Abstract
Osteoarthritis and osteoporosis are the two most common age-related chronic disorders of articular joints and skeleton, representing a major public health problem in most developed countries. Apart from being influenced by environmental factors, both disorders have a strong genetic component, and there is now considerable evidence from large population studies that these two disorders are inversely related. Thus, an accurate analysis of the genetic component of one of these two multifactorial diseases may provide data of interest for the other. However, the existence of confounding factors must always be borne in mind in interpreting the genetic analysis. In addition, each patient must be given an accurate clinical evaluation, including family history, history of drug treatments, lifestyle, and environment, in order to reduce the background bias. Here, we review the impact of recent work in molecular genetics suggesting that powerful molecular biology techniques will soon make possible both a rapid accumulation of data on the genetics of both disorders and the development of novel diagnostic, prognostic, and therapeutic approaches.
Keywords: candidate genes, genetics, multifactorial diseases, osteoporosis, osteoarthritis
Background
Osteoarthritis (OA) and osteoporosis (OP) are two common age-related chronic disorders of the skeleton with a complex, multifactorial pathogenesis. They are both associated with considerable morbidity and mortality. Apart from being influenced by environmental factors, OA and OP have a strong genetic component, as has been shown by twin and family studies [1,2,3,4]. Although in clinical practice, a combination of OP and OA may be coincidentally encountered, particularly in the very elderly, there is now considerable evidence from large population studies that these two disorders are inversely related [5,6,7,8]. While low bone mineral density (BMD) is an essential feature of OP, an increased BMD has been often shown in subjects with OA. Moreover, if OA patients develop osteoporotic fractures, they do so in very old age, suggesting that OA, or related factors, might have a protective effect on the progression of OP [7]. Patients with primary OP and those with OA also appear to represent anthropometrically different populations. The typical patient with OA tends to be a mesomorph, to be fatter, and to have greater muscular strength, whereas the typical OP patient tends to be an ectomorph [8]. Because of the contrasting levels of BMD in OA and OP, studies on the pathophysiology of OA may also provide some insight into the understanding of OP. In particular, the knowledge of the genetics of OA could benefit from characterization of genetic markers linked to OP risk, and vice versa. A recent co-twin control study suggested that the generalized increase in BMD observed in subjects with OA may be due in part to shared genetic factors in hip OA and high bone mass [9]. It is therefore possible that individuals may be genetically predisposed to be 'bone formers', with a higher BMD, a lower chance of osteoporotic fracture, and a greater tendency to develop OA, or 'bone losers', with a higher age-related bone loss and a lower tendency to form osteophytes. Potential candidate genes for OA and OP are listed in Table 1.
Table 1.
Potential candidate genes common to osteoporosis and osteoarthritis
| Adhesion molecules and ligands (e.g. integrins) |
| Cartilage and bone matrix proteins |
| Collagenic |
| Noncollagenic |
| Calciotropic hormones and their receptors |
| Calcitonin and calcitonin receptor |
| Vitamin D and vitamin D receptor |
| PTH and PTH receptors |
| Calcium-sensing receptor |
| Cytokines, growth factors, and their receptors (IL-6, IL-1, IGF1, etc) |
| Enzymes (aromatase, metalloproteinases, etc) |
| Sex hormones and their receptors |
| Androgen and androgen receptor |
| Estrogen and estrogen receptors |
IGF, insulin-like growth factor; IL, interleukin; PTH, parathyroid hormone.
Genetic markers in primary generalized osteoarthritis
OA is a heterogeneous cluster of diseases sharing cartilage involvement as the main feature [10]. Bone may be also affected, with the formation of osteophytes and sclerotic areas. OA is classified as idiopathic and secondary [11]. Several reports suggest that genetic influences contribute considerably to the development of OA [12]. The concept of hereditary OA has been defined as an entity encompassing primary generalized OA, familial chondrodysplasias, and familial crystal deposition disease [13]. However, the relevance of the genetic component varies among subgroups of patients, and as yet it is not clear which genes are involved. Families with primary generalized OA (PGOA) exhibit a higher incidence of OA than is seen in in the general population, with premature development of Heberden's and Bouchard's nodes and rapid cartilage degeneration at multiple joints [14]. Early family studies suggested that first-degree relatives of PGOA probands were twice as likely to have radiographically visible generalized disease as were population controls [15]. Recently, a twin study of 120 nonidentical and 130 identical female twins, examined for radiological evidence of OA, showed a striking genetic influence on the development of PGOA, with a score of 40 to 70% for an effect of hereditability [2]. This result has been recently confirmed by evidence that osteoarthritis of the hand, knee, and hip, and disc degeneration of the spine, is statistically more frequent in sibling studies [16].
The cluster of familial chondrodysplasias, which are inherited as an autosomal Mendelian trait, is characterized by induction of precocious cartilage destruction with consequent OA. Several mutations in genes encoding the components of cartilaginous extracellular matrix have been described [13]. Conversely, the multifactorial nature of PGOA and the heterogeneity that characterizes the syndrome greatly complicate the choice of putative candidate genes. Moreover, there is now substantial evidence from epidemiological, twin, and segregation studies that the genetic contribution to osteoarthritis is gender- and joint-related [17,18,19,20,21]. In PGOA, identification of genes that could lead to development of the disease is still under investigation.
Studies of collagen genes
Mutations of the COL2A1 gene have been identified in familial chondrodysplasias [13,22]. This gene also seems to be involved both in early-onset PGOA [23,24,25] and in families with crystal deposition disorder [26]. However, linkage analysis of 14 candidate genes in OA kindreds resulted in the exclusion of 10 important genes, including COL2A1 [27]. Moreover, both PGOA and familial crystal deposition disease have been related to a region of chromosome 8q [28], while nodal OA appeared significantly associated with loci on chromosome 2q23-35, where the gene encoding the a3-chain of collagen type VI is located [29]. Recently, various other chromosomal loci have reported to be associated with OA [30,31,32,33,34,35], as summarized in Table 2.
Table 2.
Quantitative trait loci (QTL) associated with osteoarthritis
| Region | |||
| Reference | QTL | affected | Phenotype |
| [28] | 8q | GOA | Early-onset OA-CPDD (1 family) |
| [29] | 2q23?35 | Hand | Nodal OA |
| [30] | 11q | Hip, knee | Female OA |
| [31] | 2q | Hip, knee | OA of the hip |
| 4q | Female OA of the hip | ||
| 6p/6q | OA of the hip | ||
| 11q | Female OA | ||
| 16p/16q | Female OA of the hip | ||
| [32] | 2q12?13 | Hand | Distal interphalangeal joint OA |
| 4q26?27 | Distal interphalangeal joint OA | ||
| 7p15?21 | Distal interphalangeal joint OA | ||
| X-cen | Distal interphalangeal joint OA | ||
| [33] | 4q35 | Hip | Premature degenerative OA of the hip |
| [34] | 6q12-13 | Hip, knee | Female OA of the hip |
| 6p21.3 | Female OA of the hip | ||
| [35] | 2q31 | Hip, knee | Familial OA of the hip |
CPDD = calcium pyrophosphate deposition disease; GOA = generalized osteoarthritis; OA = osteoarthritis.
Studies of estrogen receptor genes
Evidence that PGOA is becoming apparent in postmenopausal women [36,37] prompted investigations on the role of genes encoding for estrogen receptors (ERs). In a Japanese study, a restriction-fragment-length polymorphism at the ERa gene locus appeared to associate significantly with PGOA [38], although studies in other populations failed to confirm this association [39].
Studies of the gene for vitamin D receptor
The association of polymorphism of the gene for vitamin D receptor (VDR) with BMD [40] was followed by investigations of this gene's possible association with OA. VDR gene polymorphisms segregated significantly (showing a 2.27-fold increased relative risk) with the presence of osteophytes in knee OA [41,42] and in the spine [43]. The genetic association is substantial: subjects with the VDR allelic variant TT have a 50?60% lower risk of spinal osteophytosis and disc narrowing than the opposite (tt) genotype [43]. To date, results do not allow us to distinguish the associations between VDR and osteophytes or between VDR and disc narrowing. Polymorphisms of this receptor might directly affect the pathophysiology of OA by promoting either osteophytosis or disc narrowing. VDR is expressed in both osteoblasts and chondrocytes, both of which are found in osteophytes, suggesting a role for the vitamin D/VDR complex in the formation or progression of osteophytes, or both. It has also been hypothesized that polymorphisms in COL2A1, one of the major candidate genes for familial OA, are in linkage disequilibrium with VDR gene polymorphisms [41].
Studies of other candidate genes
In recent years, new polymorphisms in other candidate genes, such as IGFI [44], COL1A1 [39], COL2A1 [45,46,47], TGFB1 [48], and the gene for aggrecan proteoglycan [49], have been identified and found to be correlated with OA in some studies, although agreement is not universal [39,50,51]. An updated list of candidate genetic polymorphisms associated with osteoarthritis is reported in Table 3.
Table 3.
