;274:14246–14254. doi: 10.1074/jbc.274.20.14246. [DOI] [PubMed] [Google Scholar]
- Arch RH, Gedrich RW, Thompson CB. Tumor necrosis factor receptor-associated factors (TRAFs) - a family of adapter proteins that regulates life and death. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- Bulfone-paus S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. Death deflected: IL-15 inhibits TNF-α-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15α chain. FASEB J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- Dadgostar H, Cheng G. Membrane localization of TRAF3 enables JNK activation. J Biol Chem. 2000;275:2539–2544. doi: 10.1074/jbc.275.4.2539. [DOI] [PubMed] [Google Scholar]
- McWhirter SM, Pullen SS, Holton JM, Crute JJ, Kehry MR, Alber T. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc Natl Acad Sci U S A. 1999;96:8408–8413. doi: 10.1073/pnas.96.15.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Liu Z-G, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappa B signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- Deven A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z-G. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- Means TK, Golenbock DT, Fenton MJ. The biology of toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–232. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Auron PE. The interleukin 1 receptor: ligand interactions and signal transduction. Cytokine Growth Factor Rev. 1998;9:221–237. doi: 10.1016/s1359-6101(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Li X, Commane M, Burns C, Vithalani K, Cao Z, Stark GR. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol Cell Biol. 1999;19:4643–4652. doi: 10.1128/mcb.19.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- Darnay BG, Ni J, Moore PA, Aggarwal BB. Activation of NF-κB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-κB-inducing kinase. J Biol Chem. 1999;274:7724–7731. doi: 10.1074/jbc.274.12.7724. [DOI] [PubMed] [Google Scholar]
- Istova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappa B in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Akiyama S, Tsurukai T, Okahashi N, Kobayashi K, Udagawa N, Nishihara T, Takahashi N, Suda T. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J Immunol. 1999;163:434–442. [PubMed] [Google Scholar]
- Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J Biol Chem. 2000;275:31155–31161. doi: 10.1074/jbc.M001229200. [DOI] [PubMed] [Google Scholar]
- Yeh R-B, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeharn A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;71:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- Yang W-C, Collette Y, Nunes JA, Olive D. Tec kinases: A family with multiple roles in immunity. Immunity. 2000;12:378–382. doi: 10.1016/s1074-7613(00)80189-2. [DOI] [PubMed] [Google Scholar]
- Grey A, Chen Y, Paliwal I, Carlberg K, Insogna K. Evidence for a functional association between phosphatidylinositol 3-kinase and c-src in the spreading response of osteoclasts to colony-stimulating factor-1. Endocrinology. 2000;141:2129–2138. doi: 10.1210/endo.141.6.7480. [DOI] [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Xing L, Hoffmann O, Lowell CA, Garrett L, Boyce BF, Varmus HE. Rescue of osteoclast function by transgenic expression of kinase-deficient src in src-/- mutant mice. Genes Dev. 1997;11:2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomaga MA, Yeh W-C, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–1024. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai Y-T, Su Y-H, Fang S-S, Huang T-N, Qiu Y, Jou Y-S, Shih H-M, Kung H-J, Chen R-H. Etk, a BTK family tyrosine kinase, mediates cellular transformation by linking Src to Stat3 activation. Mol Cell Biol. 2000;20:2043–2054. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: Kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Abu-Amer Y, Erdmann J, Alexopoulou L, Kollias G, Ross FP, Teitelbaum SL. Tumor necrosis factor receptors types 1 and 2 differentially regulate osteoclastogenesis. J Biol Chem. 2000;275:27307–27310. doi: 10.1074/jbc.M003886200. [DOI] [PubMed] [Google Scholar]
- Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–285. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CY, Kyritsis G, Graves DT, Amar S. Interleukin-1 and tumor necrosis factor activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun. 1999;67:4231–4236. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amer Y, Ross FP, Edwards J, Teitelbaum SL. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its p55 receptor. J Clin Invest. 1997;100:1557–1565. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YC, Ye H, Hsia C, Segal D, Rich RL, Liou H-C, Myszka DG, Wu H. A novel mechanism of TRAF signaling revealed by structural and functional analyses of the TRADD-TRAF2 interaction. Cell. 2000;101:777–787. doi: 10.1016/s0092-8674(00)80889-2. [DOI] [PubMed] [Google Scholar]
- Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S. Involvement of receptor activator of nuclear factor kappa-B ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Kartsogiannis V, Quinn JMW, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–150. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]


