Abstract
T lymphocytes play a key role in the immune response to both foreign and self peptide antigens, which they recognize in combination with MHC molecules. In the past it has been difficult to analyse objectively the specificity, frequency and intensity of T cell responses. The recent application of fluorescent-labelled MHC class I multimers, however, has provided a powerful experimental approach to the direct visualisation of antigen-specific T cells. As a result, our perspective of how T cells respond to both viruses and other antigens in vivo has been greatly enhanced.
Keywords: autoimmune disease, CD8+ T cells, cytotoxic T lymphocyte, HLA Class 1, immunotherapy, MHC class I tetrameric complex technology
Introduction
MHC molecules are highly polymorphic glycoproteins that are expressed at the cell surface after assembly within the cell, together with beta 2 microglobulin and short antigenic peptide fragments. This combination can then be recognized by CD8+ cytotoxic T lymphocytes (CTLs); specificity for a particular MHC/peptide combination is conferred by the TCR, which is an immunoglobulin-like molecule. CTLs play a major role in the adaptive immune response to intra-cellular infections and tumours, specifically killing appropriate target cells and also releasing pro-inflammatory cytokines. The principal human leukocyte antigen (HLA) MHC class I alleles are denoted HLA A, HLA B and HLA C, although a number of MHC class I-like molecules have also been described. CTLs can be detected according to their function, for example in killing assays or by analysis of cytokine production, according to either their antigenic specificity (using MHC class I multimers) or their phenotype (i.e. their TCR or other expressed molecules).
The traditional method for functional analysis of antigen-specific CTLs is the cytotoxic T cell lysis assay. This method is based on the ability of CTLs to lyse target cells bearing the appropriate MHC and peptide antigen in vitro. Such assays usually require a period of in vitro culture with antigen, after which quantitation and phenotypic interpretation is difficult. The limiting dilution analysis (LDA) is a modified CTL assay that gives a quantitative measure of the number of precursor CTLs. Antigen-specific T cells are first serially diluted and then cultured until the cells have multiplied to a sufficient number to perform CTL lysis assays. The number of precursor CTLs can be estimated from the lowest dilution at which CTL activity can no longer be detected. Because the LDA only detects the portion of cells that are able to proliferate and kill targets after in vitro culture, it may result in a significant underestimation of the total number of antigen-specific CTLs.
Several other methods have also been used to estimate antigen-specific CTLs. The numbers of specific transcripts for a given T cell receptor (TCR) can be quantified using molecular techniques. This gives information about T cell phenotype and clonality, but not about antigen specificity or function [1,2]. The frequency of CD4+ or CD8+ T cells secreting IFN-? in response to a particular antigen can be detected by the enzyme-linked immunospot (ELISPOT) assay [3]. The number of T cells producing a given cytokine in the presence of specific peptides can also be measured by flow cytometry [4]. These techniques give information about both specificity and function, but both are limited because they detect only the CTLs releasing a particular cytokine. Furthermore, the results from these methods have failed to answer some questions, such as whether individual T cell clones of the same specificity have identical cell surface phenotypes and cytokine-producing profiles. The MHC class I tetrameric complex technology provides an ideal tool to address these issues and can be used to follow the course of T cell response to the challenge of a particular antigen.
Development of MHC tetrameric complex technology
The MHC class I tetrameric complex technique was introduced by Altman and Davis in Stanford, USA, and their collaborators in Oxford, UK [5]. The principle of the technique is that peptide/MHC complexes, as TCR ligands, can be used to identify T cells with particular antigenic specificity. In vitro, a single, soluble peptide/MHC complex has a low affinity for its corresponding TCR, resulting in a weakly bound complex with a rapid dissociation rate [6]. MHC class I/peptide multimers, however, can greatly enhance the avidity of the binding. Such tetrameric complexes can be tagged with fluorescent dyes, allowing individual T cells to be identified by flow cytometry analysis. If the specific T cell is co-tagged with different fluorescent-conjugated antibodies that are specific for cell surface markers (cytokine or chemokine) the phenotype of the cell can be elucidated simultaneously.