Candidate gene polymorphisms associated with osteoarthritis
| Genetic | Association | ||
| polymorphism | Phenotype | found? | Reference |
| VDR | Female knee OA | Yes | [42] |
| Knee OA (osteophytosis) | Yes | [41] | |
| Female OA (hip replacement) | No | [50] | |
| Hand, hip, knee OA | No | [51] | |
| Idiopathic OA | No | [39] | |
| COL2A1 | PGOA/chondrodysplasia | Yes | [140] |
| Nodal GOA | No | [141] | |
| GOA, finger joints OA | No | [24] | |
| GOA | Yes | [45] | |
| Female OA (hip replacement) | No | [50] | |
| GOA | Yes | [46] | |
| Knee OA (joint space narrowing) | Yes | [47] | |
| COL1A1 | Female OA (hip replacement) | No | [50] |
| Idiopathic female OA | Yes | [39] | |
| ERa | GOA | Yes | [38] |
| Idiopathic OA | No | [39] | |
| TGFB1 | Spine OA (osteophytosis) | Yes | [48] |
| IGF-I | GOA | Yes | [44] |
| Aggrecan proteoglycan | Male bilateral hand OA | Yes | [49] |
OA = osteoarthritis; COL = collagen; ER = estrogen receptor; GOA = generalized osteoarthritis; IGF = insulin-like growth factor; PGOA = primary generalized osteoarthritis; TGF = transforming growth factor.
Gene?environment interaction
Finally, a strong interaction between genes and environment plays an important role, because increasing age and body mass index are known to be associated with increased prevalence and severity of spinal degenerative disease, as are smoking and quadriceps strength for osteophytosis. Moreover, joint degeneration in the early stages of OA may be reflected in changes in structural and material properties of the articular cartilage. A recent study showed that for a given loading condition, the contact areas are higher and peak stresses are lower in a diseased joint than in a normal one [52]. Thus, loading stress conditions may play a critical role in the selection of 'genetically' susceptible joints.
OA has wide variability, both clinically and radiologically. The identification of gene(s) linked to PGOA might make it possible to construct a new OA classification based on genetic causes, independent of clinical or radiological features, to develop molecular tests for definition of OA risk, and to design a preventive therapeutic strategy based on gene therapy [53], as has already been done successfully by gene transfer of interleukin-1 receptor antagonist in the animal model [54].
Animal model
Very recently, an elegant study in mice demonstrated that mutation at the progressive ankylosis (ank) locus, mapped to proximal mouse chromosome 15, causes a generalized progressive form of arthritis with mineral deposition, formation of bony outgrowths, and joint destruction. Interestingly, the human orthologue of the ank gene, ANK, is nearly identical to the mouse gene and maps to chromosome 5p in a region showed to be linked in several human pedigrees with arthritis and chondrocalcinosis [55,56].
Genetic markers in osteoporosis
Osteoporosis is a systemic multifactorial disease characterized by decreased BMD and microarchitectural deterioration of bone structure, leading to a higher susceptibility to fractures [57]. Although there are several environmental influences on BMD, such as diet and amount of physical exercise, a genetic contribution to the pathogenesis of OP accounting for 50% to 70% of the interindividual variability in bone mass has been recognized [3,4]. Given the complex biology of the skeleton, it is likely that bone mass is under the control of a large number of genes, many of which exert relatively small effects on BMD and a few of which contribute substantially to the variation in this trait. It is also likely that complex gene?environment interactions exist. Many candidate genes have been implicated in the determination of BMD and in the pathogenesis of OP, including those encoding cytokines, calciotropic hormones and their receptors, and matrix bone proteins (see Table 1).
To date, among the genetic strategies commonly employed for the dissection of complex traits, the analysis of the genetic determinants of BMD has largely relied on association studies, in which a polymorphism in a candidate gene is analyzed in unrelated affected and unaffected individuals from a given population. However, there are some pitfalls for such an approach in late-onset disorders such as OP, mainly due to inappropriate choice of the control group, to population admixture, and to competing risk leading to selection bias [58]. Moreover, a positive association can arise for any of three reasons: a given allele might in effect be a cause of the disease; or it might not cause the trait but be in linkage disequilibrium with the actual cause; or the apparent association might be an artifact of population admixture.
Studies of vitamin D receptor gene
Among the several candidate genes, that encoding VDR was the first to be proposed as a major locus for the genetic effect on bone mass. The VDR gene possesses several polymorphic sites, of which that detected by the restriction endonuclease BsmI at intron 8 was associated with BMD in the Australian population [40]. Since that original report, conflicting data have been published on the association of the diallelic BsmI restriction-fragment-length polymorphism (RFLP) with the VDR gene and BMD in both premenopausal [59,60,61,62] and postmenopausal [63,64,65,66,67,68] women. Similarly, studies examining the relation of this polymorphism with skeletal growth [69,70,71,72], bone-turnover markers [59,63,73,74], rates of bone loss [63, 74,75,76], intestinal calcium absorption [74,77,78,79,80], and osteoporotic fractures [81,82,83] yielded conflicting results. A meta-analytical approach incorporating the results from 16 studies revealed a weak contribution of the allelic variant at the 3' end of the gene to the variation of BMD values [84], while a more recent meta-analysis concluded that BMD is associated with VDR polymorphism at high confidence levels and that both genetic and nongenetic factors can interfere with the unmasking of the effects of VDR variants on bone phenotype [85].
There are several possible explanations for the discrepancies among these studies. First, interactions of environmental factors such as dietary calcium intake appeared to represent an important confounding factor [72,78,79, 86,87,88]. Moreover, linkage disequilibrium with other bone-metabolism-related genes on chromosome 12 (i.e. collagen type 1 and retinoic acid receptor genes) cannot be excluded. Finally, the limited sizes of samples, differences in genotype distribution among different ethnic groups, and interactions with other genes all have to be considered as potential confounders. Other polymorphic genes, such as the one encoding ERa, have been shown to modulate the effect of the VDR gene in the determination of BMD, confirming the existence of gene?gene interaction [67,89]. Taken together, these findings may help to explain contrasting data among published studies, suggesting the possibility of modifying genetically determined BMD through appropriate lifestyle changes. However, polymorphisms at the 3' end of the VDR gene are anonymous polymorphisms, as they do not code for different amino acids in the VDR protein. Therefore, a major question is how these allelic differences may be related to functional differences. Current evidence suggests that these VDR restriction-fragment-length polymorphisms do not affect the abundance of VDR mRNA [90,91,92]. Recently, a new diallelic (ATG/ACG) polymorphic VDR variant has been described in exon 2 of the gene, detectable with the restriction endonuclease FokI [93]. This polymorphism is responsible for a three-amino-acid difference in VDR length between FF and ff individuals and the short form of the VDR gene (FF) gave an approximately 1.7-fold increase in transcription activation in transfected HeLa cells [94]. Mexican?American postmenopausal women with the ff genotype showed lower lumbar BMD than those with the FF genotype [93]. This relation, also found in Japanese [94], North American [95], and Italian [96] populations, was not found in French [97] and Swiss [98] women, although a significant association of this genotype with differences in urinary type I collagen cross-linked n-telopeptide was observed in the French population [97].
Studies of estrogen receptor genes
The importance of the ER genes in the determination of BMD is supported by several observations. Firstly, osteoblasts, osteoclasts, and bone marrow stromal cells bear ERs and are modulated by estrogen [99,100]. In addition, a homozygotic inactivating mutation of the ERa gene caused OP in a male patient [101]. Finally, ERa knockout mice exhibit a low BMD [102]. It is possible that common allelic variants of the ERa gene cause milder estrogen resistance, which becomes evident with aging or with menopausal hypogonadism, leading to clinical disorders such as OP. Both intronic polymorphisms (recognized by the restriction endonucleases PvuII and XbaI) and polymorphic variable numbers of (TA)n repeats upstream of the ERa have been associated with BMD in Japanese populations [103,104]. Similar studies in other populations yielded conflicting results [67,89,105,106,107]. Recently, we investigated the role of these polymorphisms at the ERa gene locus in a large sample of postmenopausal Italian women [108]. We found a strong linkage disequilibrium between intron 1 (PvuII and XbaI) polymorphic sites and also between these sites and the microsatellite (TA)n dinucleotide repeat polymorphism, with a high degree of coincidence of the short TA alleles and the presence of PvuII and XbaI restriction sites. Interestingly, a statistically significant correlation between the (TA)n repeat allelic variants and osteoporosis was observed, with subjects with a low number of repeats (TA<15) showing the lowest BMD values and the highest risk of vertebral fracture. Two studies, in American and Danish populations, recently confirmed this observation [109,110]. However, in another study in a Scottish population, no overall association between the TA repeat number and BMD was observed [111]. All the positive studies are concordant and demonstrate a significant association between reduced BMD values and the presence of a low number of TA repeats. Conversely, in the Scottish study, the small group of subjects with the highest number of TA repeats (having at least one allele TA= 26) appeared to have lower BMD values at the spine than those with fewer TA repeats [111]. The molecular mechanism underlying how bone mineralization is affected by the variation in the number of dinucleotide repeats is still unclear. However, because of the TA repeats position, between promoters A and B of the ERa gene and next to a regulatory region, it is possible that allelic variation due to different (TA)n dinucleotide repeat lengths might have physiological relevance by affecting promoter usage and/or mRNA transcription.
Studies of collagen genes
Collagen type I is the major constituent of bone matrix proteins and, therefore, collagen type I genes (COL1A1 and COL1A2) have been proposed as candidates for the determination of bone mass. Indeed, the osteoporotic phenotype of osteogenesis imperfecta is due to mutations that affect the coding regions of collagen type I genes [112]. Recently, Grant and colleagues showed that a G/T polymorphism in the first intron of COL1A1 strongly segregated with BMD and osteoporotic fractures [113]. Additional data in larger samples of different populations support these findings [114,115,116]. A recent cross-section large-scale study indicates that the unfavorable COL1A1 allele (the T variant, arbitrarily called the 's' allele) acts as a marker for accelerated age-related bone loss rather than a marker for lower peak bone mass [117]. However, a small study in a Finnish population [118] showed no significant association of COL1A1 Sp1 polymorphism with bone mass or fracture, nor did another study in twins in the USA [119]. Sp1 is a transcription factor. To date, the molecular mechanisms by which the described COL1A1 Sp1 diallelic polymorphism associates with bone mass are currently unclear. Preliminary data have recently supported evidence of allele-specific differences in binding of the Sp1 protein to the polymorphic recognition site, in collagen protein production and in bone strength in samples derived from patients with different genotypes [120].
Other studies of candidate genes and linkage analysis
Polymorphisms of other candidate genes such as those for interleukin-6 [121], transforming growth factor-? [122], apolipoprotein E [123], calcitonin receptor [124,125], androgen receptor [109], and osteocalcin [109] have been related to BMD in some isolated studies. These observations have not yet been confirmed by other independent studies; certainly other genes, with as great or even greater effects both on BMD and bone metabolism, have yet to be mapped and identified. An updated overview of candidate genes related to BMD and osteoporotic risk is depicted in Fig. 1.
Figure 1.
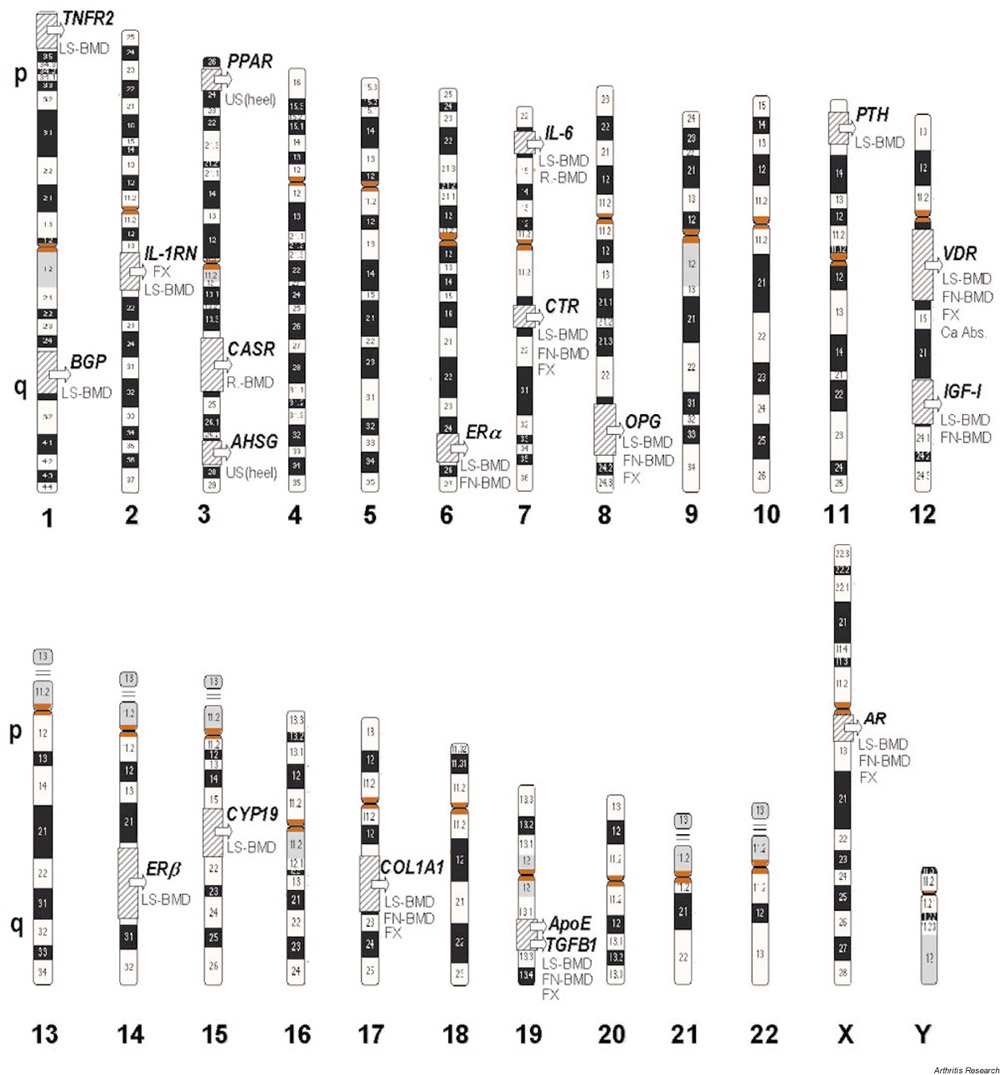
Genetics of osteoporosis: candidate genes. Numbers at the bottom are chromosome numbers. p, q = short and long arms, respectively, of the chromosome.
The absence of a clear Mendelian pattern of inheritance (at least for a subset of cases) makes it extremely difficult, if not impossible, to determine a priori the number of involved genes and their effects on the trait of interest (i.e. peak bone mass, BMD, rates of bone loss) [72]. A study in 22 French families unraveled an autosomal dominant pattern of transmission for BMD [126]. However, in the French study and in general, the term 'familial osteoporosis' is lacking a clinical definition, because of the difficulty of separating genetic from environmental factors. Criteria for definition and selection of osteoporotic kindreds are therefore essential. One possibility could be to focus on subsets of kindreds showing a clear family history of low BMD/OP and of characteristics that make the pedigree 'interesting'. Some families with apparently transmissible osteoporosis also exhibit clinical evidence of connective tissue dysplasia, with no clinical or biochemical evidence of osteogenesis imperfecta or Ehlers-Danlos syndrome [127]. This sign can itself become a hallmark for definition of the 'patients' within the kindred. The a priori chance of success for linkage studies in a family is increased by the analysis of multiple generations (a minimum of three generations could be the cut-off) exhibiting a pattern of inheritance with high penetrance. The need for at least four affected (i.e. having low BMD and osteoporosis) members in multiple generations, including males, will help to narrow the definition of an 'interesting' pedigree as a pedigree that constitutes a rare subset of a common phenotype. Linkage studies in man and experimental animals suggested the existence of multiple loci regulating bone mass, but the genes that account for such effects remain to be defined. Linkage analysis for chromosome 11q12?13 polymorphic loci indicated the possible existence of a candidate gene or genes in this region that may play an important role in the variation of BMD in a normal population [128]. Linkage studies in sib-pairs were able to define other loci controlling the BMD on different human chromosomes in different populations [129,130,131]. To date, traditional linkage analysis has been successfully used to find major contributory genes but has limited power to detect genes with only a modest effect. In the latter case, different approaches, such as nonparametric allele-sharing methods (i.e. affected sib-pair analysis, linkage disequilibrium, and transmission/disequilibrium testing) have far greater power [132,133]. In this respect, recent observations have revealed a few chromosomal regions containing genes (quantitative trait loci) modulating the BMD [128,129,130,134,135], as shown in Table 4. Recruitment of a large number of sib-pairs would be valuable for doing linkage studies of haplotype sharing and transmission/disequilibrium tests in humans [136]. Affected relatives should show excess allele sharing even in the presence of incomplete penetrance, phenocopy, genetic heterogeneity, and a high frequency of disease alleles [132]. Nonparametric linkage approaches testing multiple candidate genes in large pedigrees could also provide interesting information. Preliminary data from such a study showed a suggestive linkage of the parathyroid hormone receptor type 1 to osteoporosis [131]. A limiting factor in linkage analysis of multiple candidate genes is the lack of accurate statistical software to clearly define the threshold of significance.
Table 4.
Quantitative trait loci (QTL) associated with BMD or osteoporosis
| Genetic | |||
| Reference | QTL | analysis | Phenotype |
| [135] | 11q12?13 | Linkage | High bone mass |
| [129] | 1p36 | ||
| 2p23?24 | |||
| 4qter | |||
| 11q | Linkage | Low BMD | |
| [128] | 11q12?13 | Sib-pairs | Femoral neck BMD |
| [130] | 2p | ||
| 13q | Sib-pairs | Proximal and distal forearm BMD | |
| [134] | 1q21?23 | Sib-pairs | Lumbar or femoral BMD |
| 5q33?35 | |||
| 6p11?12 | |||
| 11q12?13 |
BMD = bone mineral density.