Perspective (what it can do)
MHC class I tertrameric complex technology has been used to study immune responses to tumours and viral infections, and to probe the mechanism underlying autoimmnune disease. Tetramers have been used to stain peripheral blood lymphocytes and other cell types [7,8], cell suspensions made from tissue samples, and tissue sections [9,10]. Evidence of a role for CTLs in the control of HIV infection has been provided using tetrameric complex technology [7,11]. Thus, HLA class I tetramer staining has shown that the frequency of HIV-1-specific CTLs in the blood of HIV-infected individuals is inversely correlated to the RNA viral load in the plasma [11].
By co-staining cells with MHC class I tetrameric complexes as well as antibodies for cell surface markers (cytokines and chemokines), a considerable amount of information about CTL phenotype can be obtained. Using CMV- and HIV-specific tetramers to stain CD8+ T cells from the same HIV-infected donor, Appay et al found that CD8+ T cells that are specific for both HIV and CMV can secrete antiviral cytokines in response to stimulation by their respective antigens [12]. However, HIV-specific CD8+ T cells express a much lower level of perforin than CMV-specific CD8+ T cells. Perforin is an important molecule for CTL lysis and promotes the cell death through pore formation in the cell membrane. This defect in perforin production may contribute to the impaired function of HIV-specific CD8+ T cells in clearing virus-infected cells [12].
Viral infection can induce massive increases in the number of T cells. Class I tetrameric complex staining has been used to determine if this expansion is antigenic specific. In peripheral blood from a patient with primary Epstein-Barr virus (EBV) infection, CD8+ CTLs that are specific for a single EBV epitope may comprise up to 44% of the total CD8+ T cells [13].
Initially it was thought that much of the increase in T cell numbers during the response to viruses is not antigen specific, but represents bystander activation or cross reactivity to the stimulation of non-specific antigens. In order to address this issue, Murali-Krishna et al used MHC class I tetrameric complexes of a lymphocytic chorio meningitis virus (LCMV) epitope to stain CTLs from samples collected during primary and secondary murine LCMV infection. Large increases in numbers of CD8+ T cells were observed, and the majority of them were LCMV antigen specific. When mice were first primed with LCMV then later challenged with heterologous vaccine virus, the number of LCMV-specific CTLs did not increase [14]. Tetramer technology has therefore shown, at least in this model, that bystander activation does not occur to a significant extent. This calls into question its importance as a general immunological phenomenon.
MHC class I multimers and the response to tumours
Direct evidence for the role of CTLs in the immune response to tumours has been obtained using MHC class I tetrameric complex technology. In patients with chronic myelogenous leukaemia treated with either IFN-a-2b or allogeneic bone marrow transplantation, tumour-specific CTLs have been detected using specific MHC class I tetramers complexed with PR1 (a peptide derived from proteinase 3). The presence of PR1-specific T cells correlates with disappearance of the tumour (by cytogenic analysis) after treatment [15]. Theoretically, CTLs with specificity against tumours could be expanded ex vivo and then reinfused as therapy for tumour patients. In practice, however, it has proved difficult to isolate sufficient CTLs due to their low frequency. Single cell FACS of MHC class I tetrameric complex-stained CTLs has proved to be an efficient method of obtaining significant numbers of melanoma-specific CD8+ CTLs, which kill in vitro, and this precedure could be utilised for therapy [8].
Detection of CTLs in autoimmune disease
Although CTLs play an important role in protecting the body from pathogens, they can occasionally attack their own body to cause self-damage, a phenomenon seen in some MHC-class-I-related autoimmune diseases. Using MHC class I tetrameric technology, Ogg et al showed that in the blood of patients with vitiligo (an autoimmune condition charactarized by loss of epidermal melanocytes) HLA-A2-restricted melanA-specific CTLs expressed high levels of cutaneous lymphocyte-associated antigen which is a skin-homing receptor. This receptor was absent from the CTLs in an HLA-A2 positive normal control. This study has lead to the suggestion that lack of homing receptors on the surface of auto-reactive CTLs could be a mechanism to maintain peripheral tolerance in vivo [16].