Information derived from cross-sectional association studies could offer potential starting points, although a complete genomic screening with high-resolution linkage maps and regional follow-up by additional markers could not be excluded.
Animal models
Comparative genetics could add information about potentially interesting genes in humans once quantitative trait loci in animal models (i.e. rodents, primates) are identified [137]. Very recently, an autosomal recessive mutation at locus sfx, mapped to central chromosome 14, was found to segregate with stage-specific bone growth failure and fracture in a new mouse model, designated spontaneous fracture (sfx) [138]. Fine mapping of this chromosomal region could define the role of this gene in the pathophysiology of the skeleton and could provide evidence of other genes co-localizing with sfx.
Together, these efforts will make it possible to map unknown OP-related genes to defined chromosomal regions, to clone them, and to identify their function.
Conclusions
OP and OA affects hundreds of millions of people throughout the world, causing pain and disability and having a great impact on individuals and on society as whole. There is evidence that the two disorders are often inversely correlated and that they have a complex genetic component. The identification of the genetic pathways involved is difficult and represents a great challenge in the near future. As in other multifactorial diseases (such as hypertension and diabetes), in both OA and OP, the initiation, progression, and severity of the disease may be influenced by multiple environmental factors with multiple genes in a given individual. The authors of some association studies have suggested the possibility that a given allelic variant in a candidate gene (i.e. VDR) may increase the risk for OP and be protective for OA, and vice versa [40,41,42,43,47,60,61,67]. However, this intriguing hypothesis remains to be confirmed in larger samples, in different populations, and by other genetic approaches. Moreover, we must take into account that the inverse correlation between OP and OA observed in several epidemiological reports may have other, nongenetic, components. Indeed, it is known that increased physical loading due to enhanced weight-bearing activity is protective for OP but seems to confer a higher risk of developing OA in the elicited joint structures.
Several large-scale investigations now under way, involving thousands of patients and genome-wide screening, may make it possible to identify multiple gene variations associated with an increased risk for OA and/or OP. However, the importance of genetic heterogeneity, including ethnicity, as well as of environmental, hormonal, and constitutional confounders (e.g. skeletal and body size) will need to be taken into serious account in future genetic studies. Gene?gene and gene?environment interactions and interactions between pharmaceuticals and the genome in humans and animal models will be critical targets for future research. Further developments in molecular genetics, such as microarray chips, will allow simultaneous large-scale differential identification of thousands of genetic polymorphisms segregating with OA or OP or both [139]. All these efforts will improve our understanding of the pathogenesis of these two disabling disorders, making possible earlier preventive strategies as well as the development of more appropriate and effective treatment options.
Abbreviations
BMD = bone mineral density; COL = collagen; ER = estrogen receptor; OA = osteoarthritis; OP= osteoporosis; PGOA = primary generalized osteoarthritis; VDR = vitamin D receptor.
Acknowledgments
Acknowledgements
This work was performed with support from the Italian National Health System Project 'Human Exposure to Xenobiotics with Potential Endocrine Activities: Evaluation of the Risks for Reproduction and Development' (2000) and of the Italian National Health System Project 'Environmental Risk of Postmenopausal Diseases' (2000) and was supported by grants Cofin Murst 1999 to ML Brandi, ex 60% 1999 'Genetics of Osteoporosis' to ML Brandi and Telethon 1999–2000 to ML Brandi.
References
- Allison AC, Bluberg BS. Familial osteoarthropathy of the fingers. J Bone Joint Surg. 1958;40B:538–540. doi: 10.1302/0301-620X.40B3.538. [DOI] [PubMed] [Google Scholar]
- Spector TD, Cicutini F, Baker J, Loughlin JA, Hart DJ. Genetic influences on osteoarthritis in females: a twin study. BMJ. 1996;312:940–943. doi: 10.1136/bmj.312.7036.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock NA, Lisman IA, Hopper I, Yeates MG, Sambrook PN, Eber S. Genetic determinants of bone mass in adults. J Clin Invest. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Hooper JL, Bach LA, Cooper ME, Parkinson E, McKay J, Jeremus C. Reduced bone mass in daughters of women with osteoporosis. N Engl J Med. 1989;320:554–558. doi: 10.1056/NEJM198903023200903. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Klineberg RJ. Epidemiological study of the relationship between arthritis of the hip and hip fractures. Ann Rheum Dis. 1993;52:707–710. doi: 10.1136/ard.52.10.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dequeker J, Boonen S, Aerssens J, Westhovens R. Inverse relationship osteoarthritis-osteoporosis: what is the evidence? What are the consequences? Br J Rheumatol. 1996;35:813–820. doi: 10.1093/rheumatology/35.9.813. [DOI] [PubMed] [Google Scholar]
- Verstaeten A, Van Ermen H, Haghebaert G, Nijs J, Geusens P, Dequeker J. Osteoarthritis retards the development of osteoporosis. Observation of the coexistence of osteoarthrosis and osteoporosis. Clin Orthop. 1991;264:169–177. [PubMed] [Google Scholar]
- Dequeker J, Goris P, Uytterhoeven R. Osteoporosis and osteoarthritis (osteoarthrosis): anthropometric distinctions. JAMA. 1983;249:1448–1451. [PubMed] [Google Scholar]
- Antoniades L, MacGregor AJ, Matson M, Spector TD. A co-twin control study of the relationship between hip osteoarthritis and bone mineral density. Arthritis Rheum. 2000;43:1450–1455. doi: 10.1002/1529-0131(200007)43:7<1450::AID-ANR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Keuttner K, Goldberg VM. Osteoarthritis Disorders Rosemont, PA: American Academy of Orthopedic Surgeons. 1995. pp. 21–25.
- Brandt KD, Mankin HJ, Shulman LE. Workshop on etiopathogenesis of osteoarthritis. J Rheumatol. 1986;13:1126–1160. [Google Scholar]
- Holderbaum D, Haqqi TM, Moskowitz RW. Genetics and osteoarthritis. Exposing the iceberg. Arthritis Rheum. 1999;42:397–405. doi: 10.1002/1529-0131(199904)42:3<397::AID-ANR1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Jimenez SA, Williams CJ, Karasick D. Hereditary osteoarthritis. In Osteoarthritis Edited by Brandt KD, Doherty M, Lohmander LS Oxford: Oxford University Press, 1988. pp. 31–49.
- Kellgren JH, Moore R. Generalized osteoarthritis and Heberden's nodes. BMJ. 1952;1:181–187. doi: 10.1136/bmj.1.4751.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS, Bier F. Genetic factors in generalized osteoarthritis. Ann Rheum Dis. 1963;22:237–255. doi: 10.1136/ard.22.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijkerk C, Houwing-Duistermaat JJ, Valkenburg HA, Meulenbelt T, Hofman A, Breedveld FC, Pols HA, van Duijn CM, Slagboom PE. Heritabilities of radiologic osteoarthritis in peripheral joints and of disc degeneration of the spine. Arthritis Rheum. 1999;42:1729–1735. doi: 10.1002/1529-0131(199908)42:8<1729::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Lindberg H. Prevalence of primary coxoarthrosis in siblings of patients with primary osteoarthrosis. Clin Orthop. 1986;203:273–275. [PubMed] [Google Scholar]
- Cooper C, McAlindon T, Snow S, Vines K, Young P, Kirwan J, Dieppe P. Mechanical and constitutional risk factors for symptomatic knee osteoarthritis: differences between medial tibiofemoral and patello-femoral disease. J Rheumatol. 1994;21:307–313. [PubMed] [Google Scholar]