The strongest association of HLA class I with rheumatic disease is that of HLA B27 with the spondyloarthritides. To determine the role that class I multimers can play in elucidating the pathogenesis of these conditions, tetramers of HLA B27, complexed with beta 2 microglobulin and an influenza epitope (Fig. 1a), have been used to stain both HLA B27-restricted CTLs (Fig. 1b) and cells transfected with TCRs from such cells [17]. If disease results from an HLA-B27-restricted response to an "arthritogenic" peptide or peptides, it should be possible to detect and monitor HLA-B27-restricted peptide-specific CTLs in the blood and joints of these patients. Unfortunately such arthritogenic peptides have not been conclusively identified, although a number of candidates have been proposed. These include peptides derived from Yersinia [18] and self-peptides [19], including one derived from HLA B27 itself. Responses to these peptides have been detected by both CTL lysis assay and ELISPOT. It should now be possible to use HLA B27/peptide tetrameric complexes to stain directly patient blood and synovial material ex vivo, giving information about the frequency and phenotype of such cells in both patients with spondyloarthropathy and healthy controls.
Figure 1.
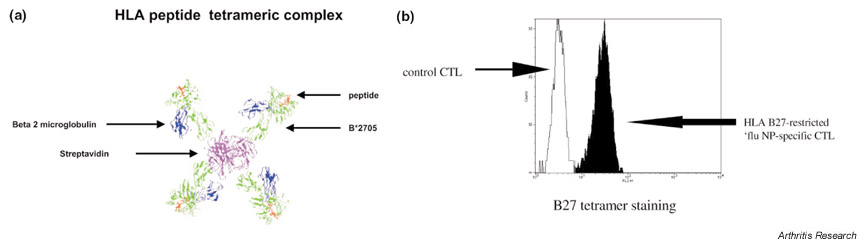
Model of an HLA/peptide tetrameric complex and its use to stain specific cytotoxic T cells. (a) Molecular model of an HLA/peptide tetrameric complex. Here HLA B*2705, the molecular subtype of HLA B27, has been refolded with beta 2 microglobulin and the influenza nucleoprotein peptide NP383-391. Four biotinylated complexes are shown together with one streptavidin molecule (here without a fluorochrome). (b) Example of HLA-B27/peptide tetrameric complex tetramer staining. Positive FACS staining (shown infilled) for an HLA B27-restricted Influenza NP-specific CTL line is shown.
MHC class I-restricted T cell responses in rheumatoid arthritis
Callan et al have used tetrameric complex technology to demonstrate the presence of a high frequency of EBV-specific HLA-class I-restricted CTL in the joints of patients with rheumatoid arthritis [20]. Whilst not necessarily implying a direct pathogenic role for such cells, this finding gives an intriguing insight into the specificity of T cells in the arthritic joint.
Other applications of tetrameric complex technology
The application of tetrameric complex technology is not limited to the identification of classical MHC-class-I-restricted T cell responses. The extension of the use of this technology has enabled the identification of the ligands for HLA-E (a non-MHC-class I molecule) as the natural killer cell receptors CD94/NKG2A, B and C [10].
MHC class I tetrameric complex technology has also been used to address some fundamental questions in immunology studies. It was well understood that secondary humoral and cellular immune responses are stronger than they are after primary antigenic challenge. Savage et al have provided evidence of a qualitative difference between primary and secondary effector T cells with respect to affinity. Kinetic analysis of tetrameric complex binding to TCR showed that, over the course of an immune response, T cell clones were selected that had higher affinity and longer duration of binding to the MHC class I-peptide complex [21].