- Kaprio J, Kujala UM, Peltonen L, Koskenvuo M. Genetic liability to osteoarthritis may be greater in women than in men. BMJ. 1996;313:232. doi: 10.1136/bmj.313.7051.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnavis J, Sinsheimer JS, Clipsham K, Loughlin J, Sykes B, Burge PD, Carr AJ. Genetic influences in end-stage osteoarthritis: sibling risks of hip and knee replacement for idiopathic osteoarthritis. J Bone Joint Surg (Br) 1997;79:660–664. doi: 10.1302/0301-620x.79b4.7437. [DOI] [PubMed] [Google Scholar]
- Felson DT, Couropmitree NN, Chaisson CE, Hannan MT, Zhang Y, McAlindon TE, La Valley M, Levy D, Myers RH. Evidence for a Mendelian gene in segregation analysis of generalized radiographic osteoarthritis. Arthritis Rheum. 1998;41:1064–1071. doi: 10.1002/1529-0131(199806)41:6<1064::AID-ART13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Jimenez SA. Heritable diseases of cartilage caused by mutations in collagen gene. J Rheumatol. 1995;43 (suppl):28–33. [PubMed] [Google Scholar]
- Palotie A, Vaisanen P, Ott J, Ryhanen L, Elima K, Vikkula M. Predisposition to familial osteoarthritis linked to type II collagen gene. Lancet. 1988;i:924–927. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- Vikkula M, Nissila M, Hirvensala E, Nuotio P, Palotie A, Aho K, Peltonen L. Multiallelic polymorphism of the cartilage gene: no association with osteoarthritis. Ann Rheum Dis. 1993;52:762–764. doi: 10.1136/ard.52.10.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivtaniemi P, Korkko J, Bonaventure J, Vikkula M, Hyland J, Paasilta P. Identification of COL2A1 mutations in patients with chondrodysplasias and familial osteoarthritis. Arthritis Rheum. 1995;38:999–1004. doi: 10.1002/art.1780380717. [DOI] [PubMed] [Google Scholar]
- Loughlin J, Irven C, Fergusson C, Sykes B. Sibling pair analysis shown no linkage of generalized osteoarthritis to the loci encoding type II collagen link protein or cartilage matrix protein. Br J Rheumatol. 1994;33:1103–1106. doi: 10.1093/rheumatology/33.12.1103. [DOI] [PubMed] [Google Scholar]
- Meulabent I, Bijkerk C, Breedveld FC, Slagboom PE. Genetic linkage analysis of 14 gene loci in a family with autosomal dominant osteoarthritis without dysplasia. J Med Gen. 1997;34:1024–1027. doi: 10.1136/jmg.34.12.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CT, Farrar LA, Dharmavaram R, Jimenez SA, Anderson Linkage of early onset osteoarthritis and chondrocalcinosis to human chromosome 8q. Am J Hum Genet. 1995;56:692–697. [PMC free article] [PubMed] [Google Scholar]
- Wright GD, Hughes AE, Regan M, Doherty M. Association of two loci on chromosome 2q with nodal osteoarthritis. Ann Rheum Dis. 1996;55:317–319. doi: 10.1136/ard.55.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K, Moustafa Z, Irven C, Carr AJ, Clipsham K, Smith A, Chitnavis J, Sinsheimer JS, Bloomfield VA, McCartney M, Cox O, Cardon LR, Sykes B, Loughlin J. Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet. 1999;65:167–174. doi: 10.1086/302465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin J, Moustafa Z, Irven C, Smith A, Carr AJ, Sykes B, Chapman K. Stratification analysis of an osteoarthritis genome screen-suggestive linkage to chromosomes 4, 6, and 16. Am J Hum Genet. 1999;65:1795–1798. doi: 10.1086/302685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppavouri J, Kujala U, Kinnunen J, Kaprio J, Nissila M, Heliovaara M, Klinger N, Partanen J, Terwilliger JD, Peltonen L. Genome scan for predisposing loci for distal interfalangeal joint osteoarthritis: evidence for a locus on 2q. Am J Hum Genet. 1999;65:1060–1067. doi: 10.1086/302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby P, Eyre S, Worthington J, Ramesar R, Cilliers H, Beighton P, Grant M, Wallis G. Autosomal dominant (Beukes) premature degenerative osteoarthropathy of the hip joint maps to an 11-cM region on chromosome 4q35. Am J Hum Genet. 1999;64:904–908. doi: 10.1086/302291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa Z, Chapman K, Irven C, Carr AJ, Clipsham K, Chitnavis J, Sinsheimer JS, Bloomfield VA, McCartney M, Cox O, Sykes Y, Loughlin J. Linkage analysis of candidate genes as susceptibility loci for osteoarthritis-suggestive linkage of COL9A1 to female hip osteoarthritis. Rheumatology. 2000;39:299–306. doi: 10.1093/rheumatology/39.3.299. [DOI] [PubMed] [Google Scholar]
- Loughlin J, Moustafa Z, Smith A, Irven C, Carr AJ, Clipsham K, Chitnavis J, Bloomfield VA, McCarney M, Cox O, Sinsheimer JS, Sykes B, Chapman K. Linkage analysis of chromosome 2q in osteoarthritis. Rheumatology. 2000;39:377–381. doi: 10.1093/rheumatology/39.4.377. [DOI] [PubMed] [Google Scholar]
- Stecher RM, Beard EE, Hersh AH. The relationship of the menopause to degenerative joint disease of the fingers. J Lab Clin Med. 1949;34-32:1193–1229. [PubMed] [Google Scholar]
- Spector TD, Campion GD. Generalized osteoarthritis: a hormonally mediated disease. Ann Rheum Dis. 1989;48:523–527. doi: 10.1136/ard.48.6.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiyama T, Ueyama H, Inoue K, Nisshioka J, Onkubo I, Hukuda S. Estrogen receptor gene polymorphism and generalized osteoarthritis. J Rheumatol. 1998;25:134–137. [PubMed] [Google Scholar]
- Loughlin J, Sinsheimer JS, Mustafa Z, Carr AJ, Clipsham K, Bloomfield VA, Chitnavis J, Bailey A, Sykes B, Chapman K. Association of the vitamin D receptor gene, the type I collagen gene COL1A1, and the estrogen receptor gene in idiopathic osteoarthritis. J Rheumatol. 2000;27:779–784. [PubMed] [Google Scholar]
- Morrison NA, Cheng JQI, Akifumi T, Kelly PJ, Nguyen TV, Sambrook PN, Eisman JA. Prediction of bone density from vitamin D receptor allele. Nature. 1994;367:284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Burger H, Huang Q, Odding E, Van Duijn CM, Hofman A, Birkenhager JC, Van Leeuwen JPTM, Pols HAP. Vitamin D receptor genotype is associated with radiographic osteoarthritis at the knee. J Clin Invest. 1997;100:259–263. doi: 10.1172/JCI119530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen RW, Hart DJ, Lanchbury JS, Spector TD. Association of early osteoarthritis of the knee with a Taq I polymorphism of the vitamin D receptor gene. Arthritis Rheum. 1997;40:1444–1449. doi: 10.1002/art.1780400812. [DOI] [PubMed] [Google Scholar]
- Jones G, White C, Sambrook P, Eisman J. Allelic variation in the vitamin D receptor, lifestyle factors and lumbar spinal degenerative disease. Ann Rheum Dis. 1998;57:94–99. doi: 10.1136/ard.57.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbelt I, Bijkerk C, Miedema HS, Breedveld FC, Hofman A, Valkenburg HA, Pols HAP, Slagboom PE, van Duijn CM. A genetic association study of the IGF-I gene and radiological osteoarthritis in a population-based cohort study (the Rotterdam study). Ann Rheum Dis. 1998;57:371–374. doi: 10.1136/ard.57.6.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin J, Irven C, Athanasou N, Carr A, Sykes B. Differential allelic expression of the type II collagen gene (COL2A1) in osteoarthritic cartilage. Am J Hum Genet. 1995;56:1186–1193. [PMC free article] [PubMed] [Google Scholar]
- Meulenbelt I, Biykerk C, De Wildt SC, Miedema HS, Breedveld FC, Pols HA, Hofman A, Van Duijn CM, Slagboom PE. Haplotype analysis of three polymorphisms of the COL2A1 gene and association with generalized radiological osteoarthritis. Ann Hum Genet. 1999;63:393–400. doi: 10.1017/S0003480099007708. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Burger H, van Duijn CM, Huang Q, Hofman A, Birkenhager JC, van Leeuwen JP, Pols HA. Adjacent genes, for COL2A1 and vitamin D receptor, are associated with separate features of radiographic osteoarthritis of the knee. Arthritis Rheum. 2000;43:1456–1464. doi: 10.1002/1529-0131(200007)43:7<1456::AID-ANR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Okuizumi H, Miyauchi A, Takagi Y, Ikeda K, Harada A. Association of transforming growth factor beta 1 genotype with spinal osteophytosis in Japanese women. Arthritis Rheum. 2000;43:452–460. doi: 10.1002/1529-0131(200002)43:2<452::AID-ANR28>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Horton WE, Jr, Lethbridge-Cejku M, Hochberg MC, Balakir R, Precht P, Plato CC, Tobin JD, Meek L, Doege K. An association between an aggrecan polymorphic allele and bilateral hand osteoarthritis in elderly white men: data from the Baltimore Longitudinal Study of Aging (BLSA). Osteoarthritis Cartilage. 1998;6:245–251. doi: 10.1053/joca.1998.0117. [DOI] [PubMed] [Google Scholar]