Tetrameric complex technology has now also been adapted, despite numerous technical difficulties, to construct MHC class II tetrameric complexes. Tetramers of HLA DR4 complexed with type II collagen, for example, have been made and used to detect DR4-restricted peptide-specific CD4+ helper T cells [22]. These results do not, however, suggest that type 2 collagen-specific T cells are driving the pathogenic process in rheumatoid arthritis.
Short technical description
A fusion protein containing the extracellular domains of a MHC class I heavy chain together with a 13-amino acid C-terminal tag (that allows site-specific biotinylation), is expressed in E. coli. This fusion protein is refolded by dilution in urea in vitro in the presence of beta 2-microglobulin and specific peptide. Biotinylation of the C-terminal tag is then carried out using the BirA enzyme (Fig. 2). Since streptavidin has 4 biotin binding sites, a tetrameric complex is generated by adding pycoerythrin-conjugated or fluorescein-conjugated extravidin streptavidin. Use of phycoerythrin as a fluorescent marker has given better definition than fluorescein. Figure 1a shows a model of a tetrameric complex.
Figure 2.
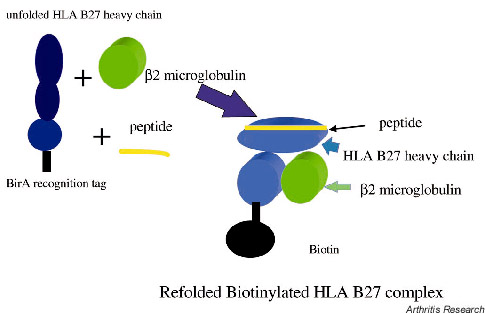
Generation of an HLA B27/beta 2 microglobulin/peptide tetramer is shown in cartoon form. Denatured class 1 heavy chain is refolded by limiting dilution in the presence of beta 2-microglobulin and excess peptide. After chromatographic purification, correctly folded complexes are biotinylated as shown. Four of these complexes are bound to fluorochrome-conjugated streptavidin (as shown in Fig. 1) for use in FACS staining of T cells.
Tetrameric complexes are relatively stable at 4?C for periods of weeks to months. After titration to determine the optimum concentration required, tetramers are used directly to stain viable cells, and the results analysed by flow cytometry. There is little or no non-specific binding at temperatures of 4?37?C.
Address of full information and technical help
A detailed technical description is given by Casares et al [23] and Ogg [24].
An example
An example of FACS staining of a human HLA B27-restricted CTL line with an HLA B27 tetramer is shown in figure 1b.
Limitations
Individual MHC/peptide tetrameric complexes have to be produced separately, and production is both technically difficult and time consuming. These complexes have a limited storage stability. Flow cytometry equipment is required for analysis of tetrameric staining.
Future developments
MHC class I tetrameric complex technology is becoming an indispensable tool for studying T cell responses to both foreign and self antigens. New applications in the elucidation of mechanisms of tumour evasion and autoimmunity are rapidly being developed, which will be directly applicable to arthritis research. Examples might include the development of tetrameric-complex-based libraries to attempt to identify arthritogenic peptide antigens in spondyloarthritis. Tetrameric complexes tagged with toxins could be used to specifically target infected, autoimmune or malignant cells [23].
Conclusion
MHC class I tetrameric complex technology is proving to be a valuable tool that can be used to study antigen-specific T cell response in viral infection, tumour evasion and autoimmune disease. In the near future, MHC class I mutimers may have a role in immunotherapy; fighting against infections, autoimmune disease and cancer.
Abbreviations
CTL = cytotoxic T lymphocyte; EBV = Epstein-Barr virus; ELISPOT = enzyme-linked immunospot; FACS = fluorescence-activated cell sorting; HLA= human leukocyte antigen; IFN = interferon; LCMV = lymphocytic chorio meningitis virus; LDA = limiting dilution analysis; MHC = major histocompatibility complex; TCR = T-cell receptor.