- Aerssens J, Dequeker J, Peeters J, Breemans S, Boonen S. Lack of association between osteoarthritis of the hip and gene polymorphisms of VDR, COL1A1, and COL2A1 in postmenopausal women. Arthritis Rheum. 1998;41:1946–1950. doi: 10.1002/1529-0131(199811)41:11<1946::AID-ART8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Huang J, Hushiyama T, Inoue K, Kawasaki T, Hukuda S. Vitamin D receptor gene polymorphisms and osteoarthritis of the hand, hip, and knee: a case-control study in Japan. Rheumatology. 2000;39:79–84. doi: 10.1093/rheumatology/39.1.79. [DOI] [PubMed] [Google Scholar]
- Wu JZ, Herzog W, Epstein M. Joint contact mechanisms in the early stages of osteoarthritis. Med Eng Phys. 2000;22:1–12. doi: 10.1016/s1350-4533(00)00012-6. [DOI] [PubMed] [Google Scholar]
- Kang R, Marui T, Ghivizzani SC, Nita IM, Georgescu HI, Suh JK, Robbins PD, Evans CH. Ex vivo gene transfer to chondrocyte in full-thickness articular cartilage defects: a feasibility study. Osteoarthritis Cartilage. 1997;5:139–143. doi: 10.1016/s1063-4584(97)80007-6. [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Caron JP, Evans C, Robbins FD, Georgescu HI, Jovanovic D, Fernandes JC, Martel-Pelletier J. In vivo suppression of early experimental osteoarthritis by IL1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1007/s004030050191. [DOI] [PubMed] [Google Scholar]
- Rojas K, Serrano de la Pena L, Gallardo T, Simmons A, Nyce K, McGrath R, Considino E, Vasko AJ, Peterson E, Grady D, Cox R, Andrew LJ, Lovett M, Overhauser J, Williams CJ. Physical map and characterization of transcripts in the candidate interval for familial chondrocalcinosis at chromosome 5p15.1. Genomics. 1999;62:177–183. doi: 10.1006/geno.1999.5997. [DOI] [PubMed] [Google Scholar]
- Consensus Development Conference. Prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–110. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- Econs MJ, Speer MC. Genetic studies of complex diseases: let the reader beware. J Bone Miner Res. 1996;11:1835–1840. [PubMed] [Google Scholar]
- Garnero P, Borel O, Sornay-Rendu E, Delmas PD. Vitamin D receptor gene polymorphisms do not predict bone turnover and bone mass in healthy premenopausal women. J Bone Miner Res. 1995;10:1283–1288. doi: 10.1002/jbmr.5650100902. [DOI] [PubMed] [Google Scholar]
- Salamone LM, Ferrell R, Black DM, Palermo L, Epstein RS, Petro N, Steadman N, Kuller LH, Cauley A. The association between vitamin D receptor gene polymorphisms and bone mineral density at the spine, hip and whole body in premenopausal women. Osteoporosis Int. 1996;6:63–68. doi: 10.1007/BF01626540. [DOI] [PubMed] [Google Scholar]
- Tokita A, Matsumoto H, Morrison NA, Tawa T, Miura Y, Fuka-mauchi K, Mitsuhashi N, Irimoto M, Yamamori S, Miura M, Watanabe T, Kuwabara Y, Yabuta K, Eisman JA. Vitamin D receptor alleles, bone mineral density and turnover in premenopausal Japanese women. J Bone Miner Res. 1996;11:1003–1009. doi: 10.1002/jbmr.5650110718. [DOI] [PubMed] [Google Scholar]
- Hansen TS, Abrahamsen B, Henriksen FL, Hermann AP, Jensen LB, Holder M, Gram J. Vitamin D receptor alleles do not predict bone mineral density or bone loss in Danish perimenopausal women. Bone. 1998;22:571–575. doi: 10.1016/S8756-3282(98)00028-3. [DOI] [PubMed] [Google Scholar]
- Garnero P, Borel P, Sonray-Rendu E, Arlot ME, Delmas PD. Vitamin D receptor gene polymorphisms are not related to bone turnover, rate of bone loss and bone mass in postmenopausal women: the OFELY study. J Bone Miner Res. 1996;11:827–834. doi: 10.1002/jbmr.5650110614. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Pols HAP, Burger H, Huang Q, Van Daele PLA, Van Duijn CM, Hofman A, Birkenhager JC, Van Leeuwen JPTM. A large scale population-based study of the association of vitamin D receptor gene polymorphisms with bone mineral density. J Bone Miner Res. 1996;11:1241–1248. doi: 10.1002/jbmr.5650110908. [DOI] [PubMed] [Google Scholar]
- Vandevyver C, Wyling T, Cassiman JJ, Raus J, Geusens P. Influence of vitamin D receptor gene alleles on bone mineral density in postmenopausal and osteoporotic women. J Bone Miner Res. 1997;12:241–247. doi: 10.1359/jbmr.1997.12.2.241. [DOI] [PubMed] [Google Scholar]
- Tamai M, Yokouchi M, Komiya S, Mochizuki , Hidaka S, Narita S, Inoue A, Itoh K. Correlation between vitamin D receptor genotypes and bone mineral density in Japanese patients with osteoporosis. Calcif Tissue Int. 1997;60:229–232. doi: 10.1007/s002239900219. [DOI] [PubMed] [Google Scholar]
- Gennari L, Becherini L, Masi L, Mansani R, Gonnelli S, Cepollaro C, Martini S, Montagnani A, Lentini G, Becorpi AM, Brandi ML. Vitamin D and estrogen receptor allelic variants in postmenopausal women: evidence of multiple gene contribution on bone mineral density. J Clin Endocrinol Metab. 1998;83:939–944. doi: 10.1210/jcem.83.3.4649. [DOI] [PubMed] [Google Scholar]
- Jorgensen HL, Scholler J, Sand JC, Bjuring M, Hassager C, Christiansen C. Relation of common allelic variation at vitamin D receptor locus to bone mineral density and postmenopausal bone loss: cross sectional and longitudinal population study. BMJ. 1998;313:586–590. doi: 10.1136/bmj.313.7057.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnes M, Berg JP, Halse J, Lehmann EH. Lack of relationship between vitamin D receptor genotype and forearm bone gain in healthy children, adolescents, and young adults. J Clin Endocrinol Metab. 1997;82:851–855. doi: 10.1210/jcem.82.3.3814. [DOI] [PubMed] [Google Scholar]
- Suarez F, Zeghoud F, Rossignol C, Walrant O, Garabedian M. Association between vitamin D receptor gene polymorphism and sex-dependent growth during the first two years of life. J Clin Endocrinol Metab. 1997;82:2966–2970. doi: 10.1210/jcem.82.9.4232. [DOI] [PubMed] [Google Scholar]
- Sainz J, Van Tornout JM, Loro ML, Sayre J, Roe TF, Gilsanz V. Vitamin D receptor gene polymorphisms and bone density in prepubertal American girls of Mexican descent. N Engl J Med. 1997;337:77–82. doi: 10.1056/NEJM199707103370202. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Rizzoli R, Slosman DO, Bonjour JP. Do dietary calcium and age explain the controversy surrounding the relationship between bone mineral density and vitamin D receptor gene polymorphisms? J Bone Miner Res. 1998;13:363–370. doi: 10.1359/jbmr.1998.13.3.363. [DOI] [PubMed] [Google Scholar]
- Howard G, Nguyen T, Morrison N, Watanabe T, Sambrook P, Eisman J, Kelly P. Genetic influences on bone density: physiological correlates of vitamin D receptor gene alleles in pre-menopausal women. J Clin Endocrinol Metab. 1995;80:2800–2805. doi: 10.1210/jcem.80.9.7673427. [DOI] [PubMed] [Google Scholar]
- Zmuda JM, Cauley JA, Danielson ME, Wolf RL, Ferrell RE. Vitamin D receptor gene polymorphisms, bone turnover, and rates of bone loss in older African-American women. J Bone Miner Res. 1997;12:1446–1452. doi: 10.1359/jbmr.1997.12.9.1446. [DOI] [PubMed] [Google Scholar]
- Keen RW, Major PJ, Lanchbury JS, Spector TD. Vitamin D receptor gene polymorphisms and bone loss. Lancet. 1995;345:990. [PubMed] [Google Scholar]
- Krall EA, Parry P, Lichter JB, Dawson-Hughes Vitamin D receptor alleles and rates of bone loss: influences of years since menopause and calcium intake. J Bone Miner Res. 1995;10:978–984. doi: 10.1002/jbmr.5650100620. [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Finneran S. Calcium absorption on high and low calcium intake in relation to vitamin D receptor genotype. J Clin Endocrinol Metab. 1995;80:3657–3661. doi: 10.1210/jcem.80.12.8530616. [DOI] [PubMed] [Google Scholar]
- Kinyamu UK, Gallagher JC, Knezetic JA, De Luca HF, Prahl JM, Lanspa SJ. Effect of vitamin D receptor genotypes on calcium absorption, duodenal vitamin D receptor concentration, and serum 1,25 dihydroxyvitamin D levels in normal women. Calcif Tissue Int. 1997;60:491–495. doi: 10.1007/s002239900269. [DOI] [PubMed] [Google Scholar]
- Wishart JM, Horowitz M, Need AG, Scopacasa F, Morris HA, Clifton PM, Nordin BEC. Relations between calcium intake, calcitriol, polymorphisms of vitamin D receptor gene, and calcium absorption in premenopausal women. Am J Clin Nutr. 1997;65:798–802. doi: 10.1093/ajcn/65.3.798. [DOI] [PubMed] [Google Scholar]