References
- Kalams SA, Johnson RP, Trocha AK, Dynan MJ, Ngo HS, D'Aquila RT, Kurnick JT, Walker BD. Longitudinal analysis of T cell receptor (TCR) gene usage by human immunodeficiency virus 1 envelope-specific cytotoxic T lymphocyte clones reveals a limited TCR repertoire. J Exp Med. 1994;179:1261–1271. doi: 10.1084/jem.179.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss PA, Rowland-Jones SL, Frodsham PM, McAdam S, Giangrande P, McMichael AJ, Bell JI. Persistent high frequency of human immunodeficiency virus-specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- Kern F, Surel IP, Brock C, Freistedt B, Radtke H, Scheffold A, Blasczyk R, Reinke P, Schneider-Mergener J, Radbruch A, Walden P, Volk HD. T-cell epitope mapping by flow cytometry. Nat Med. 1998;4:975–978. doi: 10.1038/nm0898-975. [DOI] [PubMed] [Google Scholar]
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-Ek complexes: correlation of the dissociation rate with T-cell responsiveness. Proc Natl Acad Sci USA. 1994;91:12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ. T cell responses and viral escape. Cell. 1998;93:673–676. doi: 10.1016/s0092-8674(00)81428-2. [DOI] [PubMed] [Google Scholar]
- Dunbar PR, Chen JL, Chao D, Rust N, Teisserenc H, Ogg GS, Romero P, Weynants P, Cerundolo V. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J Immunol. 1999;162:6959–6962. [PubMed] [Google Scholar]
- Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: In situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Appay V, Nixon DF, Donahoe SM, Gillespie GM, Dong T, King A, Ogg GS, Spiegel HM, Conlon C, Spina CA, Havlir DV, Richman DD, Waters A, Easterbrook P, McMichael AJ, Rowland-Jones SL. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J Exp Med. 2000;192:63–75. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O'Callaghan CA, Steven N, McMichael AJ, Rickinson AB. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowness P, Allen RL, Barclay DN, Jones EY, McMichael AJ. Importance of a conserved TCR J alpha-encoded tyrosine for T cell recognition of an HLA B27/peptide complex. Eur J Immunol. 1998;28:2704–2713. doi: 10.1002/(SICI)1521-4141(199809)28:09<2704::AID-IMMU2704>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ugrinovic S, Mertz A, Wu P, Braun J, Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715–5723. [PubMed] [Google Scholar]
- Fiorillo MT, Maragno M, Butler R, Dupuis ML, Sorrentino R. CD8(+) T-cell autoreactivity to an HLA-B27-restricted self-epitope correlates with ankylosing spondylitis. J Clin Invest. 2000;106:47–53. doi: 10.1172/JCI9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LC, Mowat AG, Fazou C, Rostron T, Roskell H, Dunbar PR, Tournay C, Romagne F, Peyrat MA, Houssaint E, Bonneville M, Rickinson AB, McMichael AJ, Callan MF. Specificity of T cells in synovial fluid: high frequencies of CD8+ T cells that are specific for certain viral epitopes. Arthritis Research. 2000;2:154–164. doi: 10.1186/ar80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Kotzin BL, Falta MT, Crawford F, Rosloniec EF, Bill J, Marrack P, Kappler J. Use of soluble peptide-DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:291–296. doi: 10.1073/pnas.97.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casares S, Stan AC, Bona CA, Brumeanu TD. Antigen-specific downregulation of T cells by doxorubicin delivered through a recombinant MHII-peptide chimera. Nat Biotechnol. 2001;19:142–147. doi: 10.1038/84404. [DOI] [PubMed] [Google Scholar]
- Ogg GS. HLA-peptide tetrameric complexes. In Lymphocytes A Practical Approach Second edition Edited by Rowland-Jones SL, McMichael AJ Oxford: Oxford Univerity Press, 2000.