- Gennari L, Becherini L, Masi L, Gonnelli S, Cepollaro C, Martini S, Mansani R, Brandi ML. Vitamin D receptor genotypes and intestinal calcium absorption in postmenopausal women. Calcif Tissue Int. 1997;61:460–463. doi: 10.1007/s002239900368. [DOI] [PubMed] [Google Scholar]
- Houston LA, Grant SFA, Reid DM, Ralston SH. Vitamin D receptor polymorphism, bone mineral density and osteoporotic vertebral fracture: studies in a UK population. Bone. 1996;18:249–252. doi: 10.1016/8756-3282(95)00483-1. [DOI] [PubMed] [Google Scholar]
- Berg JP, Falch JA, Haug E. Fracture rate, pre- and postmenopausal bone mass and early and late postmenopausal bone loss are not associated with vitamin D receptor genotype in a high-endemic area of osteoporosis . Europ J Endocrinol. 1996;135:96–100. doi: 10.1530/eje.0.1350096. [DOI] [PubMed] [Google Scholar]
- Yanagy H, Tomura H, Kawanami K, Hosokawa M, Tanaka M, Kobayashi K, Tsuchiya S, Amagai H, Hayashi K, Hamaguchi H. Vitamin D receptor gene polymorphisms are associated with osteoporosis in Japanese women. J Clin Endocrinol Metab. 1996;81:4179–4180. doi: 10.1210/jcem.81.11.8923886. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Umbach DM. Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. J Bone Miner Res. 1996;11:1841–1849. doi: 10.1002/jbmr.5650111203. [DOI] [PubMed] [Google Scholar]
- Gong G, Stern HS, Cheng SC, Fong N, Mordeson J, Deng HW, Recker RR. The association of bone mineral density with vitamin D receptor gene polymorphisms. Osteoporosis Int. 1999;9:55–64. doi: 10.1007/s001980050116. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Rizzoli R, Chevallery T, Slosman D, Eisman JA, Bonjour JP. Vitamin D receptor gene polymorphisms and change in lumbar spine bone mineral density. Lancet. 1995;345:423–424. doi: 10.1016/s0140-6736(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Salamone LM, Glynn NW, Black DM, Ferrell RE, Palermo L, Epstein RS, Kuller LH, Cauley JA. Determinants of postmenopausal bone mineral density: the interplay of genetic and lifestyle factors. J Bone Miner Res. 1996;11:1557–1565. doi: 10.1002/jbmr.5650111024. [DOI] [PubMed] [Google Scholar]
- Kiel DP, Myers RH, Cupples LA, Kong XF, Zhu XH, Ordovas J, Schaefer EJ, Felson DT, Rush D, Wilson PW, Eisman JA, Holick MF. The BsmI vitamin D receptor restriction fragment length polymorphism (bb) influences the effect of calcium intake on bone mineral density. J Bone Miner Res. 1997;12:1049–1057. doi: 10.1359/jbmr.1997.12.7.1049. [DOI] [PubMed] [Google Scholar]
- Willing M, Sowers M, Aron D, Clark MK, Burns T, Bunten C, Crutchfield M, D'Agostino D, Jannausch M. Bone mineral density and its change in white women: estrogen and vitamin D receptor genotypes and their interaction. J Bone Miner Res. 1998;13:695–705. doi: 10.1359/jbmr.1998.13.4.695. [DOI] [PubMed] [Google Scholar]
- Mocharla H, Butch AW, Pappas AA, Flick JT, Weistein RS, De Togni P, Jilka RL, Roberson PK, Parfitt AM, Manolagas SC. Quantification of vitamin D receptor mRNA by competitive polymerase chain reaction in PBMC: lack of correspondence with common allelic variants. J Bone Miner Res. 1997;12:726–733. doi: 10.1359/jbmr.1997.12.5.726. [DOI] [PubMed] [Google Scholar]
- VerbeeK W, Gombart AF, Shiohara M, Campbell M, Koeffler HP. Vitamin D receptor: no evidence for allele-specific mRNA stability in cells that are heterozygous for the Taq I restriction enzyme polymorphism. Biochem Biophys Res Commun. 1997;238:77–80. doi: 10.1006/bbrc.1997.7239. [DOI] [PubMed] [Google Scholar]
- Gross C, Musiol IM, Eccleshall TR, Mallory PJ, Feldman D. Vitamin D receptor gene polymorphism: analysis of ligand binding and hormone responsiveness in cultured fibroblasts. Biochem Biophys Res Commun. 1998;242:467–473. doi: 10.1006/bbrc.1997.7986. [DOI] [PubMed] [Google Scholar]
- Gross C, Eccleshall TR, Malloy PJ, Villa ML, Marcus R, Feldman D. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal Mexican-American women. J Bone Miner Res. 1996;11:1850–1855. doi: 10.1002/jbmr.5650111204. [DOI] [PubMed] [Google Scholar]
- Arai H, Myamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S, Takeda E. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12:915–921. doi: 10.1359/jbmr.1997.12.6.915. [DOI] [PubMed] [Google Scholar]
- Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D. The vitamin D receptor start codon polymorphism (Fok I) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997;12:1043–1048. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- Gennari L, Becherini L, Mansani R, Masi L, Falchetti A, Morelli A, Colli E, Gonnelli S, Cepollaro C, Brandi ML. Fok I polymorphism at translation initiation site of the vitamin D receptor gene predicts bone mineral density and vertebral fractures in postmenopausal Italian women. J Bone Miner Res. 1999;14:1379–86. doi: 10.1359/jbmr.1999.14.8.1379. [DOI] [PubMed] [Google Scholar]
- Eccleshall TR, Garnero P, Gross C, Delmas PD, Feldman D. Lack of correlation between start codon polymorphism of the vitamin D receptor gene and bone mineral density in pre-menopausal French women: the OFELY study. J Bone Miner Res. 1998;13:31–35. doi: 10.1359/jbmr.1998.13.1.31. [DOI] [PubMed] [Google Scholar]
- Ferrari SL, Rizzoli R, Manen D, Slosman D, Bonjour JP. Vitamin D receptor gene start codon polymorphisms (Fok I) and bone mineral density: interaction with age, dietary calcium, and 3'-end region polymorphisms. J Bone Miner Res. 1998;13:925–930. doi: 10.1359/jbmr.1998.13.6.925. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TG, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Pensler JM, Radosevich JA, Higbee R, Langman CB. Osteoclasts isolated from membranous bone in children exhibit nuclear estrogen and progesterone receptors. J Bone Miner Res. 1990;5:797–802. doi: 10.1002/jbmr.5650050802. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyod J, Frank GR, Talahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- Sano M, Inoue S, Hosoi T, Ouchi Y, Emi M, Shiraki M, Orimo H. Association of estrogen receptor dinucleotide repeat polymorphism with osteoporosis. Biochem Biophys Res Commun. 1995;217:378–383. doi: 10.1006/bbrc.1995.2787. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Inoue S, Hosoi T, Ouchi Y, Shiraki M, Orimo H. Association of bone mineral density with polymorphism of estrogen receptor gene. J Bone Miner Res. 1996;11:306–311. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- Han KO, Moon IG, Kang YS, Chung HY, Min HK, Han IK. Non association of estrogen receptor genotypes with bone mineral density and estrogen responsiveness to hormone replacement therapy in Korean postmenopausal women. J Clin Endocrinol Metab. 1997;82:991–995. doi: 10.1210/jcem.82.4.3879. [DOI] [PubMed] [Google Scholar]
- Ho AY, Yeung SS, Kung AW. PvuII polymorphisms of the estrogen receptor gene alpha and bone mineral density in healthy Southern Chinese women. Calcif Tissue Int. 2000;66:405–408. doi: 10.1007/s002230010082. [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Jorgensen HL, Heegaard AM, Bayer L, Hansen L, Hassager C. No major effect of estrogen receptor polymorphisms on bone mineral density or bone loss in postmenopausal Danish women. Bone. 2000;26:111–116. doi: 10.1016/S8756-3282(99)00261-6. [DOI] [PubMed] [Google Scholar]
- Becherini L, Gennari L, Masi L, Mansani R, Massart F, Morelli A, Falchetti A, Gonnelli S, Fiorelli G, Tanini A, Brandi ML. Evidence of a linkage disequilibrium between polymorphisms in the human estrogen receptor alpha gene and their relationship to bone mass variation in postmenopausal Italian women. Hum Mol Genet. 2000;9:2043–2050. doi: 10.1093/hmg/9.13.2043. [DOI] [PubMed] [Google Scholar]
- Sowers M, Willing M, Burns T, Deschenes S, Hollis B, Crutchfield M, Jannusch M. Genetic markers, bone mineral density and serum osteocalcin levels. J Bone Miner Res. 1999;14:1411–1419. doi: 10.1359/jbmr.1999.14.8.1411. [DOI] [PubMed] [Google Scholar]
- Langdahl BL, Lokke E, Carstens M, Stenkjaer LL, Eriksen EF. A TA repeat polymorphism in the estrogen receptor gene is associated with osteoporotic fractures but polymorphisms in the first exon and intron are not. J Bone Mineral Res. 2000;15:2222–2230. doi: 10.1359/jbmr.2000.15.11.2222. [DOI] [PubMed] [Google Scholar]
- Albagha OM, McGuigan FE, Reid DM, Ralston SH. Estrogen receptor alpha gene polymorphisms and bone mineral density: haplotype analysis in women from the United Kingdom. J Bone Mineral Res. 2001;16:128–134. doi: 10.1359/jbmr.2001.16.1.128. [DOI] [PubMed] [Google Scholar]
- Sykes B. Bone disease cracks genetics. Nature. 1990;348:18–20. doi: 10.1038/348018a0. [DOI] [PubMed] [Google Scholar]
- Grant SFA, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with polymorphic SP1 site in the collagen type I alpha 1 gene. Nat Genet. 1996;14:203–205. doi: 10.1038/ng1096-203. [DOI] [PubMed] [Google Scholar]
- Uitterlinden AG, Burger H, Huang Q, Yue F, McGuian FEA, Grant SFA, Hofman A, van Leeuwen JPTM, Pols HAP, Ralston SH. Relation of alleles of the collagen type Ia1 gene to bone density and the risk of osteoporotic fractures in postmenopausal women. N Engl J Med. 1998;338:1016–1021. doi: 10.1056/NEJM199804093381502. [DOI] [PubMed] [Google Scholar]
- Garnero P, Borel O, Grant SFA, Ralston SH, Delmas PD. Collagen Ia1 Sp1 polymorphism, bone mass, and bone turnover in healthy French postmenopausal women: the OFELY study. J Bone Miner Res. 1998;13:813–817. doi: 10.1359/jbmr.1998.13.5.813. [DOI] [PubMed] [Google Scholar]
- Langdahl BL, Ralston SH, Grant SFA, Eriksen EF. An Sp1 binding site polymorphism in the COLIA1 gene predicts osteoporotic fractures in men and women. J Bone Miner Res. 1998;13:1384–1389. doi: 10.1359/jbmr.1998.13.9.1384. [DOI] [PubMed] [Google Scholar]
- Keen RW, Woodford-Richens KL, Grant SFA, Ralston SH, Lanchbury JS, Spector TD. Association of polymorphism at the type I collagen (COLIA1) locus is associated with reduced bone mineral density, increased collagen turnover. Arthritis Rheum. 1999;42:285–290. doi: 10.1002/1529-0131(199902)42:2<285::AID-ANR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Liden M, Wilen B, Ljunghall S, Melhus H. Polymorphism at the Sp1 binding site in the collagen type I alpha gene does not predict bone mineral density in postmenopausal women in Sweden. Calcif Tissue Int. 1998;63:293–295. doi: 10.1007/s002239900529. [DOI] [PubMed] [Google Scholar]
- Hustmyer FG, Lui G, Johnston CC, Christian J, Peacock M. Polymorphism at the Sp1 binding site of COLIA1 and bone mineral density in pre-menopausal female twins and elderly fracture patients. Osteoporosis Int. 1999;9:346–350. doi: 10.1007/s001980050157. [DOI] [PubMed] [Google Scholar]
- Dean V, Smith FG, Robins SP, Ralston SH. Relationship between COLIA1 Sp1 alleles, gene transcription, collagen production and bone strength [abstract]. Bone. 1998;S160 [Google Scholar]
- Murray RE, McGuian F, Grant SFA, Reid DM, Ralston SH. Polymorphisms of the interleukin-6 gene are associated with bone mineral density. Bone. 1997;21:89–92. doi: 10.1016/S8756-3282(97)00081-1. [DOI] [PubMed] [Google Scholar]
- Langdahl BL, Knudsen JY, Jensen HK, Gregersen N, Eriksen EF. A sequence variation: 713-delC in the transforming growth factor-?1 gene has higher prevalence in osteoporotic women than in normal women and is associated with very low bone mass in osteoporotic women and increased bone turnover in both osteoporotic and normal women. Bone. 1997;20:289–294. doi: 10.1016/S8756-3282(96)00363-8. [DOI] [PubMed] [Google Scholar]
- Shiraki M, Shiraki Y, Aoki C, Inoue S, Kaneki M, Ouchi Y. Association of bone mineral density with apolipoprotein E phenotype. J Bone Miner Res. 1997;12:1438–1445. doi: 10.1359/jbmr.1997.12.9.1438. [DOI] [PubMed] [Google Scholar]
- Masi L, Becherini L, Gennari L, Colli E, Mansani R, Cepollaro C, Gonnelli S, Tanini A, Brandi ML. Allelic variant of human calcitonin receptor: distribution and association with bone mass in postmenopausal Italian women. Biochem Biophys Res Commun. 1998;245:622–626. doi: 10.1006/bbrc.1998.8445. [DOI] [PubMed] [Google Scholar]
- Masi L, Becherini L, Colli E, Gennari L, Mansani R, Falchetti A, Becorpi AM, Cepollaro C, Gonnelli S, Tanini A, Brandi ML. Polymorphisms of the calcitonin receptor gene are associated with bone mineral density in postmenopausal Italian women. Biochem Biophys Res Commun. 1998;248:190–195. doi: 10.1006/bbrc.1998.8880. [DOI] [PubMed] [Google Scholar]
- De Venejoul MC, Cohen-Solal ME, Beaudreuil J. Analysis of 22 families suggests the feasibility of linkage studies in osteoporosis [abstract]. J Bone Miner Res. p. S494.
- Shapiro JR, Rowe DW, Burn V. Familial osteoporosis pedigrees [abstract]. J Bone Miner Res. 1987;2(S2):344A. [Google Scholar]
- Koller DL, Rodriguez LA, Christian JC, Slemenda CW, Econs MJ, Hui SL, Morin P, Conneally PN, Joslyn G, Curran ME, Peacock M, Johnston CC, Foroud T. Linkage of a QTL contributing to normal variation in bone mineral density to chromosome 11q12-13. J Bone Miner Res. 1998;13:1903–1908. doi: 10.1359/jbmr.1998.13.12.1903. [DOI] [PubMed] [Google Scholar]
- Devoto M, Shimoya K, Caminis J, Ott J, Tenenhouse A, Whyte MP, Sereda L, Hall S, Considine E, Williams CJ, Tromp G, Kuivaniemi H, Ala-Kokko L, Prockop DJ, Spotila LD. First-stage autosomal genome screen in extended pedigrees suggests genes predisposing to low bone mineral density on chromosomes 1p, 2p and 4q. Eur J Hum Genet. 1998;6:151–157. doi: 10.1038/sj/ejhg/5200169. [DOI] [PubMed] [Google Scholar]
- Niu T, Chen C, Cordell H, Yang Y, Wang B, Wang Z, Fang Z, Schork NJ, Rosen CJ, Xu X. A genome-wide scan for loci linked to forearm bone mineral density. Hum Genet. 1999;104:226–233. doi: 10.1007/s004390050940. [DOI] [PubMed] [Google Scholar]
- Duncan EN, Brown MA, Sinsheimer J, Bell J, Carr AJ, Wordsworth BP, Wass JAH. Suggestive linkage of the parathyroid receptor type 1 to osteoporosis. J Bone Miner Res. 1999;14:1993–1999. doi: 10.1359/jbmr.1999.14.12.1993. [DOI] [PubMed] [Google Scholar]
- Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Koller DL, Econs MJ, Morin PA, Christian JC, Hui SL, Parry P, Curran ME, Rodriguez LA, Conneally PM, Joslyn G, Peacock M, Johnston CC, Foroud T. Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab. 2000;85:3116–3120. doi: 10.1210/jcem.85.9.6778. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Gong G, Kimberling W, Recker SM, Kimmel DB, Recker RB. Linkage of a gene causing high bone mass to human chromosome 11 (11q12-13). Am J Hum Genet. 1997;60:1326–1332. doi: 10.1086/515470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman SR, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDMM). Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- VandeBerg JL, Williams-Blangero S. Advantages and limitations of non human primates as animal models in genetic research on complex disease. J Med Primatol. 1997;26:113–119. doi: 10.1111/j.1600-0684.1997.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Beamer WG, Rosen CJ, Bronson RT, Gu W, Donahue LR, Baylink DJ, Richardson CC, Crawford GC, Barker JE. Spontaneous fracture (sfx): a mouse genetic model of defective peripubertal bone formation. Bone. 2000;72:619–626. doi: 10.1016/s8756-3282(00)00369-0. [DOI] [PubMed] [Google Scholar]
- Velculescu VE, Madden SL, Zhang L, Madden SL, Zhang L, Lash AE, Yu J, Rago C, Lal A, Wang CJ, Beaudry GA, Ciriello KM, Cook BP, Dufault MR, Ferguson AT, Gao Y, He TC, Hermeking H, Hiraldo SK, Hwang PM, Lopez MA, Luderer HF, Mathews B, Petroziello JM, Polyak K, Zawel L, Kinzler KW, et al. Analysis of human transcriptomes. Nature Genet. 1999;23:387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- Knowlton RG, Katzenstein PL, Moskowitz RW, Weaver EJ, Malemud CJ, Pathria MN, Jimenez SA, Prockop DJ. Genetic linkage of a polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990;22:526–530. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- Priestley L, Fergusson C, Ogilvie D, Wordsworth P, Smith R, Pattrick M, Doherty M, Sykes B. A limited association of generalized osteoarthritis with alleles at the type II collagen locus: COL2A1. Br J Rheumatol. 1991;30:272–275. doi: 10.1093/rheumatology/30.4.272. [DOI] [PubMed] [Google Scholar]


